A Metagenomic and in Silico Functional Prediction of Gut Microbiota Profiles May Concur in Discovering New Cystic Fibrosis Patient-Targeted Probiotics
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Anamnestic and Laboratory Features
2.3. DNA Extraction and Next Generation Sequencing (NGS) Analysis
2.4. Statistical Analysis
3. Results
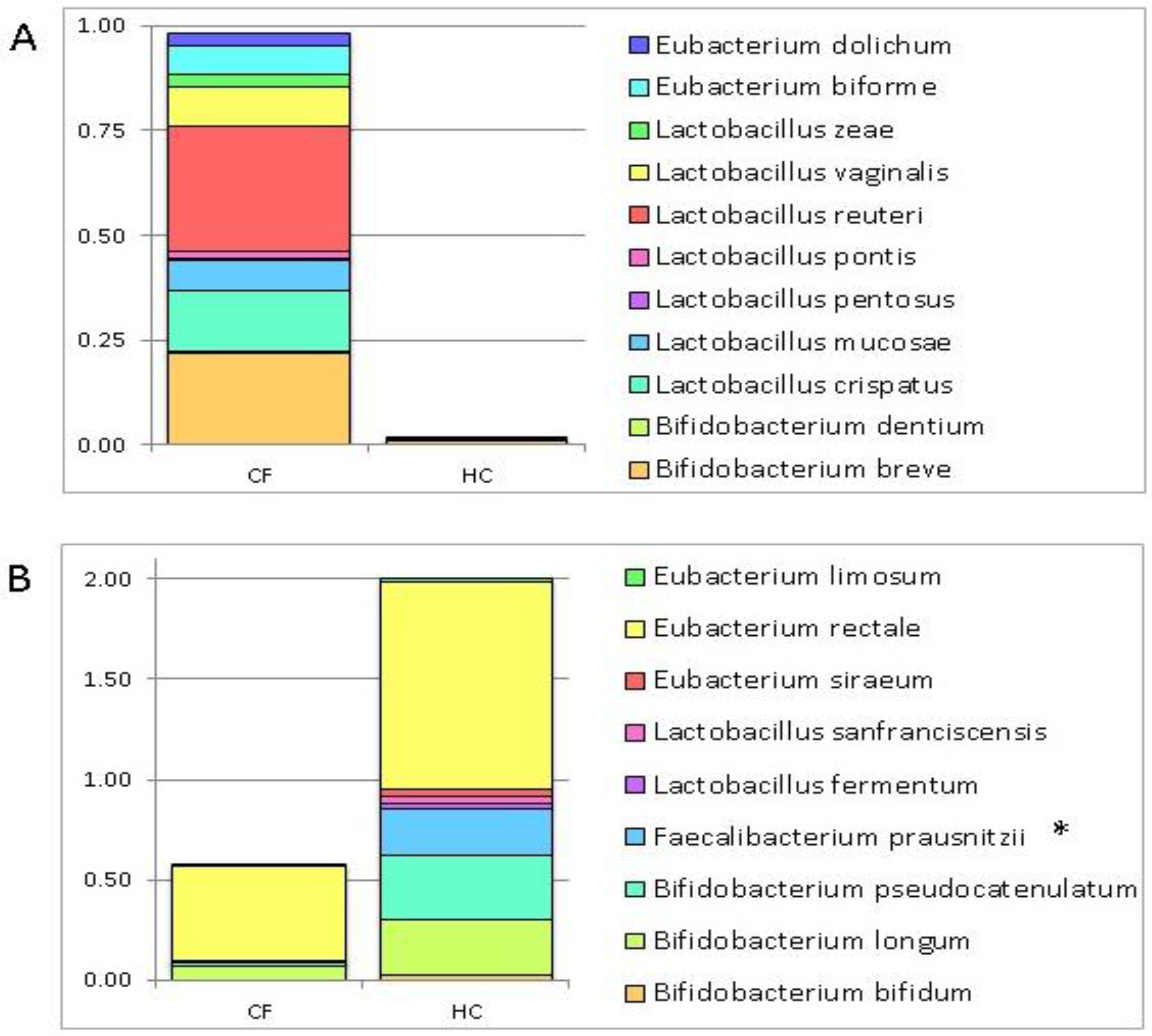
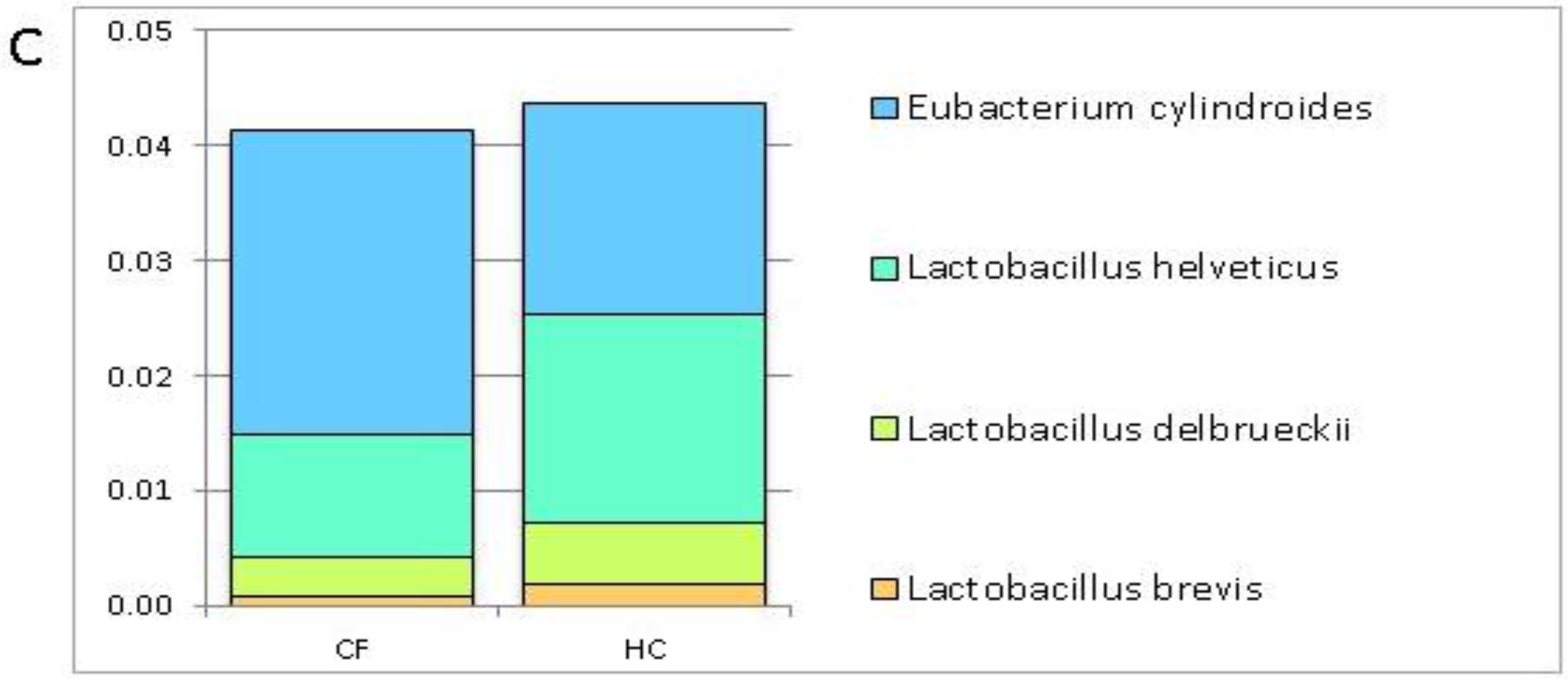
3.1. Putative Probiotic Distribution in the GM Profiles
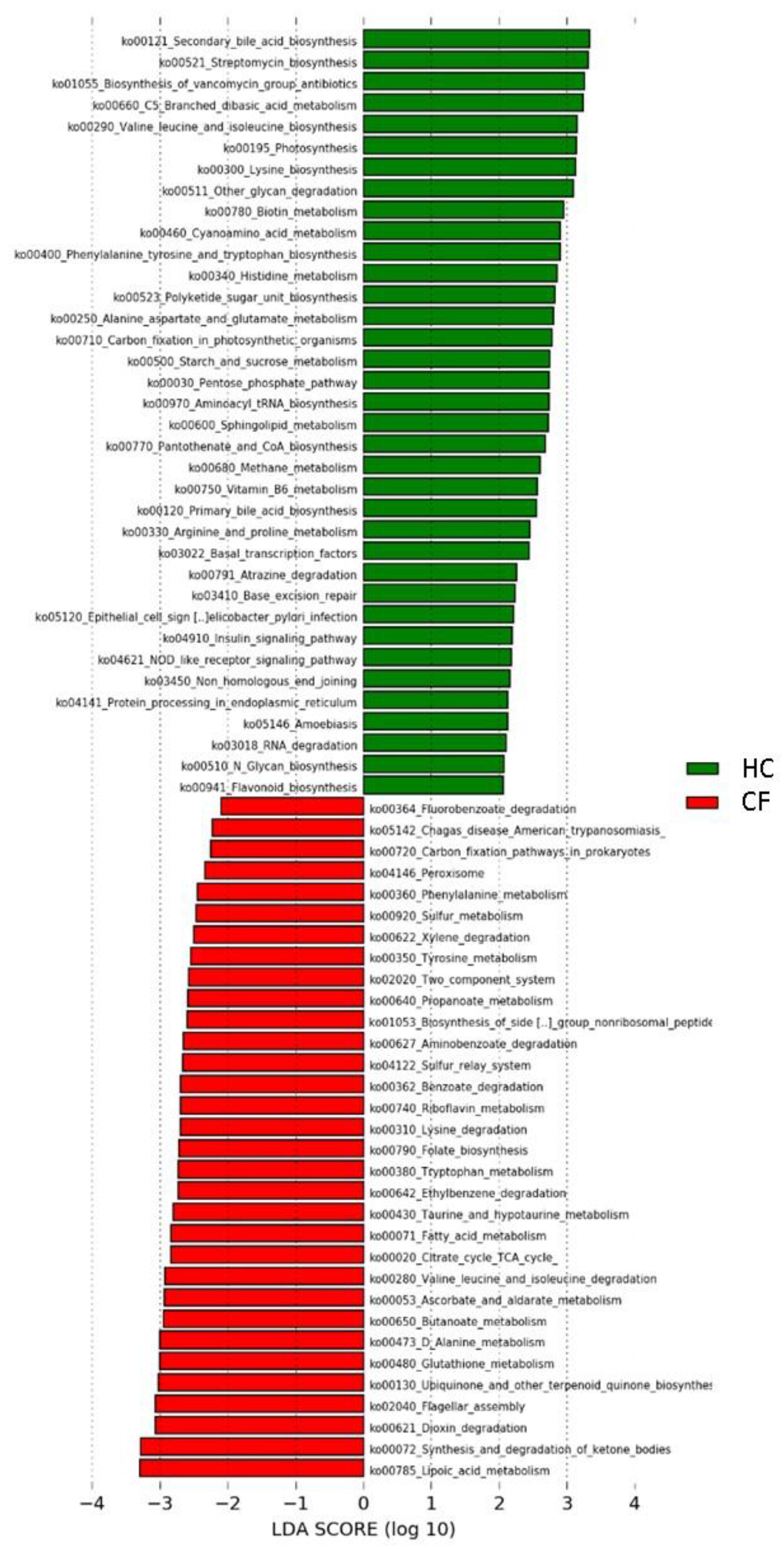
3.2. Metabolic Pathways of Probiotics
4. Discussion
4.1. Putative Probiotic Distribution in the GM Profiles
4.2. Metabolic Pathways of Probiotics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garcia, M.A.S.; Yang, N.; Quinton, P.M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J. Clin. Investig. 2009, 119, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Somerset, S. The clinical significance of the gut microbiota in cystic fibrosis and the potential for dietary therapies. Clin. Nutr. Edinb. Scotl. 2014, 33, 571–580. [Google Scholar] [CrossRef]
- Jafari, S.-A.; Mehdizadeh-Hakkak, A.; Kianifar, H.-R.; Hebrani, P.; Ahanchian, H.; Abbasnejad, E. Effects of probiotics on quality of life in children with cystic fibrosis; a randomized controlled trial. Iran. J. Pediatr. 2013, 23, 669–674. [Google Scholar]
- Claeys, S.; Van Hoecke, H.; Holtappels, G.; Gevaert, P.; De Belder, T.; Verhasselt, B.; Van Cauwenberge, P.; Bachert, C. Nasal polyps in patients with and without cystic fibrosis: A differentiation by innate markers and inflammatory mediators. Clin. Exp. Allergy 2005, 35, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Infante, P.D.; Redecillas, F.S.; Torrent, V.A.; Segarra, C.O.; Maldonado, S.M.; Gartner, T.L.; Hidalgo, A.E. Improvement of intestinal function in cystic fibrosis patients using probiotics. An. Pediatr. 2008, 69, 501–505. [Google Scholar]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4607–4614. [Google Scholar] [CrossRef]
- Dray, X.; Kanaan, R.; Bienvenu, T.; Desmazes-Dufeu, N.; Dusser, D.; Marteau, P.; Hubert, D. Malnutrition in adults with cystic fibrosis. Eur. J. Clin. Nutr. 2005, 59, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cymberknoh, M.; Shoseyov, D.; Kerem, E. Managing cystic fibrosis: Strategies that increase life expectancy and improve quality of life. Am. J. Respir. Crit. Care Med. 2011, 183, 1463–1471. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Petrucca, A.; Paci, P.; Fuentes, S.; Praticò, G.; Capuani, G.; Masotti, A.; Reddel, S.; Russo, A.; et al. Phylogenetic and Metabolic Tracking of Gut Microbiota during Perinatal Development. PLoS ONE 2015, 10, e0137347. [Google Scholar] [CrossRef]
- Putignani, L.; Dallapiccola, B. Foodomics as part of the host-microbiota-exposome interplay. J. Proteom. 2016, 147, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Miles, C.; Tierney, A.C. Effect of probiotics on respiratory, gastrointestinal and nutritional outcomes in patients with cystic fibrosis: A systematic review. J. Cyst. Fibros. 2017, 16, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organisation of the United Nations and WHO Working Group. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Rogers, G.B.; Carroll, M.P.; Hoffman, L.R.; Walker, A.W.; Fine, D.A.; Bruce, K.D. Comparing the microbiota of the cystic fibrosis lung and human gut. Gut Microbes 2010, 1, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, K.; Quigley, E.M.M. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr. Opin. Gastroenterol. 2013, 29, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, E.S.; Marsland, B.J. Microbiota abnormalities in inflammatory airway diseases—Potential for therapy. Pharmacol. Ther. 2014, 141, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Fiocchi, A.G.; El Hachem, M.; Dallapiccola, B.; Rossi, P.; Putignani, L. Understanding probiotics’ role in allergic children: The clue of gut microbiota profiling. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Fatemeh, F.; Mehri, N.; Maryam, S. Probiotics for the treatment of pediatric helicobacter pylori infection: A randomized double blind clinical trial. Iran. J. Pediatr. 2013, 23, 79–84. [Google Scholar] [PubMed]
- Bruzzese, E.; Raia, V.; Gaudiello, G.; Polito, G.; Buccigrossi, V.; Formicola, V.; Guarino, A. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment. Pharmacol. Ther. 2004, 20, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Bujanover, Y.; Yahav, Y.; Vilozni, D.; Fireman, E.; Efrati, O. Probiotic supplementation affects pulmonary exacerbations in patients with cystic fibrosis: A pilot study. Pediatr. Pulmonol. 2010, 45, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, N.A.; Cope, E.K.; Lynch, S.V. Probiotic strategies for treatment of respiratory diseases. Trends Microbiol. 2013, 21, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.E.; Cooke, R.E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 1959, 23, 545–549. [Google Scholar] [PubMed]
- Farrell, P.M. The prevalence of cystic fibrosis in the European Union. J. Cyst. Fibros. 2008, 7, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; Stefanis, C.D.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Reeder, J.; Knight, R. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 2010, 7, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Navas-Molina, J.A.; Peralta-Sánchez, J.M.; González, A.; McMurdie, P.J.; Vázquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abubucker, S.; Segata, N.; Goll, J.; Schubert, A.M.; Izard, J.; Cantarel, B.L.; Rodriguez-Mueller, B.; Zucker, J.; Thiagarajan, M.; Henrissat, B.; et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput. Biol. 2012, 8, e1002358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Jackson, M.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, F.; Mayeur, C.; Bruneau, A.; Noordine, M.-L.; Meylheuc, T.; Langella, P.; Messing, B.; Duée, P.-H.; Cherbuy, C.; Thomas, M. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 2010, 92, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Salonen, A.; Kekkonen, R.A.; Salojärvi, J.; Jalanka-Tuovinen, J.; Palva, A.; Orešič, M.; de Vos, W.M. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ 2013, 1, e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Mandal, S. Bifidobacteria—Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol. Res. 2016, 192, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.D.; da Silva, G.A.P.; de Lira, P.I.C.; de Carvalho Lima, M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2011, 93, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Takei, Y.; Yamashina, S.; Ikejima, K.; Kitamura, T.; Sato, N. Anti-inflammatory strategies in alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. 1), S59–S61. [Google Scholar] [CrossRef]
- D’Ettorre, G.; Ceccarelli, G.; Giustini, N.; Serafino, S.; Calantone, N.; De Girolamo, G.; Bianchi, L.; Bellelli, V.; Ascoli-Bartoli, T.; Marcellini, S.; et al. Probiotics Reduce Inflammation in Antiretroviral Treated, HIV-Infected Individuals: Results of the “Probio-HIV” Clinical Trial. PLoS ONE 2015, 10, e0137200. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 2013, 8, e59370. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Kim, K.-A.; Jang, S.-E.; Woo, J.-Y.; Han, M.J.; Kim, D.-H. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE 2015, 10, e0116533. [Google Scholar] [CrossRef]
- Kanauchi, O.; Fukuda, M.; Matsumoto, Y.; Ishii, S.; Ozawa, T.; Shimizu, M.; Mitsuyama, K.; Andoh, A. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J. Gastroenterol. 2006, 12, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Callegari, M.L.; Raia, V.; Viscovo, S.; Scotto, R.; Ferrari, S.; Morelli, L.; Buccigrossi, V.; Lo Vecchio, A.; Ruberto, E.; et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: A randomised clinical trial. PLoS ONE 2014, 9, e87796. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- De Angelis, M.; Di Cagno, R.; Gallo, G.; Curci, M.; Siragusa, S.; Crecchio, C.; Parente, E.; Gobbetti, M. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 2007, 114, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Lenoir, M.; Mayorga-Reyes, L.; Allain, T.; Sokol, H.; Langella, P.; Sánchez-Pardo, M.E.; Bermúdez-Humarán, L.G. Identification of novel anti-inflammatory probiotic strains isolated from pulque. Appl. Microbiol. Biotechnol. 2016, 100, 385–396. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Garcia, F.; Olivares, M.; Rodríguez-Cabezas, M.E.; Gálvez, J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef]
- Moya-Pérez, A.; Neef, A.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e0126976. [Google Scholar] [CrossRef]
- Duytschaever, G.; Huys, G.; Bekaert, M.; Boulanger, L.; De Boeck, K.; Vandamme, P. Dysbiosis of bifidobacteria and Clostridium cluster XIVa in the cystic fibrosis fecal microbiota. J. Cyst. Fibros. 2013, 12, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjögren, Y.M.; Tomicic, S.; Lundberg, A.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E.; Jenmalm, M.C. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy 2009, 39, 1842–1851. [Google Scholar] [CrossRef]
- Chenoll, E.; Rivero, M.; Codoñer, F.M.; Martinez-Blanch, J.F.; Ramón, D.; Genovés, S.; Moreno Muñoz, J.A. Complete Genome Sequence of Bifidobacterium longum subsp. infantis Strain CECT 7210, a Probiotic Strain Active against Rotavirus Infections. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Rivière, A.; Gagnon, M.; Weckx, S.; Roy, D.; De Vuyst, L. Mutual Cross-Feeding Interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 Explain the Bifidogenic and Butyrogenic Effects of Arabinoxylan Oligosaccharides. Appl. Environ. Microbiol. 2015, 81, 7767–7781. [Google Scholar] [CrossRef]
- Egan, M.; Motherway, M.O.; Kilcoyne, M.; Kane, M.; Joshi, L.; Ventura, M.; van Sinderen, D. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC Microbiol. 2014, 14, 282. [Google Scholar] [CrossRef]
- Nobili, V.; Putignani, L.; Mosca, A.; Chierico, F.D.; Vernocchi, P.; Alisi, A.; Stronati, L.; Cucchiara, S.; Toscano, M.; Drago, L. Bifidobacteria and lactobacilli in the gut microbiome of children with non-alcoholic fatty liver disease: Which strains act as health players? Arch. Med. Sci. 2016. [Google Scholar] [CrossRef]
- Fouhy, F.; Ronan, N.J.; O’Sullivan, O.; McCarthy, Y.; Walsh, A.M.; Murphy, D.M.; Daly, M.; Flanagan, E.T.; Fleming, C.; McCarthy, M.; et al. A pilot study demonstrating the altered gut microbiota functionality in stable adults with Cystic Fibrosis. Sci. Rep. 2017, 7, 6685. [Google Scholar] [CrossRef] [PubMed]
- Weizman, Z.; Durie, P.R.; Kopelman, H.R.; Vesely, S.M.; Forstner, G.G. Bile acid secretion in cystic fibrosis: Evidence for a defect unrelated to fat malabsorption. Gut 1986, 27, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.A.; Lim, Y.W.; Maughan, H.; Conrad, D.; Rohwer, F.; Whiteson, K.L. Biogeochemical forces shape the composition and physiology of polymicrobial communities in the cystic fibrosis lung. mBio 2014, 5, e00956-13. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.A.; Nixon, L.S.; Luzio, S.; Lewis-Jenkins, V.; Evans, W.D.; Stone, M.D.; Owens, D.R.; Routledge, P.A.; Shale, D.J. Pulmonary function, body composition, and protein catabolism in adults with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002, 165, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Mashburn, L.M.; Singh, P.K.; Whiteley, M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 2005, 187, 5267–5277. [Google Scholar] [CrossRef]
- Bacci, G.; Mengoni, A.; Fiscarelli, E.; Segata, N.; Taccetti, G.; Dolce, D.; Paganin, P.; Morelli, P.; Tuccio, V.; De Alessandri, A.; et al. A Different Microbiome Gene Repertoire in the Airways of Cystic Fibrosis Patients with Severe Lung Disease. Int. J. Mol. Sci. 2017, 18, 1654. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.D.; Prigge, S.T. Lipoic acid metabolism in microbial pathogens. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Price-Whelan, A.; Dietrich, L.E.P.; Newman, D.K. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007, 189, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, G.; Huys, G.; Bekaert, M.; Boulanger, L.; De Boeck, K.; Vandamme, P. Cross-sectional and longitudinal comparisons of the predominant fecal microbiota compositions of a group of pediatric patients with cystic fibrosis and their healthy siblings. Appl. Environ. Microbiol. 2011, 77, 8015–8024. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Evangelista, J.S.; Schmieder, R.; Bailey, B.; Haynes, M.; Furlan, M.; Maughan, H.; Edwards, R.; Rohwer, F.; Conrad, D. Clinical insights from metagenomic analysis of sputum samples from patients with cystic fibrosis. J. Clin. Microbiol. 2014, 52, 425–437. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Males | Mean Age | Mean W/L or BMI Z-Score * | Pancreatic Insufficiency: Yes/Not | Mean Value of Sweat Test | Chronic Use of Antibiotic: Yes/No | Disease Severity: Mild/Severe |
|---|---|---|---|---|---|---|---|
| CF | 11/28 (39%) | 3.5 | ±0.9 | 22/6 79/21 (%) | 93 | 12/16 43/57 (%) | 4/24 14/86 (%) |
| HC | 20/31 (64.5%) | 3.06 | ±0.51 | nda ** | nda | nda | nda |
| Bacteria | Group of Subjects |
|---|---|
| Bifidobacterium bifidum | HC |
| Bifidobacterium longum | |
| Bifidobacterium pseudocatenulatum | |
| Faecalibacterium prausnitzii | |
| Lactobacillus fermentum | |
| Lactobacillus sanfranciscensis | |
| Eubacterium siraeum | |
| Eubacterium rectale | |
| Eubacterium limosum | |
| Bifidobacterium breve | CF |
| Bifidobacterium dentium | |
| Lactobacillus crispatus | |
| Lactobacillus mucosae | |
| Lactobacillus pentosus | |
| Lactobacillus pontis | |
| Lactobacillus reuteri | |
| Lactobacillus vaginalis | |
| Lactobacillus zeae | |
| Eubacterium biforme | |
| Eubacterium dolichum |
| KEGG Pathways | Class * | Subclass | Group | KEGG Pathways | Class | Subclass | Group |
|---|---|---|---|---|---|---|---|
| Carbon fixation in photosynthetic organisms | 1 | Energy metabolism | HC | Lysine degradation | 1 | Amino acid metabolism | CF |
| Alanine aspartate and glutamate metabolism | Amino acid metabolism | Phenylalanine metabolism | |||||
| Arginine and proline metabolism | Tryptophan metabolism | ||||||
| Histidine metabolism | Tyrosine metabolism | ||||||
| Lysine biosynthesis | Valine leucine and isoleucine degradation | ||||||
| Phenylalanine tyrosine and tryptophan biosynthesis | Ascorbate and aldarate metabolism | Carbohydrate metabolism | |||||
| Valine leucine and isoleucine biosynthesis | Butanoate metabolism | ||||||
| Flavonoid biosynthesis | Biosynthesis of other secondary metabolites | Citrate cycle TCA cycle | |||||
| Streptomycin biosynthesis | Carbon fixation pathways in prokaryotes | Energy metabolism | |||||
| C5 Branched dibasic acid metabolism | Carbohydrate metabolism | Sulfur metabolism | |||||
| Pentose phosphate pathway | |||||||
| Propanoate metabolism | Fatty acid metabolism | Lipid metabolism | |||||
| Starch and sucrose metabolism | |||||||
| Methane metabolism | Energy metabolism | Synthesis and degradation of ketone bodies | |||||
| Photosynthesis | |||||||
| N Glycan biosynthesis | Glycan biosynthesis and metabolism | Folate biosynthesis | Metabolism of cofactors and vitamins | ||||
| Other glycan degradation | Lipoic acid metabolism | ||||||
| Primary bile acid biosynthesis | Lipid metabolism | ||||||
| Secondary bile acid biosynthesis | Ubiquinone and other terpenoid quinone biosynthesis | ||||||
| Sphingolipid metabolism | |||||||
| Biotin metabolism | Metabolism of cofactors and vitamins | Glutathione metabolism | Metabolism of other amino acids | ||||
| Pantothenate and CoA biosynthesis | |||||||
| Riboflavin metabolism | Taurine and hypotaurine metabolism | ||||||
| Vitamin B6 metabolism | |||||||
| Cyanoamino acid metabolism | Metabolism of other amino acids | Biosynthesis of siderophore group nonribosomal peptides | Metabolism of terpenoids and polyketides | ||||
| D Alanine metabolism | |||||||
| Biosynthesis of vancomycin group antibiotics | Metabolism of terpenoids and polyketides | Aminobenzoate degradation | Xenobiotics biodegradation and metabolism | ||||
| Polyketide sugar unit biosynthesis | Benzoate degradation | ||||||
| Atrazine degradation | Xenobiotics biodegradation and metabolism | Dioxin degradation | |||||
| Protein processing in endoplasmic reticulum | 2 | Folding, sorting and degradation | Ethylbenzene degradation | ||||
| RNA degradation | Fluorobenzoate degradation | ||||||
| Base excision repair | Replication and repair | Xylene degradation | |||||
| Non homologous end joining | Sulfur relay system | 2 | Folding, sorting and degradation | ||||
| Basal transcription factors | Transcription | ||||||
| Aminoacyl tRNA biosynthesis | Translation | Two component system | 3 | Signal transduction | |||
| Insulin signaling pathway | 5 | Endocrine system | Flagellar assembly | 4 | Cell motility | ||
| Nucleotide oligomerization domain (NOD) like receptor signaling pathway | Immune system | Peroxisome | Transport and catabolism | ||||
| Amoebiasis | 6 | Infectious diseases | Chagas disease American trypanosomiasis | 6 | Infectious diseases | ||
| Epithelial cell signaling in Helicobacter pylori infection |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernocchi, P.; Del Chierico, F.; Quagliariello, A.; Ercolini, D.; Lucidi, V.; Putignani, L. A Metagenomic and in Silico Functional Prediction of Gut Microbiota Profiles May Concur in Discovering New Cystic Fibrosis Patient-Targeted Probiotics. Nutrients 2017, 9, 1342. https://doi.org/10.3390/nu9121342
Vernocchi P, Del Chierico F, Quagliariello A, Ercolini D, Lucidi V, Putignani L. A Metagenomic and in Silico Functional Prediction of Gut Microbiota Profiles May Concur in Discovering New Cystic Fibrosis Patient-Targeted Probiotics. Nutrients. 2017; 9(12):1342. https://doi.org/10.3390/nu9121342
Chicago/Turabian StyleVernocchi, Pamela, Federica Del Chierico, Andrea Quagliariello, Danilo Ercolini, Vincenzina Lucidi, and Lorenza Putignani. 2017. "A Metagenomic and in Silico Functional Prediction of Gut Microbiota Profiles May Concur in Discovering New Cystic Fibrosis Patient-Targeted Probiotics" Nutrients 9, no. 12: 1342. https://doi.org/10.3390/nu9121342





