Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management
Abstract
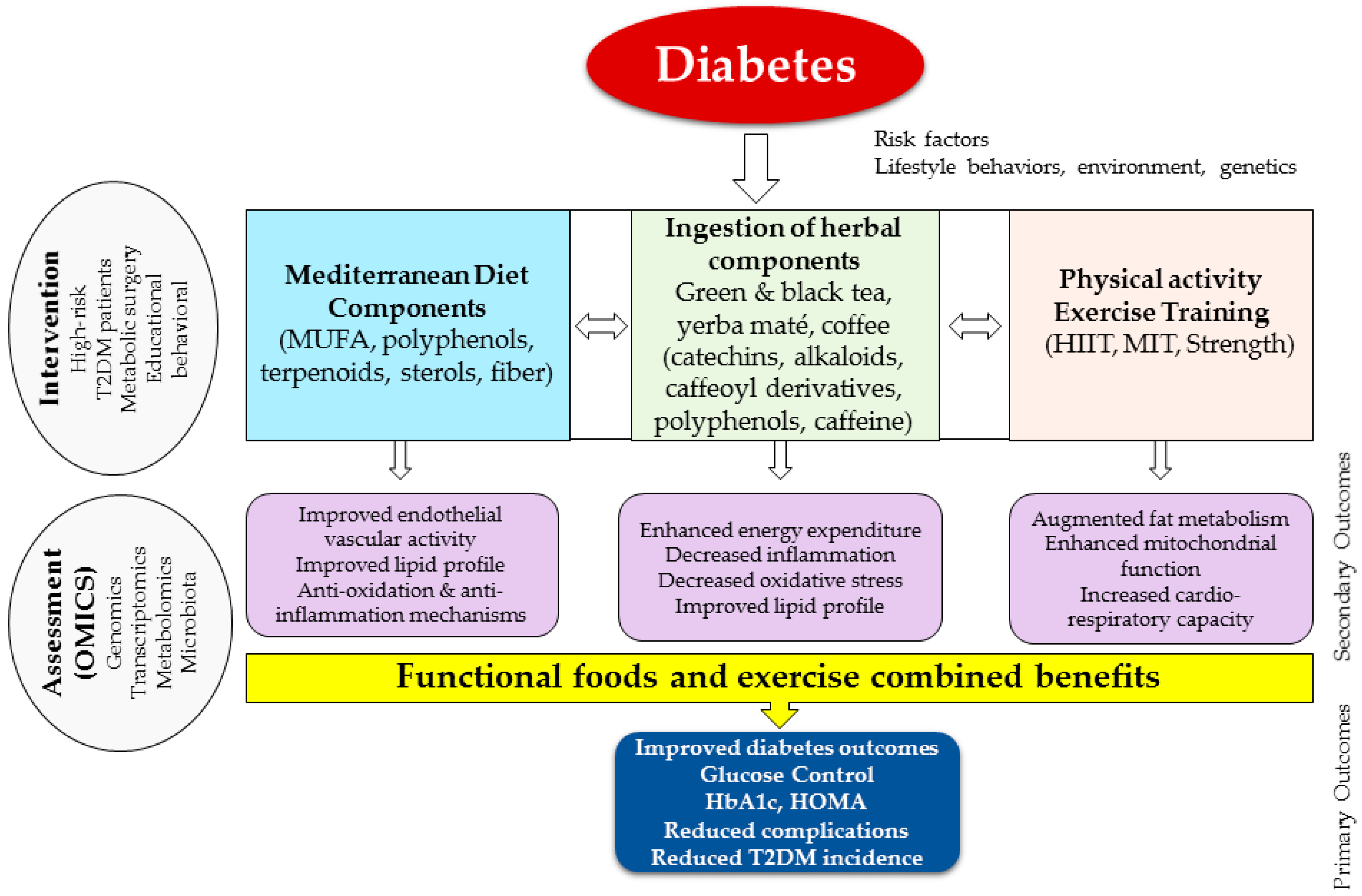
:1. Overview and Background
2. Mediterranean Diet Components as a Model for Functional Foods to Prevent and Manage Diabetes
3. Preventive Role of Exercise and Physical Activity in Augmenting Functional Food Effects
4. Protective Role of Polyphenols in T2DM
5. Clinical Role for Herbal Ingestions in T2DM Prevention and Management
6. The Use of Omics in Detecting the Inter-Individual Functional Food Effects
7. Metabolic Surgery Outcomes and Functional Foods in T2DM Management
8. Importance of Education and Counselling in Diabetes Prevention and Management
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. World Health Organization (WHO) Global Report on Diabetes. 2016. Available online: http://www.who.int/diabetes/global-report/en/ (accessed on 9 October 2017).
- IDF. Diabetes Atlas. 2015-7th Edition. Available online: http://www.diabetesatlas.org/ (accessed on 12 September 2017).
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Pittler, M.H. Assessment of therapeutic safety in systematic reviews: Literature review. BMJ 2001, 323, 546. [Google Scholar] [CrossRef] [PubMed]
- ADA. 2017. Available online: http://www.diabetes.org/food-and-fitness/food/planning-meals/diabetes-meal-plans-and-a-healthy-diet.html (accessed on 30 September 2017).
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Fernandez-Ballart, J.; Ros, E.; Martinez-Gonzalez, M.A.; Fito, M.; Estruch, R.; Corella, D.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martinez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remon, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet for type 2 diabetes: Cardiometabolic benefits. Endocrine 2017, 56, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Vecchio, S.; Giuliani, G.; Martinelli, F.; Marcucci, R.; Gori, A.M.; Fedi, S.; Casini, A.; Surrenti, C.; Abbate, R.; et al. Dietary habits, lifestyle and cardiovascular risk factors in a clinically healthy Italian population: The ‘Florence’ diet is not Mediterranean. Eur. J. Clin. Nutr. 2005, 59, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Vidra, N.; Farmaki, A.E.; Koinaki, S.; Belogianni, K.; Sofrona, S.; Magkanari, F.; Yannakoulia, M. Adherence rates to the Mediterranean diet are low in a representative sample of Greek children and adolescents. J. Nutr. 2008, 138, 1951–1956. [Google Scholar] [PubMed]
- Jimenez-Redondo, S.; Beltran de Miguel, B.; Gomez-Pavon, J.; Cuadrado Vives, C. Food consumption and risk of malnutrition in community-dwelling very old Spanish adults (≥80 years). Nutr. Hosp. 2016, 33, 263. [Google Scholar] [PubMed]
- Alkhatib, A.; Klonizakis, M. Effects of exercise training and Mediterranean diet on vascular risk reduction in post-menopausal women. Clin. Hemorheol. Microcirc. 2014, 57, 33–47. [Google Scholar] [PubMed]
- Middleton, G.; Keegan, R.; Smith, M.F.; Alkhatib, A.; Klonizakis, M. Brief report: Implementing a Mediterranean diet intervention into a RCT: Lessons learned from a non-Mediterranean based country. J. Nutr. Health Aging 2015, 19, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Barera, A.; Rizzo, C.; Di Carlo, M.; Caruso, C.; Panotopoulos, G. Mediterranean diet and longevity: An example of nutraceuticals? Curr. Vasc. Pharmacol. 2014, 12, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Greca, M.; Zarrelli, A. Nutraceuticals and Mediterranean diet. Med. Aromat. Plants 2012, 1, e126. [Google Scholar]
- Georgoulis, M.; Kontogianni, M.D.; Yiannakouris, N. Mediterranean diet and diabetes: Prevention and treatment. Nutrients 2014, 6, 1406–1423. [Google Scholar] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C. The epidemiology of Type 2 diabetes mellitus in Greek adults: The ATTICA study. Diabetes Med. 2005, 22, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Giugliano, D. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: Follow-up of a randomized trial. Diabetes Care 2014, 37, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Eguaras, S.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Carlos, S.; de la Rosa, P.; Martinez-Gonzalez, M.A. May the Mediterranean diet attenuate the risk of type 2 diabetes associated with obesity: The Seguimiento Universidad de Navarra (SUN) cohort. Br. J. Nutr. 2017, 117, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.M.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, A.; Ortega, R.M.; Maldonado, J. Wholegrain cereals and bread: A duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr. 2011, 14, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Tighe, P.; Duthie, G.; Vaughan, N.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Mutch, W.; Wahle, K.; Horgan, G.; Thies, F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 92, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Riboli, E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Micha, R.; Imamura, F.; Pan, A.; Biggs, M.L.; Ajaz, O.; Djousse, L.; Hu, F.B.; Mozaffarian, D. Omega-3 fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107 (Suppl. 2), S214–S227. [Google Scholar] [CrossRef] [PubMed]
- Tinker, L.F.; Bonds, D.E.; Margolis, K.L.; Manson, J.E.; Howard, B.V.; Larson, J.; Perri, M.G.; Beresford, S.A.; Robinson, J.G.; Rodriguez, B.; et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: The Women’s Health Initiative randomized controlled dietary modification trial. Arch. Intern. Med. 2008, 168, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferre, M.; Merino, J.; Sun, Q.; Fito, M.; Salas-Salvado, J. Dietary polyphenols, Mediterranean diet, prediabetes, and Type 2 diabetes: A narrative review of the evidence. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive polyphenols and the metabolic syndrome. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Bidel, S.; Tuomilehto, J. Coffee and Type 2 Diabetes Risk. In Coffee Emerging Health Effects and Disease Prevention; Chu, Y.F., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 141–179. [Google Scholar]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A. Effective intervention strategies combining Mediterranean diet and exercise for reducing obesity, metabolic and cardiovascular risks in high-risk populations: Mini review. Obes. Res. Open J. 2015, 1, 4–9. [Google Scholar] [CrossRef]
- Bibiloni, M.D.M.; Julibert, A.; Argelich, E.; Aparicio-Ugarriza, R.; Palacios, G.; Pons, A.; Gonzalez-Gross, M.; Tur, J.A. Western and Mediterranean dietary patterns and physical activity and fitness among Spanish older adults. Nutrients 2017, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Alkhatib, A.; Middleton, G.; Smith, M.F. Mediterranean diet- and exercise-induced improvement in age-dependent vascular activity. Clin. Sci. 2013, 124, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Couture, P.; Desroches, S.; Lamarche, B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity 2013, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Alkhatib, A.; Middleton, G. Long-term effects of an exercise and Mediterranean diet intervention in the vascular function of an older, healthy population. Microvasc. Res. 2014, 95, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Little, J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Weltman, A. High-intensity exercise training for the prevention of type 2 diabetes mellitus. Phys. Sportsmed. 2014, 42, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.; Kenno, K.A.; Milne, K.J. The effects of a 2 week modified high intensity interval training program on the homeostatic model of insulin resistance (HOMA-IR) in adults with type 2 diabetes. J. Sports Med. Phys. Fit. 2014, 54, 203–209. [Google Scholar]
- Biddle, S.J.; Batterham, A.M. High-intensity interval exercise training for public health: A big HIT or shall we HIT it on the head? Int. J. Behav. Nutr. Phys. Act. 2015, 12, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talegawkar, S.A.; Bandinelli, S.; Bandeen-Roche, K.; Chen, P.; Milaneschi, Y.; Tanaka, T.; Semba, R.D.; Guralnik, J.M.; Ferrucci, L. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J. Nutr. 2012, 142, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Arjmandi, B.H.; Wong, A.; Sanchez-Gonzalez, M.A.; Simonavice, E.; Daggy, B. Effects of hypocaloric diet, low-intensity resistance exercise with slow movement, or both on aortic hemodynamics and muscle mass in obese postmenopausal women. Menopause 2013, 20, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Landaeta-Diaz, L.; Fernandez, J.M.; Da Silva-Grigoletto, M.; Rosado-Alvarez, D.; Gomez-Garduno, A.; Gomez-Delgado, F.; Lopez-Miranda, J.; Perez-Jimenez, F.; Fuentes-Jimenez, F. Mediterranean diet, moderate-to-high intensity training, and health-related quality of life in adults with metabolic syndrome. Eur. J. Prev. Cardiol. 2013, 20, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Saura-Calixto, F.; Goni, I. Definition of the Mediterranean diet based on bioactive compounds. Crit. Rev. Food Sci. Nutr. 2009, 49, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130 (Suppl. 8S), 2073S–2085S. [Google Scholar] [PubMed]
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; Gonzalez, C.A.; Buijsse, B.; Guevara, M.; van der Schouw, Y.T.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: The EPIC-InterAct study. Diabetes Care 2013, 36, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Miranda, J.; Bullo, M.; Casas, R.; Salas-Salvado, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutierrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Morgantini, C.; Natali, A.; Boldrini, B.; Imaizumi, S.; Navab, M.; Fogelman, A.M.; Ferrannini, E.; Reddy, S.T. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 2011, 60, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Hui, R.; Wu, Y.J.; Huang, X.H. Effects of green tea catechins with or without caffeine on glycemic control in adults: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Van De Water, J.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Cocoa procyanidins and human cytokine transcription and secretion. J. Nutr. 2000, 130 (Suppl. 8S), 2093S–2099S. [Google Scholar] [PubMed]
- Matsui, T.; Ogunwande, I.A.; Abesundara, K.J.; Matsumoto, K. Anti-hyperglycemic potential of natural products. Mini Rev. Med. Chem. 2006, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [PubMed]
- Rodriguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martin, M.A. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J. Nutr. Biochem. 2011, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhatib, A.; Atcheson, R. Yerba Mate (Ilex paraguariensis) metabolic, satiety, and mood state effects at rest and during prolonged exercise. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Wamil, M.; Seckl, J.R. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov. Today 2007, 12, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, J.; Stapelfeld, C.; Loerz, C.; Martin, H.J.; Maser, E. Green tea and one of its constituents, Epigallocatechine-3-gallate, are potent inhibitors of human 11beta-hydroxysteroid dehydrogenase type 1. PLoS ONE 2014, 9, e84468. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A. Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J. Nutr. Sci. 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Tsang, C.; Ostertag, L.M.; Fyfe, L.; Al-Dujaili, E.A. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Funct. 2012, 3, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Diabetes UK: Herbal and Natural Therapies. Available online: http://www.diabetes.co.uk/Diabetes-herbal.html (accessed on 19 November 2017).
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of Type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, S.; Wang, S. Can highly cited herbs in ancient Traditional Chinese medicine formulas and modern publications predict therapeutic targets for diabetes mellitus? J. Ethnopharmacol. 2017, 213, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.I.; de Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Luximon-Ramma, A.; Gunness, T.K.; Sookar, D.; Bhoyroo, S.; Jugessur, R.; Reebye, D.; Googoolye, K.; Crozier, A.; Aruoma, O.I. Black tea reduces uric acid and C-reactive protein levels in humans susceptible to cardiovascular diseases. Toxicology 2010, 278, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Alan Crozier, A.; Aruoma, O.I. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef] [PubMed]
- Toolsee, N.A.; Aruoma, O.I.; Gunness, T.K.; Kowlessur, S.; Dambala, V.; Murad, F.; Googoolye, K.; Daus, D.; Indelicato, J.; Rondeau, P.; et al. Effectiveness of green tea in a randomized human cohort: Relevance to diabetes and its complications. Biomed. Res. Int. 2013, 2013, 412379. [Google Scholar] [CrossRef] [PubMed]
- Toolsee, N.A.; Aruoma, O.I.; Rondeau, P.; Bourdon, E.; Bahorun, T. Modulatory effects of green tea on HEK-293 cell energy metabolism: Implications in diabetic nephropathy. Arch. Med. Biomed. Res. 2014, 1, 156–162. [Google Scholar] [CrossRef]
- Ramlagan, P.; Rondeau, P.; Planesse, C.; Neergheen-Bhujun, V.S.; Bourdon, E.; Bahorun, T. Comparative suppressing effects of black and green teas on the formation of advanced glycation end products (AGEs) and AGE-induced oxidative stress. Food Funct. 2017, 8, 4194–4209. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A. Yerba Mate (Illex paraguariensis) ingestion augments fat oxidation and energy expenditure during exercise at various submaximal intensities. Nutr. Metab. 2014, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Seijo, M.; Larumbe, E.; Naclerio, F. Acute effectiveness of a “fat-loss” product on substrate utilization, perception of hunger, mood state and rate of perceived exertion at rest and during exercise. J. Int. Soc. Sports Nutr. 2015, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Snyder, M. Promise of personalized omics to precision medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 73–82. [Google Scholar] [CrossRef] [PubMed]
- De Toro-Martin, J.; Arsenault, B.J.; Despres, J.P.; Vohl, M.C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.; Peyrot, N.; Caderby, T.; Verkindt, C.; Dalleau, G. Energy expenditure in people with diabetes mellitus: A review. Front. Nutr. 2016, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Tiss, A.; Khadir, A.; Abubaker, J.; Abu-Farha, M.; Al-Khairi, I.; Cherian, P.; John, J.; Kavalakatt, S.; Warsame, S.; Al-Ghimlas, F.; et al. Immunohistochemical profiling of the heat shock response in obese non-diabetic subjects revealed impaired expression of heat shock proteins in the adipose tissue. Lipids Health Dis. 2014, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Khadir, A.; Tiss, A.; Abubaker, J.; Abu-Farha, M.; Al-Khairi, I.; Cherian, P.; John, J.; Kavalakatt, S.; Warsame, S.; Al-Madhoun, A.; et al. MAP kinase phosphatase DUSP1 is overexpressed in obese humans and modulated by physical exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E71–E83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Barrere-Cain, R.E.; Yang, X. Nutritional systems biology of type 2 diabetes. Genes Nutr. 2015, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.; Boque, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrient 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Williams, E.G.; Dubuis, S.; Mottis, A.; Jovaisaite, V.; Houten, S.M.; Argmann, C.A.; Faridi, P.; Wolski, W.; Kutalik, Z.; et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 2014, 158, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Parks, B.W.; Nam, E.; Org, E.; Kostem, E.; Norheim, F.; Hui, S.T.; Pan, C.; Civelek, M.; Rau, C.D.; Bennett, B.J.; et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013, 17, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American society for metabolic and bariatric surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Nor Hanipah, Z.; Rubino, F. Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world’s leading diabetes organizations. Clevel. Clin. J. Med. 2017, 84 (Suppl. 1), S47–S56. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.M. What bariatric surgery can teach us about endoluminal treatment of obesity and metabolic disorders. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Mingrone, G.; Ikramuddin, S.; Wolfe, B. Clinical outcomes of metabolic surgery: Efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016, 39, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Cigdem Arica, P.; Kocael, A.; Tabak, O.; Taskin, M.; Zengin, K.; Uzun, H. Plasma ghrelin, leptin, and orexin-A levels and insulin resistance after laparoscopic gastric band applications in morbidly obese patients. Minerva Med. 2013, 104, 309–316. [Google Scholar] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Moize, V.; Pi-Sunyer, X.; Vidal, J.; Miner, P.; Boirie, Y.; Laferrere, B. Effect on nitrogen balance, thermogenesis, body composition, satiety, and circulating branched chain amino acid levels up to one year after surgery: Protocol of a randomized controlled trial on dietary protein during surgical weight loss. JMIR Res. Protoc. 2016, 5, e220. [Google Scholar] [CrossRef] [PubMed]
- Bobbioni-Harsch, E.; Morel, P.; Huber, O.; Assimacopoulos-Jeannet, F.; Chassot, G.; Lehmann, T.; Volery, M.; Golay, A. Energy economy hampers body weight loss after gastric bypass. J. Clin. Endocrinol. Metab. 2000, 85, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Richard, D. Effects of Bariatric Surgery on Energy Homeostasis. Can. J. Diabetes 2017, 41, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Ionut, V.; Burch, M.; Youdim, A.; Bergman, R.N. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity 2013, 21, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, H. Central modulation of energy homeostasis and cognitive performance after bariatric surgery. Adv. Neurobiol. 2017, 19, 213–236. [Google Scholar] [PubMed]
- Bays, H.E.; Laferrere, B.; Dixon, J.; Aronne, L.; Gonzalez-Campoy, J.M.; Apovian, C.; Wolfe, B.M. Adiposopathy and bariatric surgery: Is ‘sick fat’ a surgical disease? Int. J. Clin. Pract. 2009, 63, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can. J. Diabetes 2017, 41, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jorgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and loss of control related to eating: Two high-risk factors following bariatric surgery. Obesity 2008, 16, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Munzberg, H.; Laque, A.; Yu, S.; Rezai-Zadeh, K.; Berthoud, H.R. Appetite and body weight regulation after bariatric surgery. Obes. Rev. 2015, 16 (Suppl. 1), 77–90. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.D.; Kops, N.L.; de Castro, M.L.; Friedman, R. Food consumption in patients referred for bariatric surgery with and without binge eating disorder. Eat. Behav. 2015, 19, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Verger, E.O.; Bounaix, C.; Dao, M.C.; Oppert, J.M.; Bouillot, J.L.; Chevallier, J.M.; Clement, K. Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS ONE 2016, 11, e0149588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frame-Peterson, L.A.; Megill, R.D.; Carobrese, S.; Schweitzer, M. Nutrient deficiencies are common prior to bariatric surgery. Nutr. Clin. Pract. 2017, 32, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Giusti, V.; Theytaz, F.; Di Vetta, V.; Clarisse, M.; Suter, M.; Tappy, L. Energy and macronutrient intake after gastric bypass for morbid obesity: A 3-y observational study focused on protein consumption. Am. J. Clin. Nutr. 2016, 103, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional recommendations for adult bariatric surgery patients: Clinical practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Larsson, I.; Peltonen, M.; Lindroos, A.K.; Carlsson, L.M. Changes in total energy intake and macronutrient composition after bariatric surgery predict long-term weight outcome: Findings from the Swedish Obese Subjects (SOS) study. Am. J. Clin. Nutr. 2017, 106, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Deloose, E. Complications of bariatric surgery: Dumping syndrome, reflux and vitamin deficiencies. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; de Oliveira, B.A.; de Pinhel, M.A.; Donati, B.; Marchini, J.S.; Salgado Junior, W.; Nonino, C.B. Influence of excess weight loss and weight regain on biochemical indicators during a 4-year follow-up after Roux-en-Y gastric bypass. Obes. Surg. 2015, 25, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Pinhel, M.A.S.; Nonino, C.B. Bariatric surgery and precision nutrition. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, V.; Carrasco, F.; Cuevas, A.; Valenzuela, B.; Munoz, G.; Ghiardo, D.; Burr, M.; Lehmann, Y.; Leiva, M.J.; Berry, M. Mechanisms of long-term weight regain in patients undergoing sleeve gastrectomy. Nutrition 2016, 32, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Novais, P.F.; Weber, T.K.; Lemke, N.; Verlengia, R.; Crisp, A.H.; Rasera-Junior, I.; de Oliveira, M.R. Gene polymorphisms as a predictor of body weight loss after Roux-en-Y gastric bypass surgery among obese women. Obes. Res. Clin. Pract. 2016, 10, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Larsson, I.; Peltonen, M.; Lindroos, A.K.; Carlsson, L.M. Sociodemographic and lifestyle factors as determinants of energy intake and macronutrient composition: A 10-year follow-up after bariatric surgery. Surg. Obes. Relat. Dis. 2017, 13, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Lahiri, A. Herbal medications and plastic surgery: A hidden danger. Aesthet. Plast. Surg. 2014, 38, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A. Benefits of the Mediterranean diet beyond the Mediterranean Sea and beyond food patterns. BMC Med. 2016, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- ADA. American Diabetes Association position statement: Standards of medical care in diabetes—2017. Diabetes Care 2017, 40 (Suppl. 1), S1–S138. [Google Scholar]
- Wolsdorf, J. Intensive Diabetes Management, 4th ed.; American Medical Association: Alexandria, VA, USA, 2009. [Google Scholar]
- Mitchell, L.J.; Ball, L.E.; Ross, L.J.; Barnes, K.A.; Williams, L.T. Effectiveness of dietetic consultations in primary health care: A systematic review of randomized controlled trials. J. Acad. Nutr. Dietet. 2017, 117, 1941–1962. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, T.I.; Puhkala, J.; Raitanen, J.; Ahonen, S.; Aittasalo, M.; Virtanen, S.M.; Luoto, R. Effects of dietary counselling on food habits and dietary intake of Finnish pregnant women at increased risk for gestational diabetes—A secondary analysis of a cluster-randomized controlled trial. Matern. Child Nutr. 2014, 10, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.; Lawrence, I.; Mansell, P.; Thompson, G.; Amiel, S.; Campbell, M.; Heller, S. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: The U.K. DAFNE experience. Diabetes Care 2012, 35, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Heller, S.; Skinner, T.C.; Campbell, M.J.; Carey, M.E.; Cradock, S.; Dallosso, H.M.; Daly, H.; Doherty, Y.; Eaton, S.; et al. Effectiveness of the diabetes education and self-management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: Cluster randomised controlled trial. BMJ 2008, 336, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Shuttlewood, E.; De Zoysa, N.; Rankin, D.; Amiel, S. A qualitative evaluation of DAFNE-HART: A psychoeducational programme to restore hypoglycaemia awareness. Diabetes Res. Clin. Pract. 2015, 109, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, S.; Kulkarni, K. Complete Guide to Carb Counting; American Diabetes Association: Arlington, VA, USA, 2004. [Google Scholar]
- Souto, D.L.; Zajdenverg, L.; Rodacki, M.; Rosado, E.L. Impact of advanced and basic carbohydrate counting methods on metabolic control in patients with type 1 diabetes. Nutrition 2014, 30, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Kaartinen, N.E.; Rissanen, H.; Knekt, P.; Eriksson, J.G.; Sääksjärvi, K.; Sundvall, J.; Männistö, S. Associations of the Baltic Sea diet with cardiometabolic risk factors—A meta-analysis of three Finnish studies. Br. J. Nutr. 2014, 112, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Egede, L.E.; Ye, X.; Zheng, D.; Silverstein, M.D. The prevalence and pattern of complementary and alternative medicine use in individuals with diabetes. Diabetes Care 2002, 25, 324–329. [Google Scholar] [CrossRef] [PubMed]
- John, L.J.; Shantakumari, N. Herbal medicines use during pregnancy: A review from the Middle East. Oman Med. J. 2015, 30, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, R.; Cheema, S.; MacRae, B.; Alrouh, H.; Lopez, T.; ElHajj, M.; Mahfoud, Z. Herbal and nutritional supplement use among college students in Qatar. East Mediterr. Health J. 2015, 21, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Raidoo, D.M.; Harries, C.S. The prevalence, patterns of usage and people’s attitude towards complementary and alternative medicine (CAM) among the Indian community in Chatsworth, South Africa. BMC Complement. Altern. Med. 2004, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, E.M. Interactions between herbal and conventional medicines. Expert Opin. Drug Saf. 2005, 4, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. https://doi.org/10.3390/nu9121310
Alkhatib A, Tsang C, Tiss A, Bahorun T, Arefanian H, Barake R, Khadir A, Tuomilehto J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients. 2017; 9(12):1310. https://doi.org/10.3390/nu9121310
Chicago/Turabian StyleAlkhatib, Ahmad, Catherine Tsang, Ali Tiss, Theeshan Bahorun, Hossein Arefanian, Roula Barake, Abdelkrim Khadir, and Jaakko Tuomilehto. 2017. "Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management" Nutrients 9, no. 12: 1310. https://doi.org/10.3390/nu9121310




