Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age
Abstract
:1. Introduction
2. Methods
2.1. Study Overview
2.2. Participants
2.3. Anthropometric and Body Composition Variables
2.4. Breast-Milk Collection
2.5. Breast-Milk Analyses
Preparation of Fructose Quantification Sample
2.6. Statistical Analysis
3. Results
3.1. Variation in Breast Milk Sugar Composition within the First Six Months of Life
3.2. Relationships between Breast Milk Sugar Content and Infant Growth
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gillespie, C.; Welsh, J.A.; Hu, F.B.; Yang, Q. Usual intake of added sugars and lipid profiles among the U.S. adolescents: National Health and Nutrition Examination Survey, 2005–2010. J. Adolesc. Health 2015, 56, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.; Cunningham, S.A.; Vos, M.B. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011, 123, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Huang, Y.; Reilly, K.H.; Li, S.; Zheng, R.; Barrio-Lopez, M.T.; Martinez-Gonzalez, M.A.; Zhou, D. Sugar-sweetened beverages and risk of hypertension and CVD: A dose–response meta-analysis. Br. J. Nutr. 2015, 113, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 2015, 63, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Calorie-sweetened beverages and fructose: What have we learned 10 years later. Pediatr. Obes. 2013, 8, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.-M.; Havel, P.J. Adverse metabolic effects of dietary fructose: Results from the recent epidemiological, clinical, and mechanistic studies. Curr. Opin. Lipidol. 2013, 24, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Dumke, K.; Bouret, S.G.; Kayser, B.; Walker, R.W.; Blumberg, B. The obesogenic effect of high fructose exposure during early development. Nat. Rev. Endocrinol. 2013, 9, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, R.; Park, S.; Galuska, D.A.; Sherry, B.; Freedman, D.S. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014, 134, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Pan, L.; Sherry, B.; Li, R. The association of sugar-sweetened beverage intake during infancy with sugar-sweetened beverage intake at 6 years of age. Pediatrics 2014, 134, S56–S62. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioctive Factors. Pediatr. Clin. N. Am. 2013, 60, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Demerath, E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr. Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Schneider, C.R.; Pavela, G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity 2016, 24, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.A.; Gurr, M.I.; van Duijvenvoorde, P.M.; Rolls, B.J.; Rowe, E.A. Lactation in lean and obese rats: Effect of cafeteria feeding and of dietary obesity on milk composition. Physiol. Behav. 1986, 38, 185–190. [Google Scholar] [CrossRef]
- Purcell, R.H.; Sun, B.; Pass, L.L.; Power, M.L.; Moran, T.H.; Tamashiro, K.L.K. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol. Behav. 2011, 104, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.R.; Hartmann, P.E. Milk secretion in the rat: Progressive changes in milk composition during lactation and weaning and the effect of diet. Comp. Biochem. Physiol. A Physiol. 1991, 98, 535–542. [Google Scholar] [CrossRef]
- Bayol, S.A.; Farrington, S.J.; Stickland, N.C. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. Br. J. Nutr. 2007, 98, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.N. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. AJP Regul. Integr. Comp. Physiol. 2006, 291, R768–R778. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Schellong, K.; Schulz, S.; Stupin, J.H. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Franke, K.; Kohlhoff, R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care 2002, 25, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S.H. Lactation in insulin-dependent diabetes. Prog. Food Nutr. Sci. 1990, 14, 333–370. [Google Scholar] [PubMed]
- Jovanovic-Peterson, L.; Fuhrmann, K.; Hedden, K.; Walker, L.; Peterson, C.M. Maternal milk and plasma glucose and insulin levels: Studies in normal and diabetic subjects. J. Am. Coll. Nutr. 1989, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Scano, P.; Murgia, A.; Demuru, M.; Consonni, R.; Caboni, P. Metabolite profiles of formula milk compared to breast milk. Food Res. Int. 2016, 87, 76–82. [Google Scholar] [CrossRef]
- Bass, E.F.; Baile, C.A.; Lewis, R.D.; Giraudo, S.Q. Bone quality and strength are greater in growing male rats fed fructose compared with glucose. Nutr. Res. 2013, 33, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Jenness, R. The composition of human milk. Semin. Perinatol. 1979, 3, 225–239. [Google Scholar] [PubMed]
- Hagerman, D.D.; Villee, C.A. The transport of fructose by human placenta. J. Clin. Investig. 1952, 31, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, N.G.; Kaplan, B.; Karvonen, M.J.; Lind, J.; Malm, M. Permeability of human placenta to glucose, fructose, and xylose. Acta Physiol. Scand. 1956, 36, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.A.; Smith, A.H.; Tokar, E.J.; Graziano, J.H.; Kim, K. Mechanisms Underlying Latent Disease Risk Associated with Early-Life Arsenic Exposure: Current Research Trends and Scientific Gaps. Environ. Health Perspect. 2016, 124, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V.A.; Perera, F.P.; Horton, M.K.; Whyatt, R.M.; Bansal, R.; Hao, X.; Liu, J.; Barr, D.B.; Slotkin, T.A.; Peterson, B.S. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci. USA 2012, 109, 7871–7876. [Google Scholar] [CrossRef] [PubMed]
- Whyatt, R.M.; Rauh, V.; Barr, D.B.; Camann, D.E.; Andrews, H.F.; Garfinkel, R.; Hoepner, L.A.; Diaz, D.; Dietrich, J.; Reyes, A.; et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 2004, 112, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Heaney, A.P. Regulation of Adipose Differentiation by Fructose and GluT5. Mol. Endocrinol. 2012, 26, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Gunn, J.P.; Yuan, K.; Park, S.; Merritt, R. Sodium and sugar in complementary infant and toddler foods sold in the United States. Pediatrics 2015, 135, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Goran, M. Laboratory determined sugar content and composition of commercial infant formulas, baby foods and common grocery items targeted to children. Nutrients 2015, 7, 5850–5867. [Google Scholar] [CrossRef] [PubMed]

| 1 Month | 6 Months | Change a | |
|---|---|---|---|
| Mother | |||
| Age (years) | 29.64 (4.87) | ||
| BMI (kg/m2) | 28.14 (7.32) | ||
| Infant | |||
| Age (days) | 39.28 (3.51) | ||
| Sex (F/M) | 8/17 | ||
| Weight (g) | 4766.36 (765.45) | 7250.84 (1243.35) | 2484.48 (736.89) ‡ |
| Length (cm) | 55.82 (2.44) | 65.38 (2.92) | 9.56 (2.07) ‡ |
| Lean Mass (g) b | 3753.32 (476.02) | 4880.05 (749.21) | 1126.72 (494.44) ‡ |
| Fat Mass (g) | 1222.84 (309.11) | 2485.08 (642.27) | 1262.24 (462.29) ‡ |
| Adiposity (%) c | 23.96 (3.02) | 32.74 (3.75) | 8.79 (3.26) ‡ |
| BMC (g) | 93.2 (17.85) | 134.45 (21.0) | 41.28 (19.92) ‡ |
| BMD (g/cm2) | 0.37 (0.04) | 0.36 (0.03) | −0.002 (0.03) |
| 1 Month | 6 Month a | Average b | |
|---|---|---|---|
| Insulin (pg/mL) c | 641.0 (638.0) | 640.8 (533.7) | 640.9 (469.5) |
| Fructose (μg/mL) | 7.2 (1.72) | 6.3 (1.7) | 6.7 (1.3) |
| Glucose (μg/mL) | 263.6 (87.5) | 246.8 (76.8) | 255.2 (75.3) |
| Lactose (g/dL) d | 7.8 (0.8) | 7.5 (0.7) | 7.6 (0.6) |
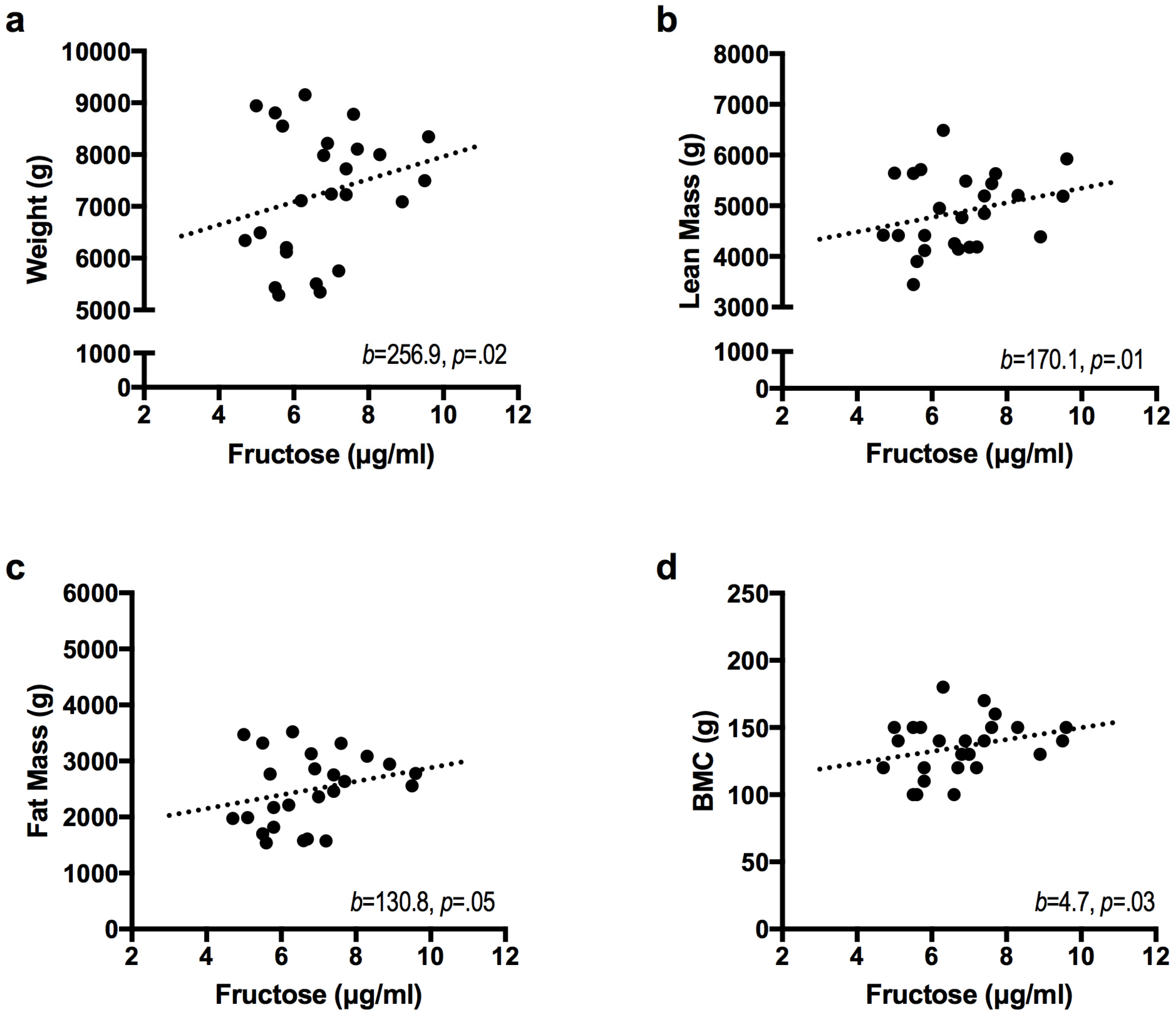
| Infant Outcome | Model | β | ΔR2 | ΔR2 p Value |
|---|---|---|---|---|
| Weight (g) | Base Model | 0.70 | 0.00 | |
| Fructose | 256.9 * | 0.08 | 0.02 | |
| Lactose | 26.2 | <0.01 | 0.92 | |
| Glucose | −0.9 | <0.01 | 0.73 | |
| Insulin | 0.04 | <0.01 | 0.91 | |
| Length (cm) | Base Model | 0.56 | 0.00 | |
| Fructose | 0.4 | 0.03 | 0.28 | |
| Lactose | −1.1 | 0.04 | 0.15 | |
| Glucose | −0.01 | 0.03 | 0.23 | |
| Insulin | <0.01 | 0.03 | 0.21 | |
| Weight-for-Length z-score | Base Model | 0.52 | 0.00 | |
| Fructose | 0.3 * | 0.12 | 0.02 | |
| Lactose | 0.5 | 0.05 | 0.14 | |
| Glucose | <0.01 | 0.01 | 0.54 | |
| Insulin | <0.01 | 0.02 | 0.39 | |
| Lean Mass (g) b | Base Model | 0.66 | 0.00 | |
| Fructose | 170.1 * | 0.09 | 0.01 | |
| Lactose | 224.3 | 0.03 | 0.18 | |
| Glucose | −1.8 | 0.02 | 0.27 | |
| Insulin | 0.30 | 0.03 | 0.23 | |
| Fat Mass (g) | Base Model | 0.59 | 0.00 | |
| Fructose | 130.8 * | 0.07 | 0.05 | |
| Lactose | −31.7 | 0.00 | 0.84 | |
| Glucose | −0.2 | <0.01 | 0.88 | |
| Insulin | −0.13 | 0.01 | 0.51 | |
| Adiposity (%) c | Base Model | 0.35 | 0.03 | |
| Fructose | 0.5 | 0.04 | 0.29 | |
| Lactose | −1.5 | 0.05 | 0.20 | |
| Glucose | 0.01 | 0.01 | 0.56 | |
| Insulin | <0.01 | 0.09 | 0.10 | |
| BMC (g) | Base Model | 0.59 | 0.00 | |
| Fructose | 4.7 * | 0.09 | 0.03 | |
| Lactose | <0.01 | 0.01 | 0.57 | |
| Glucose | <0.01 | <0.01 | 0.76 | |
| Insulin | <0.01 | <0.01 | 0.71 | |
| BMD (g/cm2) | Base Model | 0.47 | 0.00 | |
| Fructose | <0.01 | 0.03 | 0.32 | |
| Lactose | <0.01 | 0.01 | 0.62 | |
| Glucose | <0.01 | 0.07 | 0.09 | |
| Insulin | <0.01 | 0.01 | 0.60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goran, M.I.; Martin, A.A.; Alderete, T.L.; Fujiwara, H.; Fields, D.A. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients 2017, 9, 146. https://doi.org/10.3390/nu9020146
Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients. 2017; 9(2):146. https://doi.org/10.3390/nu9020146
Chicago/Turabian StyleGoran, Michael I., Ashley A. Martin, Tanya L. Alderete, Hideji Fujiwara, and David A. Fields. 2017. "Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age" Nutrients 9, no. 2: 146. https://doi.org/10.3390/nu9020146
APA StyleGoran, M. I., Martin, A. A., Alderete, T. L., Fujiwara, H., & Fields, D. A. (2017). Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients, 9(2), 146. https://doi.org/10.3390/nu9020146





