Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Red Seaweed Source
2.2. Seaweed Analysis
2.3. Rats and Diets
2.4. Systolic Blood Pressure
2.5. Oral Glucose Tolerance Test
2.6. Body Composition
2.7. Organ Weights
2.8. Histology
2.9. Biochemical Analyses of Rat Plasma
2.10. Fecal Lipid Measurements
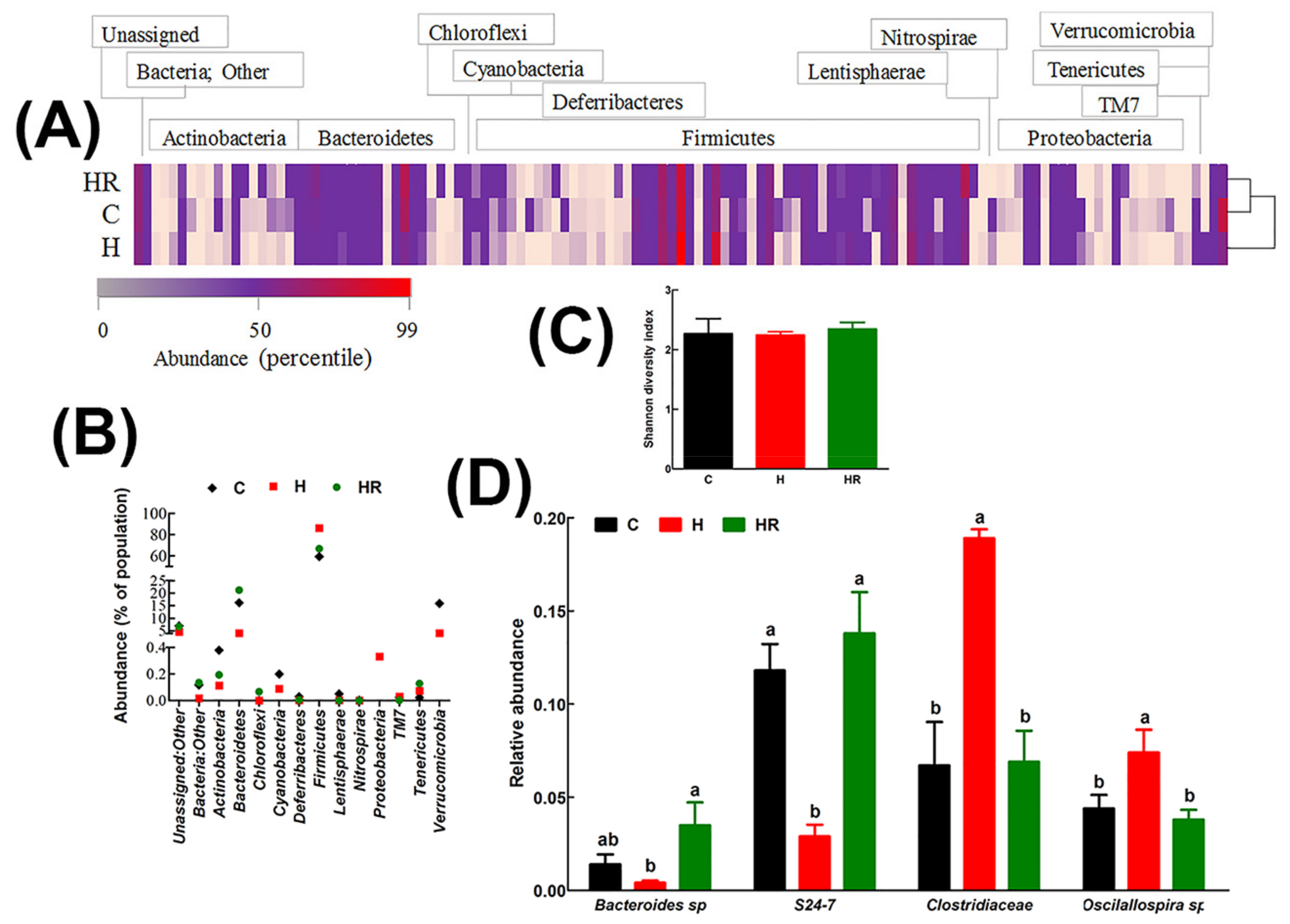
2.11. Gut Microbiota Diversity Profiling
2.12. Metal and Metalloid Liver Analyses
2.13. Statistical Analysis
3. Results
3.1. Composition of Kappaphycus
3.2. Diet Intake and Body Composition
3.3. Oral Glucose Tolerance, Plasma Biochemistry, and Fecal Lipids
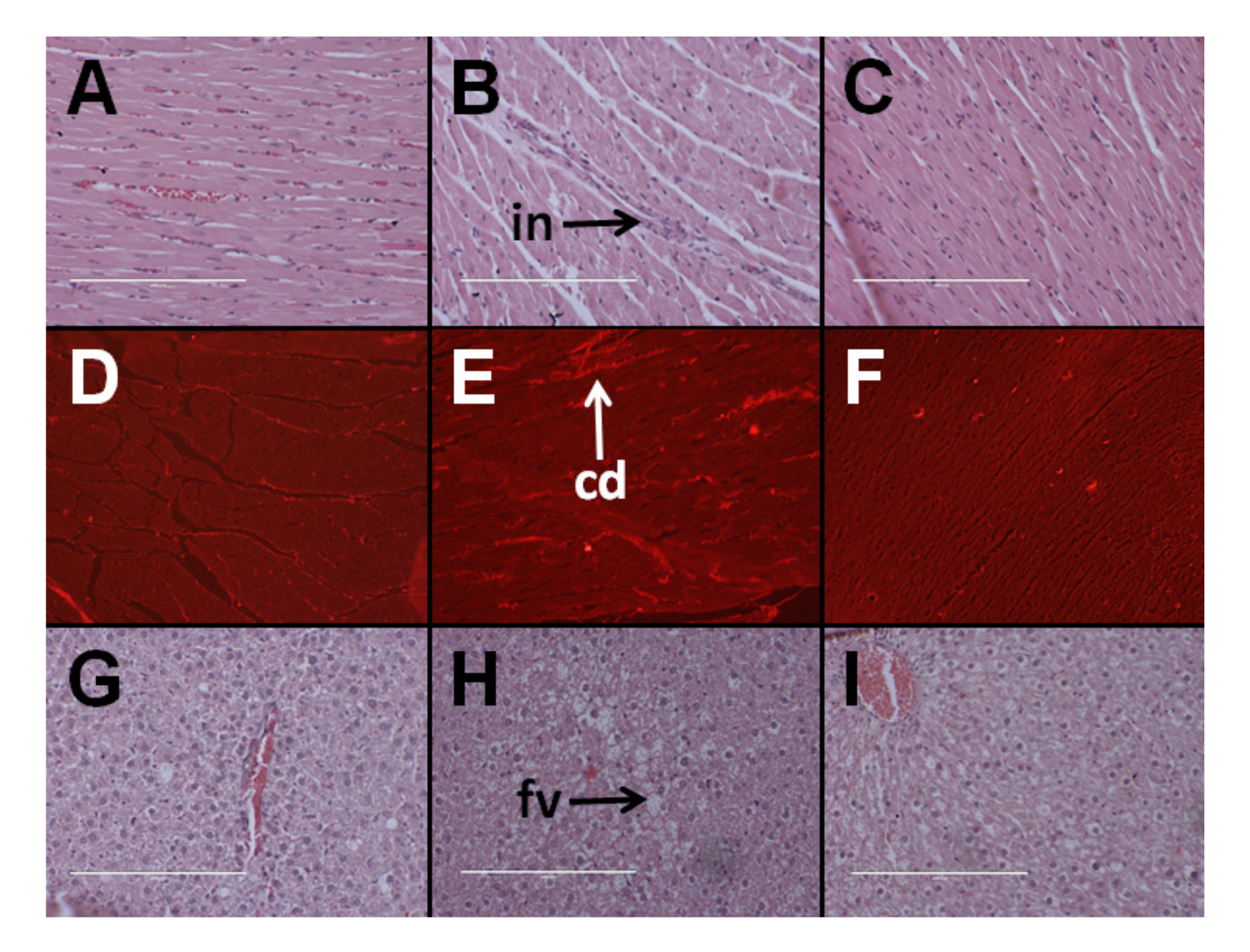
3.4. Cardiovascular Structure and Function
3.5. Hepatic Structure and Function
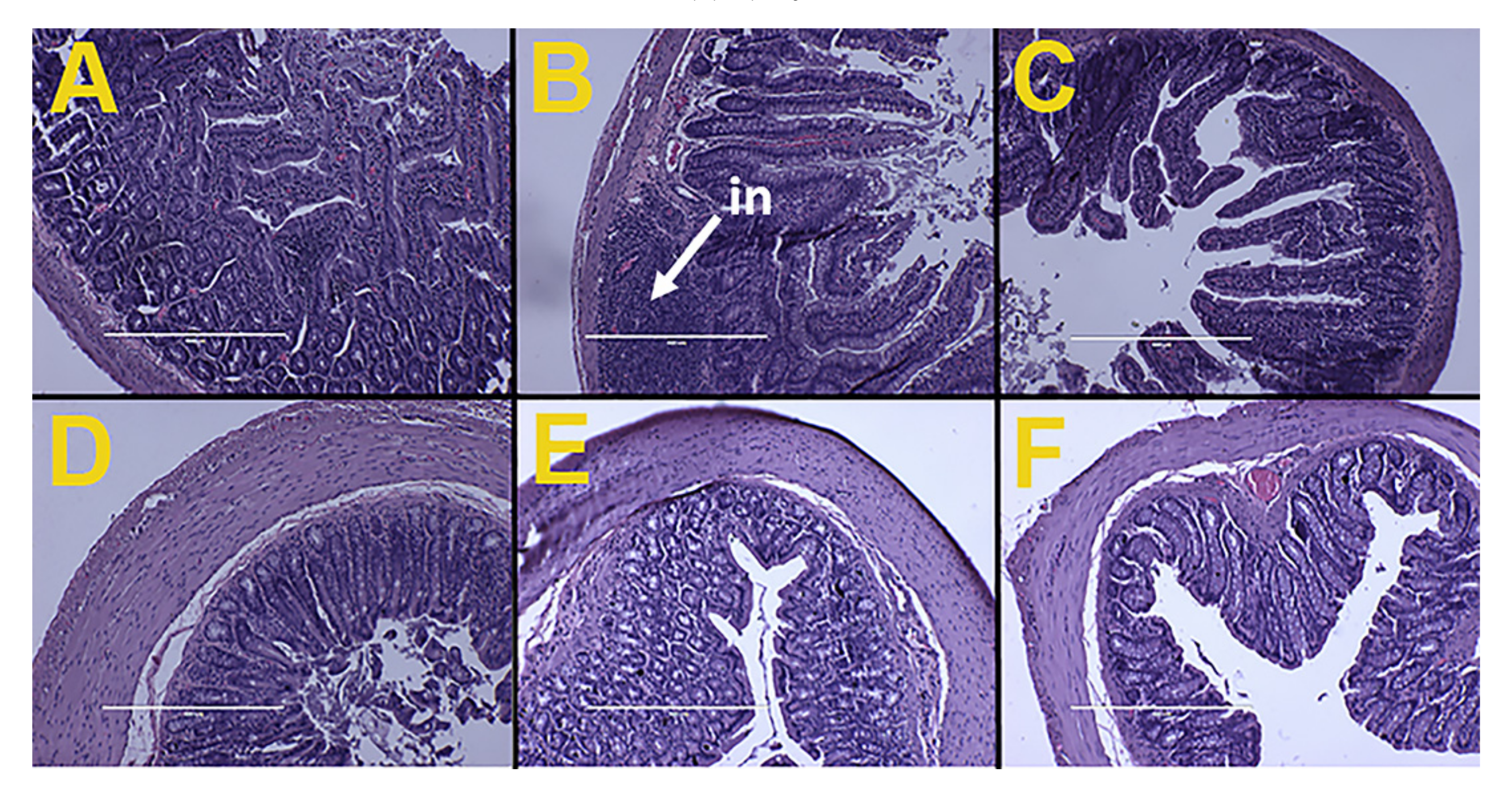
3.6. Gut Structure and Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iso, H.; Date, C.; Noda, H.; Yoshimura, T.; Tamakoshi, A.; Group, J.S. Frequency of food intake and estimated nutrient intake among men and women: The JACC study. J. Epidemiol. 2005, 15 (Suppl. 1), S24–S42. [Google Scholar] [CrossRef] [PubMed]
- Teas, J.; Baldeon, M.E.; Chiriboga, D.E.; Davis, J.R.; Sarries, A.J.; Braverman, L.E. Could dietary seaweed reverse the metabolic syndrome? Asia Pac. J. Clin. Nutr. 2009, 18, 145–154. [Google Scholar] [PubMed]
- Hoang, K.C.; Le, T.V.; Wong, N.D. The metabolic syndrome in East Asians. J. Cardiometab. Syndr. 2007, 2, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Yamane, K.; Jitsuiki, K.; Nakanishi, S.; Kamei, N.; Watanabe, H.; Kohno, N. Prevalence of metabolic syndrome compared between native Japanese and Japanese-Americans. Diabetes Res. Clin. Pract. 2008, 79, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar Drugs 2015, 13, 788–805. [Google Scholar] [CrossRef] [PubMed]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Y.; Sun-Waterhouse, D.; You, L.; Fu, X. Advantages of the polysaccharides from Gracilaria lemaneiformis over metformin in antidiabetic effects on streptozotocin-induced diabetic mice. RSC Adv. 2017, 7, 9141–9151. [Google Scholar] [CrossRef]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, K.; Ganesan, K.; Subba Rao, P.V. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty-An edible seaweed. Food Chem. 2008, 107, 289–295. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J. Med. Food 2010, 13, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Doble, M. κ-Carrageenan from marine red algae, Kappaphycus alvarezii—A functional food to prevent colon carcinogenesis. J. Funct. Food. 2015, 15, 354–364. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.H.; Khaziakhmetova, V.N.; Zigashina, L.E. Rat paw oedema modeling and NSAIDs: Timing of effects. Int. J. Risk Saf. Med. 2015, 27 (Suppl. 1), S76–S77. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. In Inflammation Protocols; Winyard, P.G., Willoughby, D.A., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 115–121. [Google Scholar]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. Variation in amino acid content and its relationship to nitrogen content and growth rate in Ulva ohnoi (Chlorophyta). J. Phycol. 2014, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Gosch, B.J.; Magnusson, M.; Paul, N.A.; de Nys, R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 2012, 4, 919–930. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh Kumar, K.; Ganesan, K.; Subba Rao, P.V. Seasonal variation in nutritional composition of Kappaphycus alvarezii (Doty) Doty-an edible seaweed. J. Food Sci. Technol. 2015, 52, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Brown, L. Seaweeds as potential therapeutic interventions for the metabolic syndrome. Rev. Endocr. Metab. Dis. 2013, 14, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. A green algae mixture of Scenedesmus and Schroederiella attenuates obesity-linked metabolic syndrome in rats. Nutrients 2015, 7, 2771–2787. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Smith, D.; Collier, G.R.; O’Dea, K. Effect of soluble dietary fibre on the viscosity of gastrointestinal contents and the acute glycaemic response in the rat. Br. J. Nutr. 1994, 71, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia 2012, 83, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Kristensen, M.; Astrup, A. Effect of alginate supplementation on weight loss in obese subjects completing a 12-wk energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, O.W.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Jung, U.; Roh, C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.T.; Kim, Y.D.; Jung, Y.M.; Park, D.C.; Lee, D.S.; Ku, S.K.; Li, X.; Lu, Y.; Chao, G.H.; Kim, K.J.; et al. Low molecular weight fucoidan improves endoplasmic reticulum stress-reduced insulin sensitivity through AMP-activated protein kinase activation in L6 myotubes and restores lipid homeostasis in a mouse model of type 2 diabetes. Mol. Pharmacol. 2013, 84, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Drago, S.R.; de Medina, F.S.; Martinez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.S.; Yong, W.T.L.; Ng, S.E.; Anton, A.; Yassir, S. Chemical composition of farmed and micropropagated Kappaphycus alvarezii (Rhodophyta, Gigartinales), a commercially important seaweed in Malaysia. J. Appl. Phycol. 2015, 27, 1271–1275. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.M.; Mostafa, S.S.; Shalaby, E.A.; Mahmoud, G.I. Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac. J. Trop. Biomed. 2012, 2, 608–615. [Google Scholar] [CrossRef]
- Yang, R.; Le, G.; Li, A.; Zheng, J.; Shi, Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006, 22, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n-3 fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499S–1504S. [Google Scholar] [PubMed]
- He, F.J.; MacGregor, G.A. Potassium intake and blood pressure. Am. J. Hypertens. 1999, 12, 849–851. [Google Scholar] [PubMed]
- Yan, Y.L.; Shapiro, J.I. The physiological and clinical importance of sodium potassium ATPase in cardiovascular diseases. Curr. Opin. Pharmacol. 2016, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; He, J.; Cutler, J.A.; Brancati, F.L.; Appel, L.J.; Follmann, D.; Klag, M.J. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. J. Am. Med. Assoc. 1997, 277, 1624–1632. [Google Scholar] [CrossRef]
- Young, D.B.; Lin, H.; McCabe, R.D. Potassium’s cardiovascular protective mechanisms. Am. J. Physiol. 1995, 268, R825–R837. [Google Scholar] [PubMed]
- WHO. WHO. Guideline: Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Bodinham, C.L.; Smith, L.; Wright, J.; Frost, G.S.; Robertson, M.D. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS ONE 2012, 7, e40834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleeth, M.; Psichas, A.; Frost, G. Weight gain and insulin sensitivity: A role for the glycaemic index and dietary fibre? Br. J. Nutr. 2013, 109, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Cummings, J.H. Mechanism of action of dietary fibre in the human colon. Nature 1980, 284, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, Q.; Li, G.; Wang, X.; Yu, G. Degradation of marine algae-derived carbohydrates by Bacteroidetes isolated from human gut microbiota. Mar. Drugs 2017, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.J.; Zhang, L.J.; Zhao, X.; He, X.X.; Li, M.M.; Zhao, X.L.; Wu, J.D.; Qiu, P.J.; Yu, G.L. Activated AMPK explains hypolipidemic effects of sulfated low molecular weight guluronate on HepG2 cells. Eur. J. Med. Chem. 2014, 85, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Bajury, D.M.; Rawi, M.H.; Sazali, I.H.; Abdullah, A.; Sarbini, S.R. Prebiotic evaluation of red seaweed (Kappaphycus alvarezii) using in vitro colon model. Int. J. Food Sci. Nutr. 2017, 68, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Xu, J.; Leip, D.D.; Chen, C.H.; Westover, B.P.; Weatherford, J.; Buhler, J.D.; Gordon, J.I. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005, 307, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Magnusdottir, S.; Ravcheev, D.; de Crecy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Burke, B.; Ford, D.; Garvin, G.; Korn, C.; Sulis, C.; Bhadelia, N. Possible association between obesity and Clostridium difficile infection. Emerg. Infect. Dis. 2013, 19, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Pfeiffer, N.; Loh, G.; Klaus, S.; Blaut, M. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. mBio 2014, 5, e01514–e01530. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Ross, R.P.; O’Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tims, S.; Derom, C.; Jonkers, D.M.; Vlietinck, R.; Saris, W.H.; Kleerebezem, M.; de Vos, W.M.; Zoetendal, E.G. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013, 7, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Saito, H.; Nakaji, S.; Yamada, M.; Ebine, N.; Tsushima, E.; Oka, E.; Kumeta, K.; Tsukamoto, T.; Tokunaga, S. Pattern of dietary fiber intake among the Japanese general population. Eur. J. Clin. Nutr. 2007, 61, 99–103. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G., III; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [CrossRef] [PubMed]
| Variables | C | H | HR |
|---|---|---|---|
| Initial body weight, g | 331 ± 1 | 334 ± 1 | 332 ± 1 |
| Final body weight, g | 350 ± 8 b | 431 ± 11 a | 348 ± 6 b |
| Body mass index, g/cm2 | 0.57 ± 0.02 b | 0.70 ± 0.03 a | 0.58 ± 0.01 b |
| Water intake, mL/day | 37.3 ± 7.3 | 21.7 ± 2.2 | 35.0 ± 2.6 |
| Food intake, g/day | 38.7 ± 3.3 a | 23.7 ± 2.3 b | 20.8 ± 2.0 b |
| Energy intake, kJ/day | 434 ± 11 b | 485 ± 8 a | 507 ± 10 a |
| Feed conversion efficiency, g/kJ | 0.04 ± 0.02 b | 0.20 ± 0.03 a | 0.03 ± 0.01 b |
| Abdominal circumference, cm | 18.4 ± 0.1 c | 20.5 ± 0.1 a | 18.9 ± 0.1 b |
| Retroperitoneal fat, mg/mm * | 149 ± 14 b | 284 ± 31 a | 157 ± 26 b |
| Epididymal fat, mg/mm * | 79 ± 10 | 141 ± 29 | 81 ± 17 |
| Omental fat, mg/mm * | 114 ± 10 b | 208 ± 20 a | 99 ± 20 b |
| Total abdominal fat, mg/mm * | 342 ± 18 b | 632 ± 73 a | 337 ± 48 b |
| Total fat mass, g | 49.4 ± 5.0 b | 98.7 ± 8.3 a | 53.4 ± 6.5 b |
| Total lean mass, g | 315 ± 4 a | 318 ± 7 a | 290 ± 6 b |
| Bone mineral content, g | 10.1 ± 0.3 b | 11.8 ± 0.3 a | 9.8 ± 0.2 b |
| Bone mineral density, g/cm2 | 0.165 ± 0.002 b | 0.180 ± 0.005 a | 0.156 ± 0.002 c |
| Basal blood glucose concentrations, mmol/L | 3.7 ± 0.3 b | 4.7 ± 0.2 a | 3.6 ± 0.2 b |
| Area under the curve, mmol/L·min | 650 ± 29 b | 799 ± 27 a | 753 ± 30 a |
| Total cholesterol, mmol/L | 1.50 ± 0.08 | 1.61 ± 0.09 | 1.76 ± 0.14 |
| Triglycerides, mmol/L | 0.61 ± 0.09 c | 1.65 ± 0.21 a | 1.31 ± 0.07 b |
| Non-esterified fatty acids, mmol/L | 1.16 ± 0.21 c | 4.09 ± 0.29 a | 1.72 ± 0.36 b |
| Fecal lipids, mg/g of feces | 0.87 ± 0.04 b | 1.31 ± 0.04 a | 0.63 ± 0.03 c |
| Systolic blood pressure, mmHg | 120 ± 2 c | 136 ± 1 a | 127 ± 3 b |
| LV + septum wet weight, mg/mm * | 17.8 ± 0.6 | 20.0 ± 0.8 | 19.2 ± 0.9 |
| RV wet weight, mg/mm * | 4.25 ± 0.30 | 4.08 ± 0.46 | 4.61 ± 0.34 |
| Liver weight, mg/mm * | 217 ± 14 | 286 ± 9 | 256 ± 30 |
| Plasma ALT activity, U/L | 28.0 ± 3.6 b | 30.1 ± 4.6 b | 42.0 ± 4.0 a |
| Plasma AST activity, U/L | 70.9 ± 3.5 | 72.1 ± 7.0 | 74.0 ± 4.0 |
| Plasma potassium, mmol/L | 5.7 ± 0.3 a | 5.0 ± 0.3 b | 6.1 ± 0.5 a |
| Metal (Symbol) | C (in ppm) | H (in ppm) | HR (in ppm) |
|---|---|---|---|
| Aluminium (Al) | 5.32 ± 0.89 | 11.83 ± 2.71 | 5.71 ± 1.50 |
| Arsenic (As) | 4.75 ± 0.71 a | 2.24 ± 0.34 b | 1.40 ± 0.16 b |
| Boron (B) | BDL | BDL | BDL |
| Barium (Ba) | 0.07 ± 0.01 b | 0.23 ± 0.04 a | 0.07 ± 0.01 b |
| Calcium (Ca) | 124 ± 3 a | 117 ± 6 a | 94 ± 9 b |
| Cadmium (Cd) | 0.06 (n =1) | BDL | 0.04 ± 0.01 (n = 2) |
| Cobalt (Co) | BDL | BDL | BDL |
| Chromium (Cr) | BDL | BDL | BDL |
| Copper (Cu) | 13.78 ± 1.32 | 10.65 ± 0.99 | 9.95 ± 1.41 |
| Iron (Fe) | 575 ± 22 a | 376 ± 12 b | 405 ± 38 b |
| Mercury (Hg) | BDL | BDL | BDL |
| Potassium (K) | 10,506 ± 256 a | 10,146 ± 309 a | 7225 ± 998 b |
| Magnesium (Mg) | 547 ± 10 | 529 ± 39 | 434 ± 40 |
| Manganese (Mn) | 4.67 ± 0.20 | 3.92 ± 0.38 | 4.41 ± 0.49 |
| Molybdenum (Mo) | 0.65 ± 0.07 ab | 0.81 ± 0.05 a | 0.42 ± 0.09 b |
| Sodium (Na) | 1581 ± 97 | 1,278 ± 44 | 1060 ± 187 |
| Nickel (Ni) | 0.14 (n = 1) | BDL | 0.57 ± 0.37 (n = 4) |
| Phosphorus (P) | 8820 ± 324 a | 8559 ± 399 a | 6090 ± 730 b |
| Lead (Pb) | 0.15 ± 0.05 | 0.11 ± 0.02 | 0.11 ± 0.03 |
| Sulfur (S) | 5233 ± 155 a | 5055 ± 239 a | 3510 ± 408 b |
| Selenium (Se) | 1.54 ± 0.03 | 1.45 ± 0.13 | 1.01 ± 0.16 |
| Strontium (Sr) | 0.20 ± 0.02 b | 0.31 ± 0.05 a | 0.17 ± 0.02 b |
| Vanadium (V) | BDL | BDL | BDL |
| Zinc (Zn) | 63.12 ± 2.81 a | 66.85 ± 2.36 a | 43.23 ± 5.13 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanyonyi, S.; Du Preez, R.; Brown, L.; Paul, N.A.; Panchal, S.K. Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats. Nutrients 2017, 9, 1261. https://doi.org/10.3390/nu9111261
Wanyonyi S, Du Preez R, Brown L, Paul NA, Panchal SK. Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats. Nutrients. 2017; 9(11):1261. https://doi.org/10.3390/nu9111261
Chicago/Turabian StyleWanyonyi, Stephen, Ryan Du Preez, Lindsay Brown, Nicholas A. Paul, and Sunil K. Panchal. 2017. "Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats" Nutrients 9, no. 11: 1261. https://doi.org/10.3390/nu9111261
APA StyleWanyonyi, S., Du Preez, R., Brown, L., Paul, N. A., & Panchal, S. K. (2017). Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats. Nutrients, 9(11), 1261. https://doi.org/10.3390/nu9111261







