Characterization of Mucosal Disaccharidases from Human Intestine
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Content and Activity of Intestinal Disaccharidases
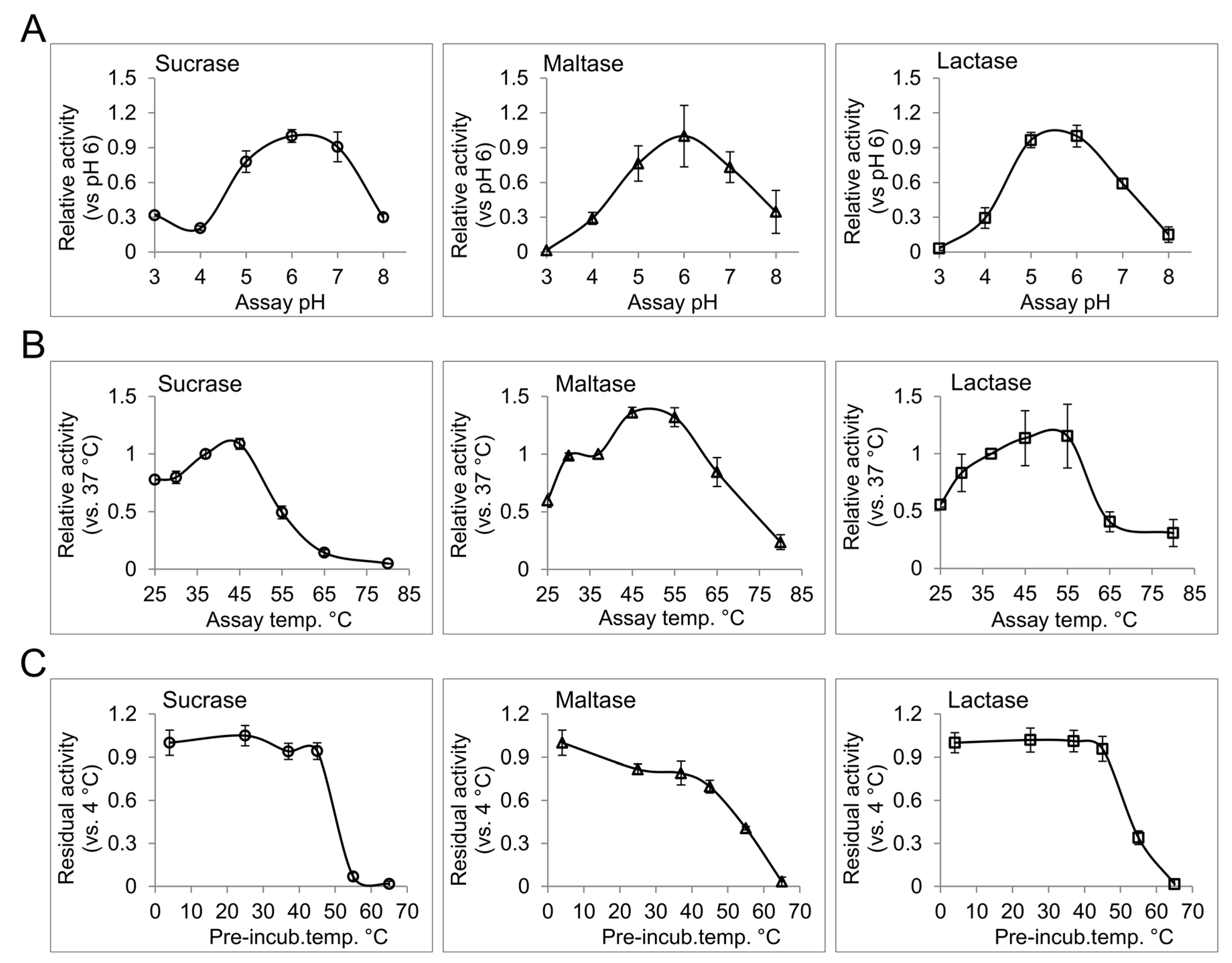
3.2. The Effect of pH and Temperature on the Function of Intestinal Disaccharidases
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Materials
Appendix B. Experimental Approach
Appendix B.1. Determination of BBM Content and Activity of the Disaccharidases
Appendix B.2. The Effects of pH and Temperature on the Activity of Intestinal Disaccharidases
References
- Naim, H.Y.; Zimmer, K.-P. Genetically determined disaccharidase deficiency. In Walker’s Pediatric Gastrointestinal Disease; Kleinman, R., Goulet, O.-J., Mieli-Vergani, G., Sanderson, I., Sherman, P., Shneider, B., Eds.; PMPH-USA: Orlando, FL, USA, 2008; Volume 2, pp. 880–887. [Google Scholar]
- Vanbeers, E.H.; Buller, H.A.; Grand, R.J.; Einerhand, A.W.C.; Dekker, J. Intestinal brush-border glycohydrolases-structure, function, and development. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 197–262. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.L.; Avery, S.; Sen, P.; Swallow, D.M.; Hahn, D.; Sterchi, E. The maltase-glucoamylase gene: Common ancestry to sucrase-isomaltase with complementary starch digestion activities. Proc. Natl. Acad. Sci. USA 2003, 100, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Naim, H.Y.; Sterchi, E.E.; Lentze, M.J. Biosynthesis of the human sucrase-isomaltase complex-differential O-glycosylation of the sucrase subunit correlates with its position within the enzyme complex. J. Biol. Chem. 1988, 263, 7242–7253. [Google Scholar] [PubMed]
- Naim, H.Y.; Sterchi, E.E.; Lentze, M.J. Structure, biosynthesis, and glycosylation of human small intestinal maltase-glucoamylase. J. Biol. Chem. 1988, 263, 19709–19717. [Google Scholar] [PubMed]
- Naim, H.Y.; Sterchi, E.E.; Lentze, M.J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem. J. 1987, 241, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Alfalah, M.; Jacob, R.; Preuss, U.; Zimmer, K.P.; Naim, H.; Naim, H.Y. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol. 1999, 9, 593–596. [Google Scholar] [CrossRef]
- Jacob, R.; Alfalah, M.; Grunberg, J.; Obendorf, M.; Naim, H.Y. Structural determinants required for apical sorting of an intestinal brush-border membrane protein. J. Biol. Chem. 2000, 275, 6566–6572. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.L.; Eldering, J.; Avery, S.; Hahn, D.; Quaroni, A.; Sterchi, E. Human small intestinal maltase-glucoamylase cdna cloning. Homology to sucrase-isomaltase. J. Biol. Chem. 1998, 273, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Radebach, I.; Wuthrich, M.; Grunberg, J.; Sterchi, E.E.; Naim, H.Y. Maturation of human intestinal lactase-phlorizin hydrolase-generation of the brush border form of the enzyme involves at least two proteolytic cleavage steps. Eur. J. Biochem. 1996, 236, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Naim, H.Y. Apical membrane proteins are transported in distinct vesicular carriers. Curr. Biol. 2001, 11, 1444–1450. [Google Scholar] [CrossRef]
- Robayo-Torres, C.C.; Quezada-Calvillo, R.; Nichols, B.L. Disaccharide digestion: Clinical and molecular aspects. Clin. Gastroenterol. Hepatol. 2006, 4, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Hauri, H.P.; Sterchi, E.E.; Bienz, D.; Fransen, J.A.M.; Marxer, A. Expression and intracellular-transport of microvillus membrane hydrolases in human intestinal epithelial-cells. J. Cell Biol. 1985, 101, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Preiser, H.; Maestracci, D.; Ghosh, B.K.; Cerda, J.J.; Crane, R.K. Purification of the human intestinal brush border membrane. Biochim. Biophys. Acta 1973, 323, 98–112. [Google Scholar] [CrossRef]
- Beaulieu, J.F.; Nichols, B.; Quaroni, A. Posttranslational regulation of sucrase-isomaltase expression in intestinal crypt and villus cells. J. Biol. Chem. 1989, 264, 20000–20011. [Google Scholar] [PubMed]
- Maiuri, L.; Raia, V.; Potter, J.; Swallow, D.; Ho, M.W.; Fiocca, R.; Finzi, G.; Cornaggia, M.; Capella, C.; Quaroni, A.; et al. Mosaic pattern of lactase expression by villous enterocytes in human adult-type hypolactasia. Gastroenterology 1991, 100, 359–369. [Google Scholar] [CrossRef]
- Wetzel, G.; Heine, M.; Rohwedder, A.; Naim, H.Y. Impact of glycosylation and detergent-resistant membranes on the function of intestinal sucrase-isomaltase. Biol. Chem. 2009, 390, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, L.; Menten, M.L.; Johnson, K.A.; Goody, R.S. The original michaelis constant: Translation of the 1913 michaelis-menten paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [PubMed]
- Press, A.G.; Hauptmann, I.A.; Hauptmann, L.; Fuchs, B.; Fuchs, M.; Ewe, K.; Ramadori, G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 1998, 12, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Gennari, F.J.; Weise, W.J. Acid-base disturbances in gastrointestinal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Ganio, M.S.; Seifert, T.; Overgaard, M.; Secher, N.H.; Crandall, C.G. Pulmonary artery and intestinal temperatures during heat stress and cooling. Med. Sci. Sports Exerc. 2012, 44, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.R.; Denissen, E.C.; McKune, A.J.; Peters, E.M. Intestinal temperature, heart rate, and hydration status in multiday trail runners. Clin. J. Sport Med. 2012, 22, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A. Method for assay of intestinal disaccharidases. Anal. Biochem. 1964, 7, 18–25. [Google Scholar] [CrossRef]
- Gupta, S.K.; Chong, S.K.; Fitzgerald, J.F. Disaccharidase activities in children: Normal values and comparison based on symptoms and histologic changes. J. Pediatric. Gastroenterol. Nutr. 1999, 28, 246–251. [Google Scholar] [CrossRef]
- Reinshagen, K.; Keller, K.M.; Haase, B.; Leeb, T.; Naim, H.Y.; Zimmer, K.P. Mosaic pattern of sucrase isomaltase deficiency in two brothers. Pediatr. Res. 2008, 63, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Gericke, B.; Amiri, M.; Scott, C.R.; Naim, H.Y. Molecular pathogenicity of novel sucrase-isomaltase mutations found in congenital sucrase-isomaltase deficiency patients. Biochim. Biophys. Acta 2017, 1863, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Naim, H.Y. Miglustat-induced intestinal carbohydrate malabsorption is due to the inhibition of α-glucosidases, but not β-galactosidases. J. Inherit. Metab. Dis. 2012, 35, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
| SI | MGAM | LPH | ||||
|---|---|---|---|---|---|---|
| Content (% total BBM protein) | 8.2 ± 0.7 | 2.7 ± 1.4 | 1.4 ± 0.5 | |||
| Substrate | Sucrose | Isomaltose | Maltose | Maltose | Lactose | |
| Specific activity (U·mg−1) | immunopr. | 9.5 ± 1.9 | 5.2 ± 1.4 | 10.3 ± 3.3 | 28.1 ± 12.4 | 1.8 ± 0.3 |
| BBM | 27.8 ± 0.5 | 16.5 ± 0.8 | 20.2 ± 0.4 | 5.6 ± 0.3 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri, M.; Naim, H.Y. Characterization of Mucosal Disaccharidases from Human Intestine. Nutrients 2017, 9, 1106. https://doi.org/10.3390/nu9101106
Amiri M, Naim HY. Characterization of Mucosal Disaccharidases from Human Intestine. Nutrients. 2017; 9(10):1106. https://doi.org/10.3390/nu9101106
Chicago/Turabian StyleAmiri, Mahdi, and Hassan Y. Naim. 2017. "Characterization of Mucosal Disaccharidases from Human Intestine" Nutrients 9, no. 10: 1106. https://doi.org/10.3390/nu9101106





