Melatonin Efficacy in Obese Leptin-Deficient Mice Heart
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment
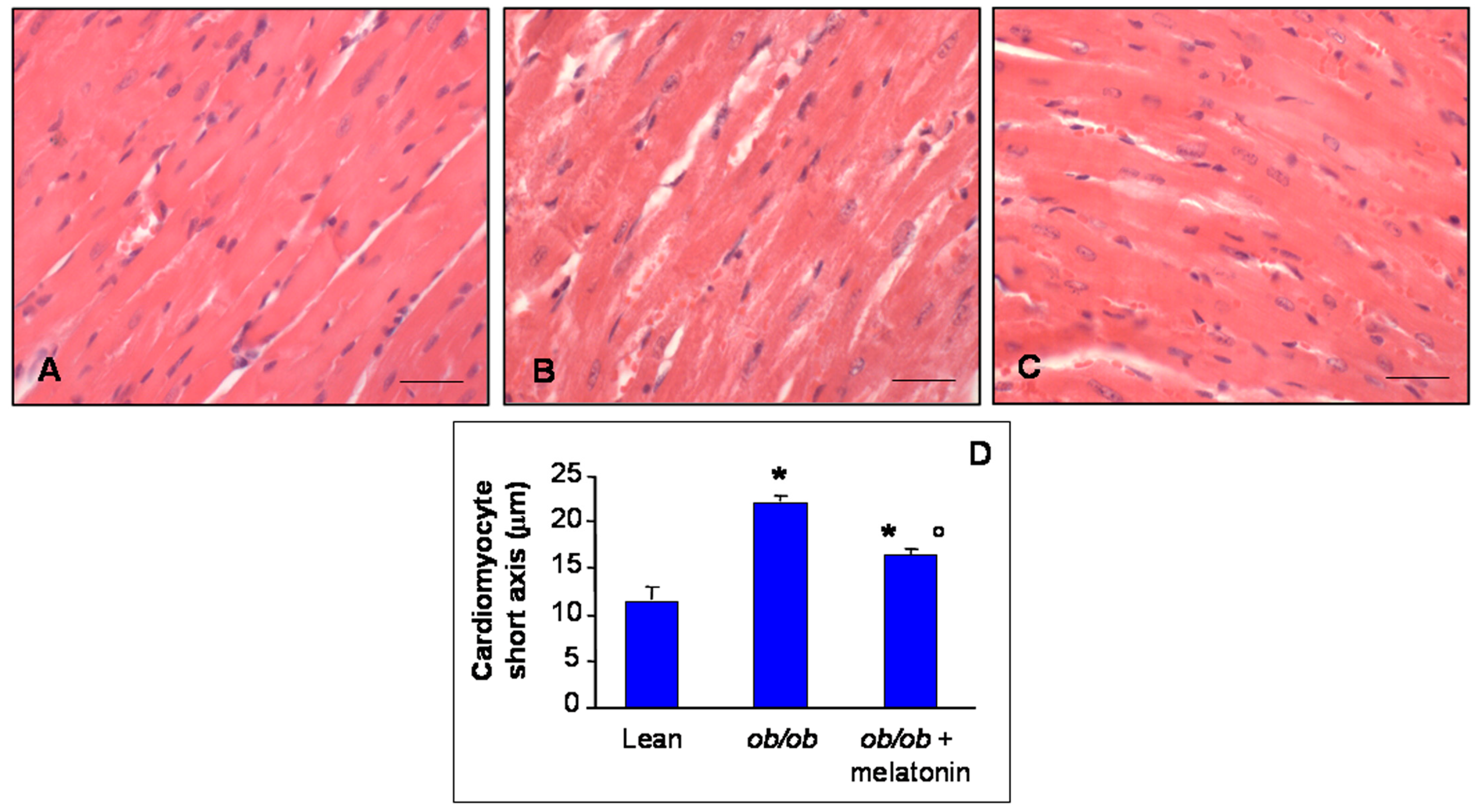
2.2. Histomorphometrical Evaluation
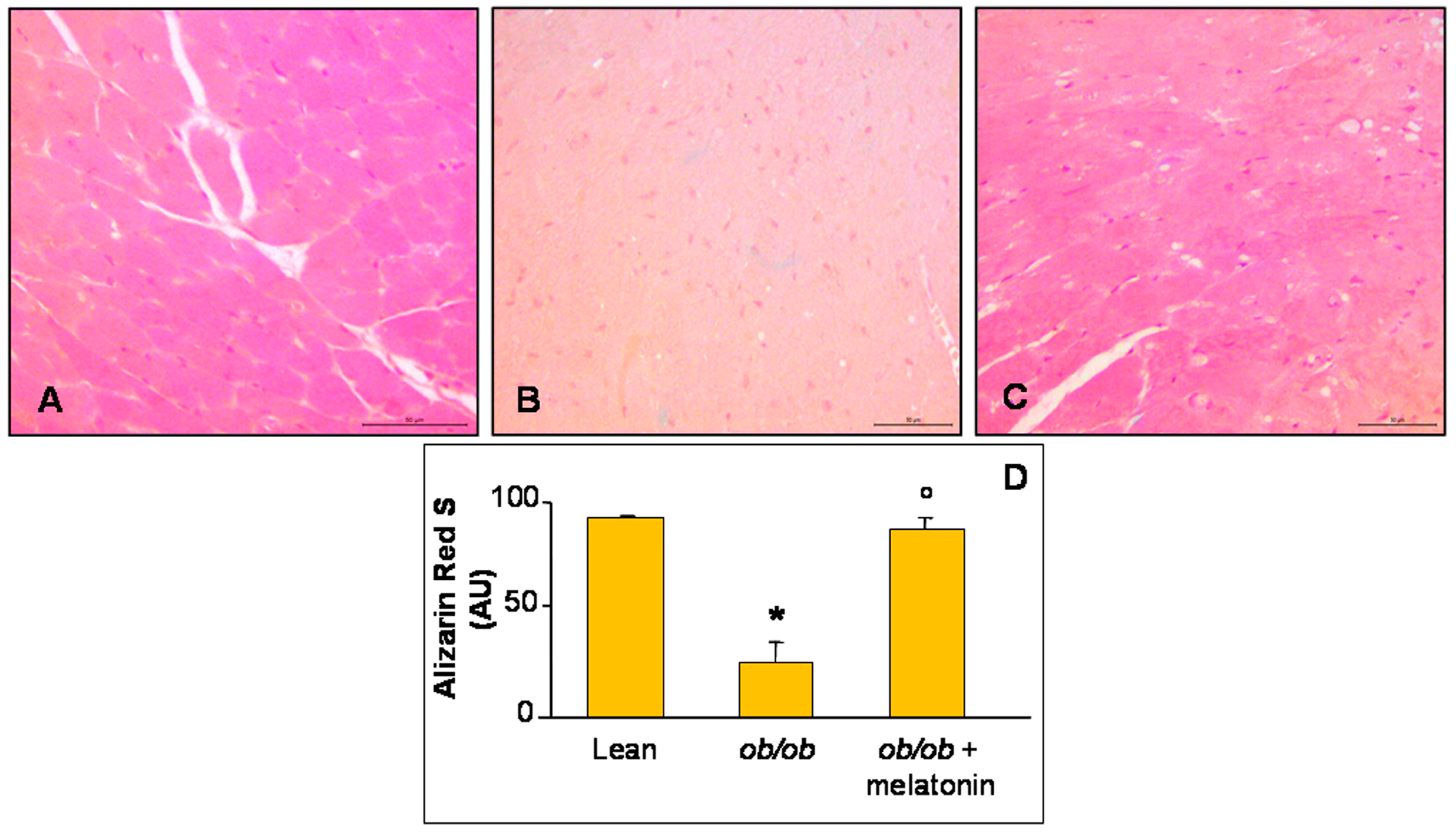
2.3. Alizarin S Red Staining
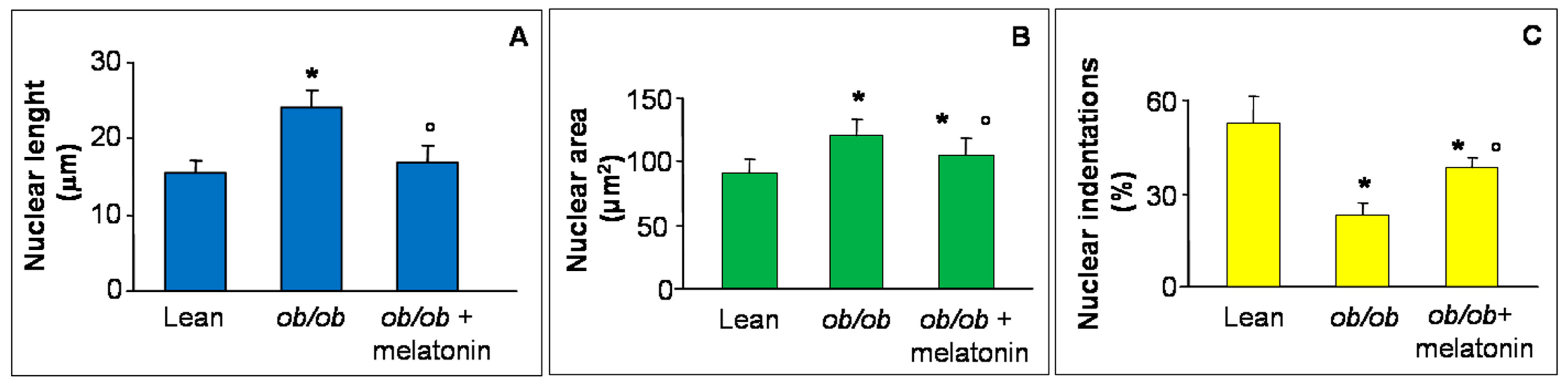
2.4. Nuclear Cardiomyocyte Morphometry
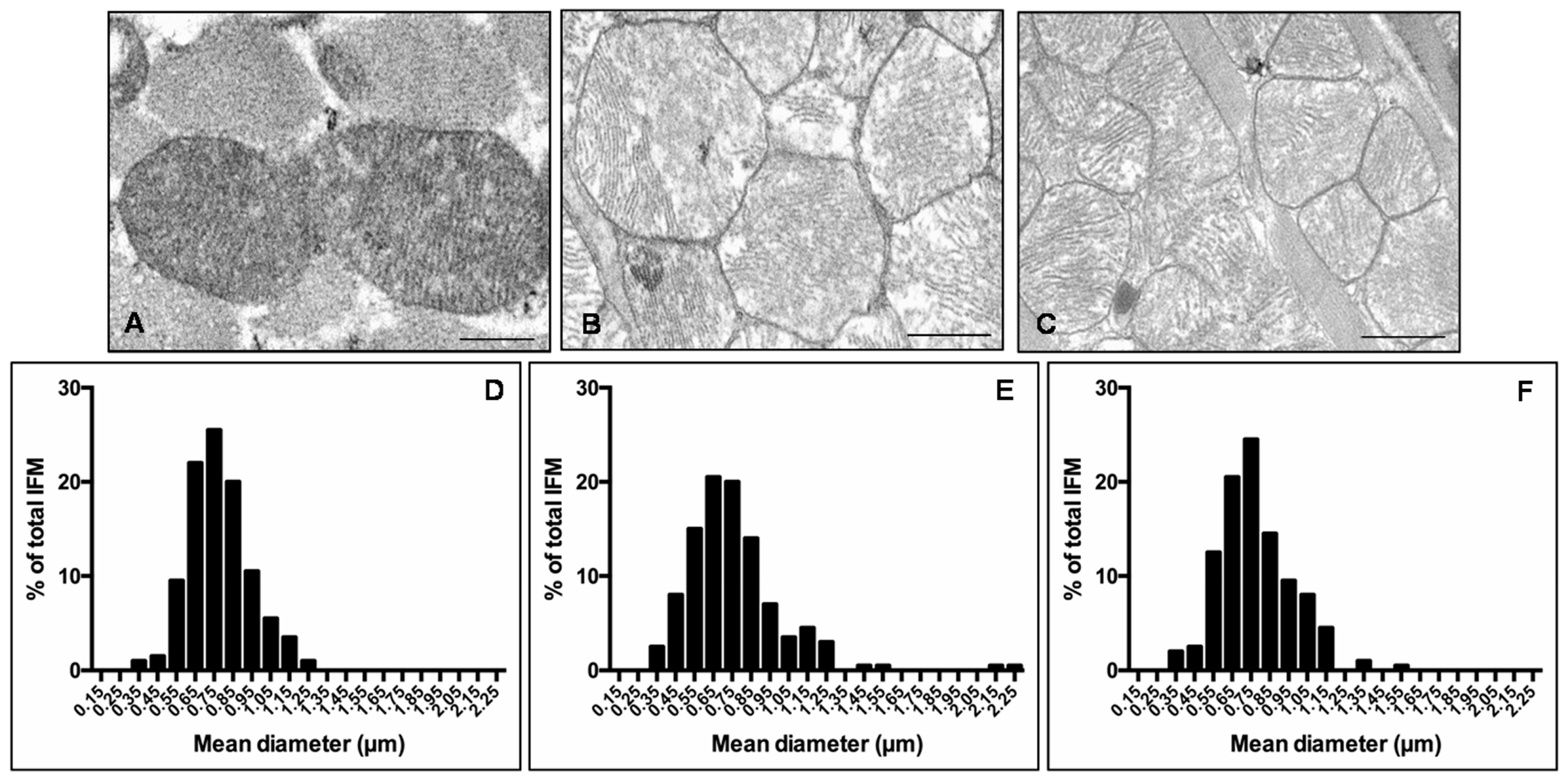
2.5. Mitochondria Evaluation
2.6. Immunohistochemical Analysis
2.7. Statistical Analysis
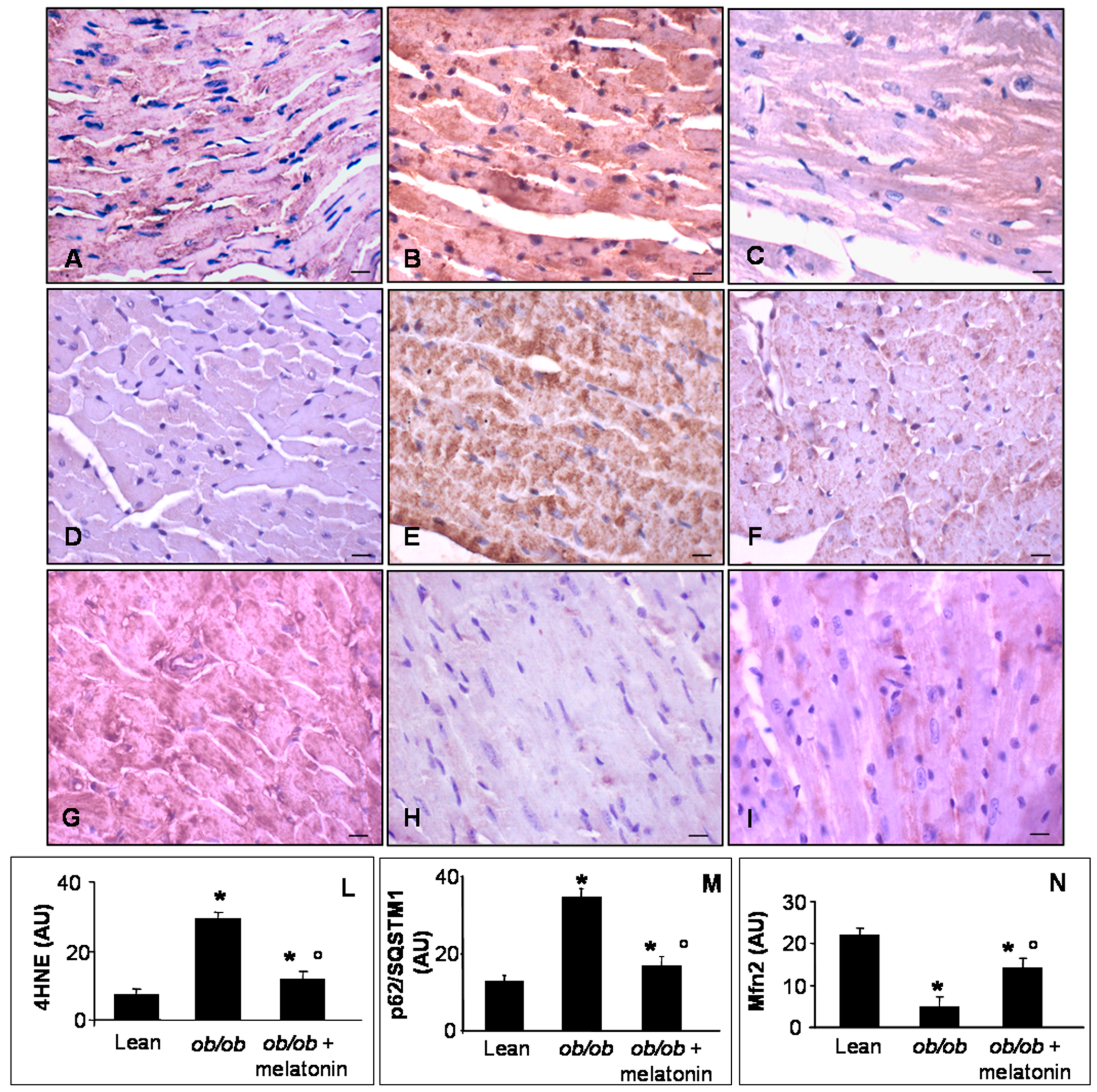
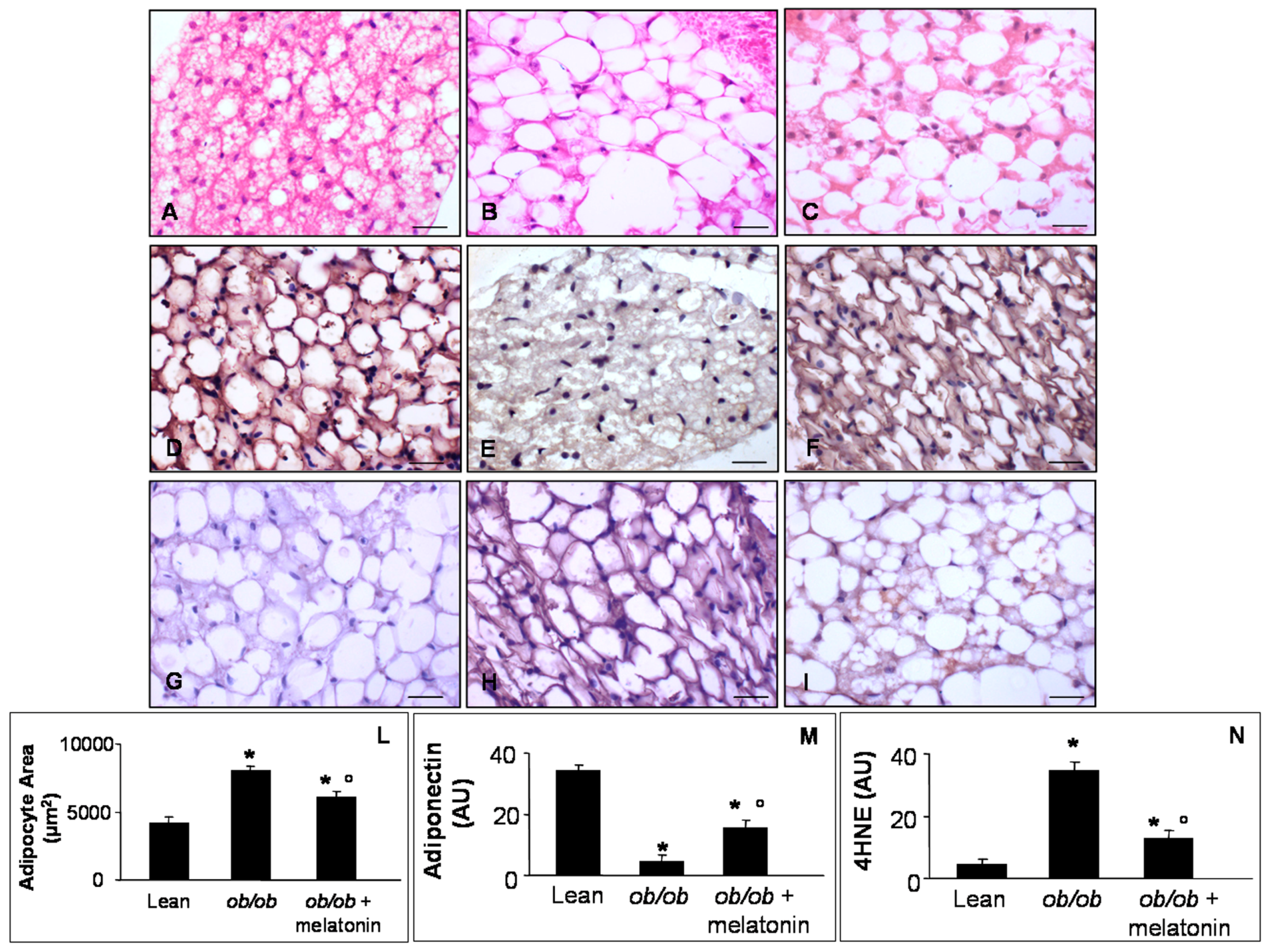
3. Results
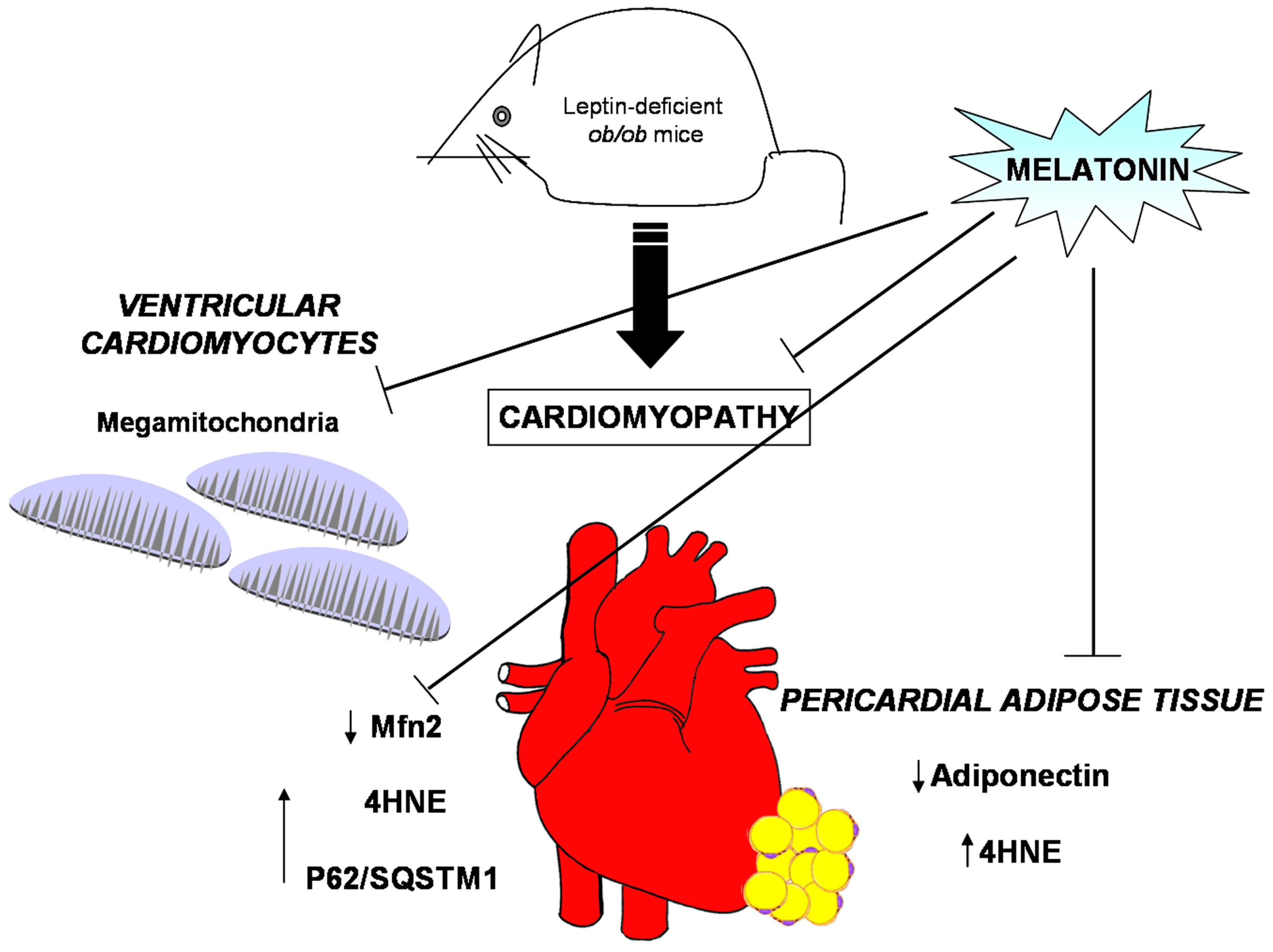
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malik, V.; Willett, W.; Hu, F. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.; Frühbeck, G.; Ryan, D.; Wilding, J. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Pagidipati, N.; Gaziano, T. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation 2013, 127, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Dugani, S.; Gaziano, T. 25 by 25: Achieving global reduction in cardiovascular mortality. Curr. Cardiol. Rep. 2016, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, N.; Aranquiz, P.; Alonso, C.; Rothermel, B.; Lavandero, S. Mitochondria in structural and functional cardiac remodeling. Adv. Exp. Med. Biol. 2017, 982, 277–306. [Google Scholar] [PubMed]
- Facundo, H.; Brainard, R.; Caldas, F.; Lucas, A. Mitochondria and cardiac hypertrophy. Adv. Exp. Med. Biol. 2017, 982, 203–226. [Google Scholar] [PubMed]
- Brown, D.; Perry, J.; Allen, M.; Sabbah, H.; Stauffer, B.; Shaikh, S.; Cleland, J.; Colucci, W.; Butler, J.; Voors, A.; et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gong, B.; Duan, W.; Fan, C.; Zhang, J.; Li, Z.; Xue, X.; Xu, Y.; Meng, D.; Li, B.; et al. Melatonin ameliorates myocardial ischemia-reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC1α-SIRT3 signaling. Sci. Rep. 2017, 7, 41337. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.; Hambly, C.; Mitchell, S.; Krol, E. The contribution of animal models to the study of obesity. Lab. Anim. 2008, 42, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, P.; O’Neill, B.; Roberts, M.; Buchanan, J.; Yun, U.; Cooksey, R.; Boudina, S.; Abel, D. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004, 53, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Villena, J.; Carvalho, A.; Garcia-Arumì, J.; Ramos, D.; Ruberte, J.; Simò, R.; Hernandez, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E. Leptin signaling, adiposity and energy balance. Ann. N. Y. Acad. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Kanoski, S.; Hayes, M.; Greenwald, H.; Fortin, S.; Gianessi, C.; Giebert, J.; Grill, H. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 2011, 36, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Catalan, V.; Rodriguez, A.; Ramirez, B.; Becerril, S.; Portincasa, P.; Gomez-Ambrosi, J. Normalization of adiponectin concentration by leptin replacement in ob/ob is accompanied by reductions in systemic oxidative stress and inflammation. Sci. Rep. 2017, 7, 2752. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.; Gustafsson, A. Mitochondrial turnover in the heart. Biochim. Biophys. Acta 2011, 1813, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, C.; Schneider, T.; Hajnoczky, G.; Csordas, G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1907–H1915. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Thapa, D.; Sheperd, D. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1–H14. [Google Scholar] [CrossRef] [PubMed]
- Holmuhamedov, E.; Oberlin, A.; Short, K.; Terzic, A.; Jahangir, A. Cardiac subsarcolemmal and interfibrillar mitochondria display distinct responsiveness to protection by diazoxide. PLoS ONE 2012, 7, e44667. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.; Dabkowski, E.; Baseler, W.; Croston, T.; Always, S.; Hollander, J. Enhanced apoptotic propensity in diabetic cardiac mitochondria: Influence of subcellular spatial location. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H633–H642. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, B.; Pasdois, P.; Duggan, S.; Bond, A.; Heesom, K.; Jackson, C.; Angelini, G.; Halestrap, A.; Suleiman, M. Hearts from mice fed a non-obesogenic high fat diet exhibit changes in their oxidative state, calcium and mitochondria in parallel with increased susceptibility to reperfusion injury. PLoS ONE 2014, 9, e100579. [Google Scholar] [CrossRef] [PubMed]
- Marin-Garcia, J.; Akhmedov, A. Mitochondrial dynamics and cell death in heart failure. Heart Fail. Rev. 2016, 21, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Dorn, G. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ. Res. 2011, 109, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Gustafsson, A. Mitochondrial autophagy—An essential quality control mechanism for myocardial homeostasis. Circ. J. 2013, 77, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Su, H.; Ranek, M.; Wang, X. Autophagy and p62 in cardiac proteinopathy. Circ. Res. 2011, 109, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.; Mazumder, P.; Hu, P.; Chakrabarti, G.; Roberts, M.; Yun, U.; Cookey, R.; Litwin, S.; Abel, E. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005, 146, 5341–5349. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, X.; Yang, X.; Esberg, L.; Yang, H.; Zhang, Z.; Culver, B.; Ren, J. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J. Endocrinol. 2006, 188, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, T.; Sam, F. Adiponectin in cardiovascular inflammation and obesity. Int. J. Inflamm. 2011, 2011, 376909. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, C.; de Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Witers, S.; Haegerty, A.; Favero, G.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J. Hypertens. 2014, 32, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Tengattini, S.; Reiter, R.; Tan, D.; Terron, M.; Rodella, L.; Rezzani, R. Cardiovascular diseases: Protective effects of melatonin. J. Pineal Res. 2008, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, O.; Amaral, F.; Campos, L.; Cipolla-Neto, J. Melatonin, mitochondria and hypertension. Cell. Mol. Life Sci. 2017, 74, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an anti-oxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.; Petrosillo, G. Protective role of melatonin in mitochondrial dysfunction and related disorders. Arch. Toxicol. 2015, 89, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Huisamen, B.; Stridom, H.; Blackhurst, D.; Lochner, A. Short-term melatonin consumption protects the heart of obese rats independently of body weight change and visceral adiposity. J. Pineal Res. 2014, 57, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 35, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Lonati, C.; Giugno, L.; Castrezzati, S.; Rodella, L.; Rezzani, R. Obesity-related dysfunction of the aorta and prevention by melatonin treatment in ob/ob mice. Acta Histochem. 2013, 115, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Lavazza, A.; Golic, I.; Aleksic, M.; Korac, A.; Rodella, L.; Rezzani, R. Hepatic macrosteatosis is partially converted to microsteatosis by melatonin supplementation in ob/ob mice non-alcoholic fatty liver disease. PLoS ONE 2016, 11, e0148115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; Korecky, B.; Rakusan, K. Remodeling of myocyte dimensions in hypertrophic and atrophic rat hearts. Circ. Res. 1991, 68, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Golic, I.; Velickovic, K.; Markelic, M.; Stancic, A.; Jankovic, A.; Vucetic, M.; Otasevic, V.; Buzadzic, B.; Korac, B.; Korac, A. Calcium-induced alteration of mitochondrial morphology and mitochondrial-endoplasmic reticulum contacts in rat brown adipocytes. Eur. J. Histochem. 2014, 58, 2377. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Yuasa, S.; Motoda, C.; Yozu, G.; Nagai, T.; Ito, S.; Lachmann, M.; Kashimura, S.; Takei, M.; Kusumoto, D.; et al. Emerin plays a crucial role in nuclear invagination and in the nuclear calcium transient. Sci. Rep. 2017, 7, 44312. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoran, S.; Munro, P.; Qiao, F.; Ong, S.; Hall, A.; Cabrera-Fuentes, H.; Chakraborty, B.; Boisvert, W.; Yellon, D.; Hausenloy, D. Unique morphological characteristics of mitochondrial subtypes in the heart: The effect of ischemia and ischemic preconditioning. Discoveries 2017, 5, e71. [Google Scholar] [CrossRef] [PubMed]
- Ljubojevic, S.; Radulovic, S.; Leitinger, G.; Sedej, S.; Sacherer, M.; Holzer, M.; Winkler, C.; Pritz, E.; Mittler, T.; Schmidt, A.; et al. Remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 2014, 130, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Sena, S.; O’Neill, B.; Tathireddy, P.; Young, M.; Abel, E. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 2005, 112, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Trincado, C.; Garcia-Carvajal, I.; Pennanenc, C.; Parra, V.; Hill, J.; Rothermel, B.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Mali, V.; Ning, R.; Chen, J.; Yang, X.; Xu, J.; Palaniyandi, S. Impairment of aldehyde dehydrogenase 2 by 4 hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp. Biol. Med. 2014, 239, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, K.; Khairallah, R.; Ngoh, G.; Chikando, A.; Luptak, I.; O’Shea, K.; Riley, D.; Lugus, J.; Colucci, W.; Lederer, W.; et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol. Cell Biol. 2011, 31, 1309–1328. [Google Scholar] [CrossRef] [PubMed]
- De Brito, O.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Li, X.; Li, L.; Dodson, M.; Zhang, D.; Zheng, H. Multifunctional p62 effects underlie diverse metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Reddy, Y. Distinction of fat around the heart. J. Am. Coll. Cardiol. 2011, 58, 1640–1641. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Coisne, A.; Marechal, X.; Staels, B. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 2016, 65, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McManus, M.; Csordas, G.; Varnai, P.; Dorn, G.; Williams, D.; Hajnoczky, G.; Wallace, D. Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat. Commun. 2015, 6, 6259. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Tandler, B.; Loffredo, F.; Vazquez, E.; Hoppel, C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am. J Physiol. Heart Circ. Physiol. 2005, 289, H868–H872. [Google Scholar] [CrossRef] [PubMed]
- Gallitelli, M.; Schultz, M.; Isenberg, G.; Rudolf, F. Twich-potentiation increases calcium in peripheral more than in central mitochondria of guinea-pig ventricular myocytes. J. Physiol. 1999, 518, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Baseler, W.; Dabkowki, E. Proteomic remodeling of mitochondria in heart failure. Congest. Heart Fail. 2011, 17, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E. Mitochondrial uncoupling: A key contributor to reduced cardiac efficiency in diabetes. Physiology 2006, 21, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T. Megamitochondria formation–Physiology and pathology. J. Cell Mol. Med. 2002, 6, 497–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.; Hotamisligil, G. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Echtay, K.; Esteves, T.; Pakay, J.; Jekabsons, M.; Lambert, A.; Portero-Otin, M.; Pamplona, R.; Vidal-Puig, A.; Wang, S.; Roebuck, S.; et al. A signaling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003, 22, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Kijima, K.; Numakura, C.; Izumino, H.; Umetsu, K.; Nezu, A.; Shiki, T.; Ogawa, M.; Ishizaki, Y.; Kitamura, T.; Shozawa, Y.; et al. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2. Hum. Genet. 2005, 116, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, X.; Han, L.; Wang, X.; Cheng, H.; Zhao, Y.; Chen, Q.; Chen, J.; Cheng, H.; Xiao, R.; et al. Central role of Mf2 in autophagosome-lysosome fusion in cardiac myocytes. J. Biol. Chem. 2012, 287, 23615–23625. [Google Scholar] [CrossRef] [PubMed]
- Zhen, M.; Xiao, R. Role of mitofusin 2 in cardiovascular oxidative injury. J. Mol. Med. 2010, 88, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Giugno, L.; Lavazza, A.; Reiter, R.; Rodella, L.; Rezzani, R. Mitochondrial and metabolic dysfunction in renal convoluted tubules of obese mice: Protective role of melatonin. PLoS ONE 2014, 9, e111141. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, H.; Liu, P.; Wang, H.; Liu, J.; Gao, C.; Liu, Y.; Lian, K.; Yang, L.; Sun, L.; et al. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC1α signaling contributing to increased vulnerability in diabetic heart. Basic Res. Cardiol. 2013, 108, 329. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Jensen, J.; Pedersen, S.; Galatius, S.; Frystyk, J.; Flyvbjerg, A.; Bjerre, M.; Mogelvang, R. Low adiponectin levels and increased risk of type 2 diabetes in patients with myocardial infarction. Diabetes Care 2014, 37, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Li, J.; Gu, D.; Li, S.; Shen, C.; Song, Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS ONE 2013, 8, e700663. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Zhou, Y.; Gan, R.; Xu, D.; Li, H. Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stacchiotti, A.; Favero, G.; Giugno, L.; Golic, I.; Korac, A.; Rezzani, R. Melatonin Efficacy in Obese Leptin-Deficient Mice Heart. Nutrients 2017, 9, 1323. https://doi.org/10.3390/nu9121323
Stacchiotti A, Favero G, Giugno L, Golic I, Korac A, Rezzani R. Melatonin Efficacy in Obese Leptin-Deficient Mice Heart. Nutrients. 2017; 9(12):1323. https://doi.org/10.3390/nu9121323
Chicago/Turabian StyleStacchiotti, Alessandra, Gaia Favero, Lorena Giugno, Igor Golic, Aleksandra Korac, and Rita Rezzani. 2017. "Melatonin Efficacy in Obese Leptin-Deficient Mice Heart" Nutrients 9, no. 12: 1323. https://doi.org/10.3390/nu9121323





