Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta‐Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Quality Assessment
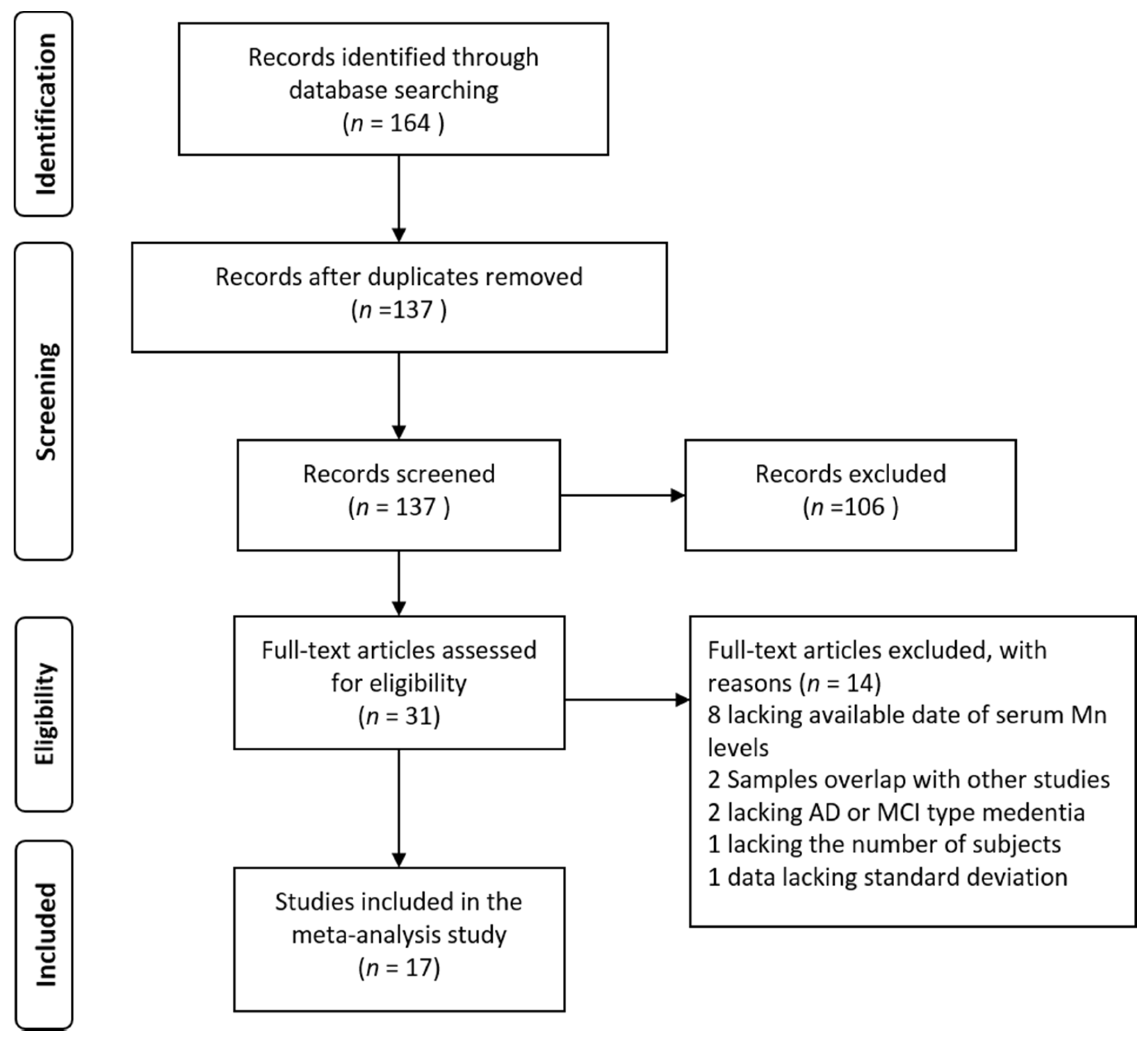
3. Results
3.1. Literature Search and Study Characteristics
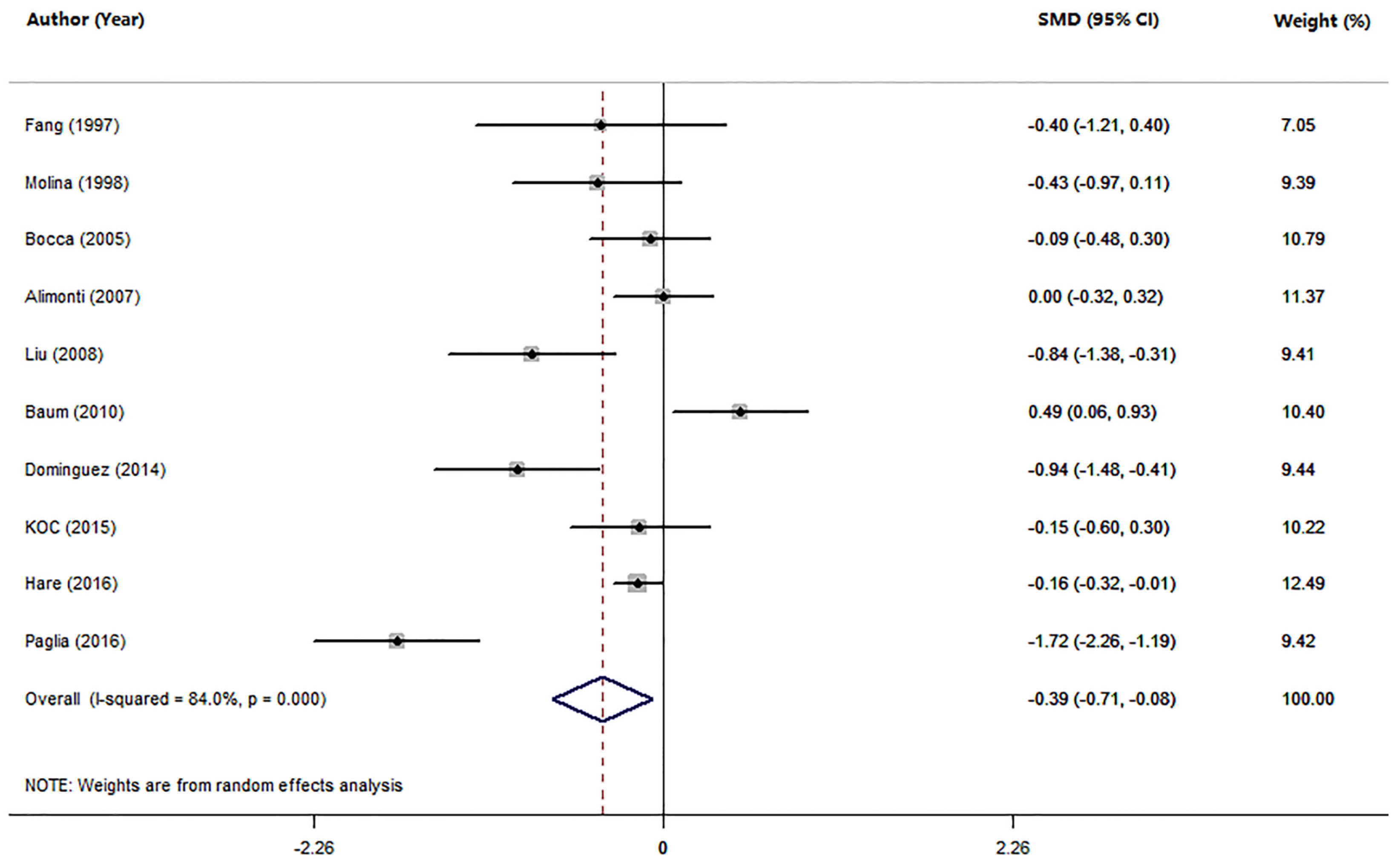
3.2. Studies on Mn Levels between Patients with AD and HC
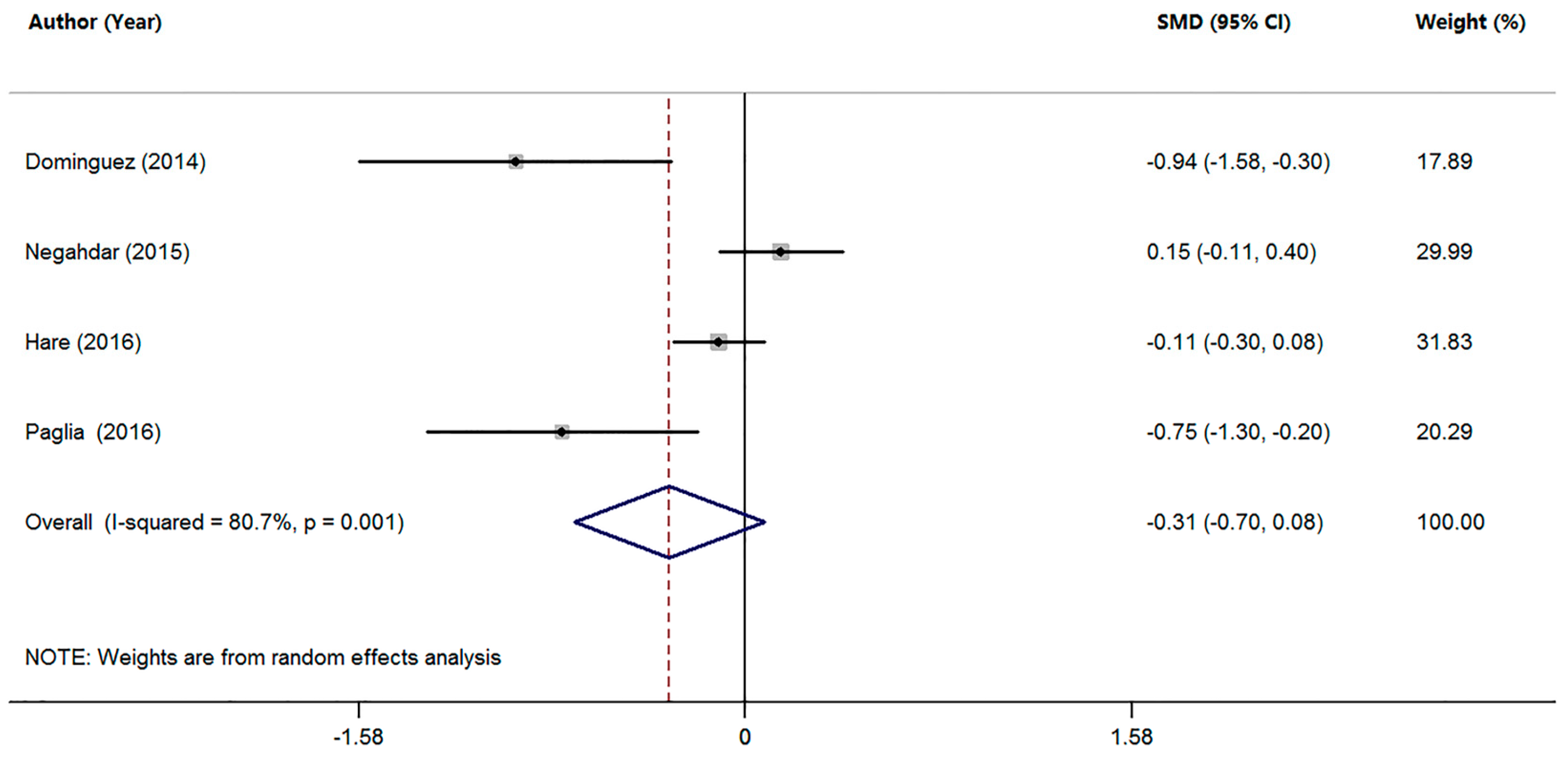
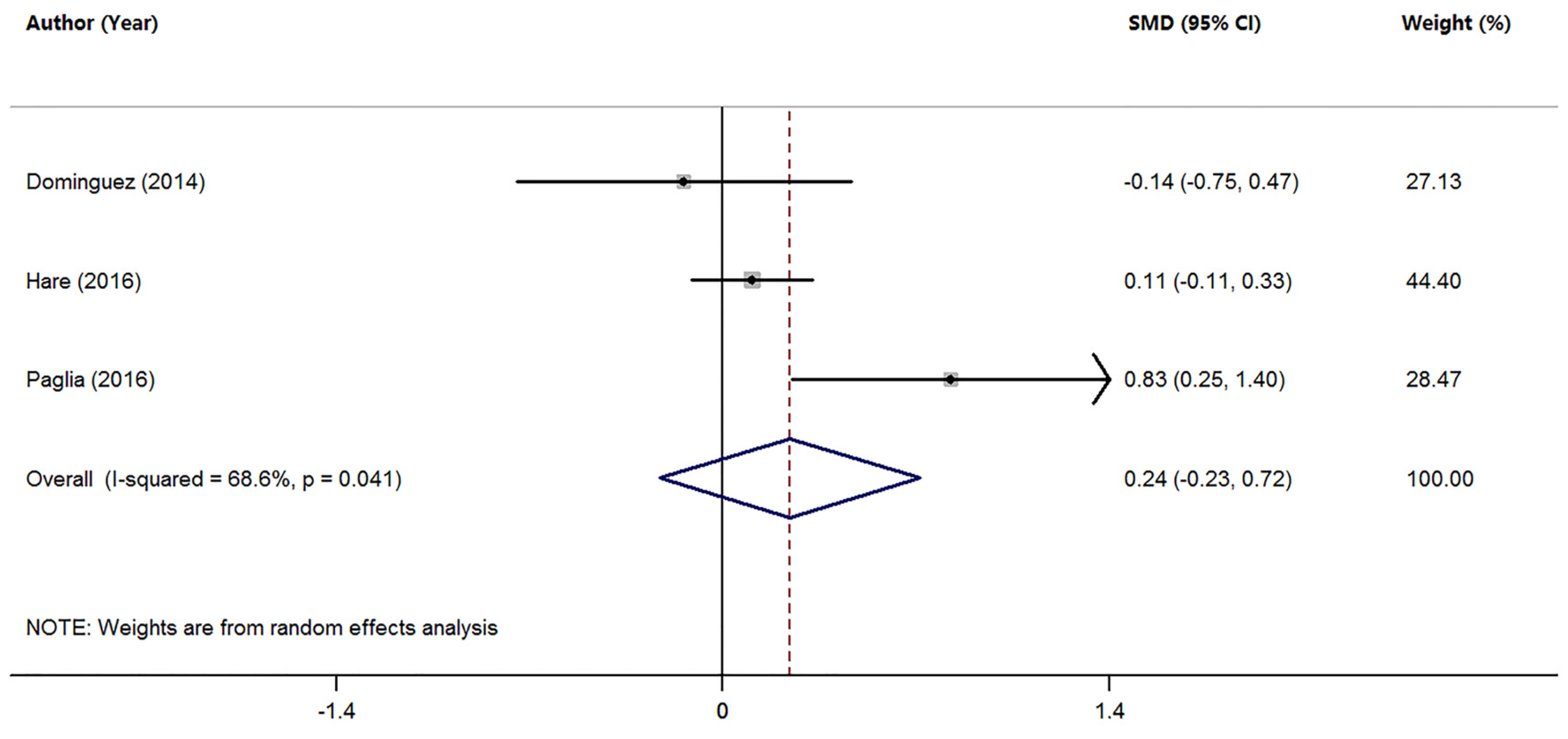
3.3. Studies on Mn Levels between Patients with MCI and HC
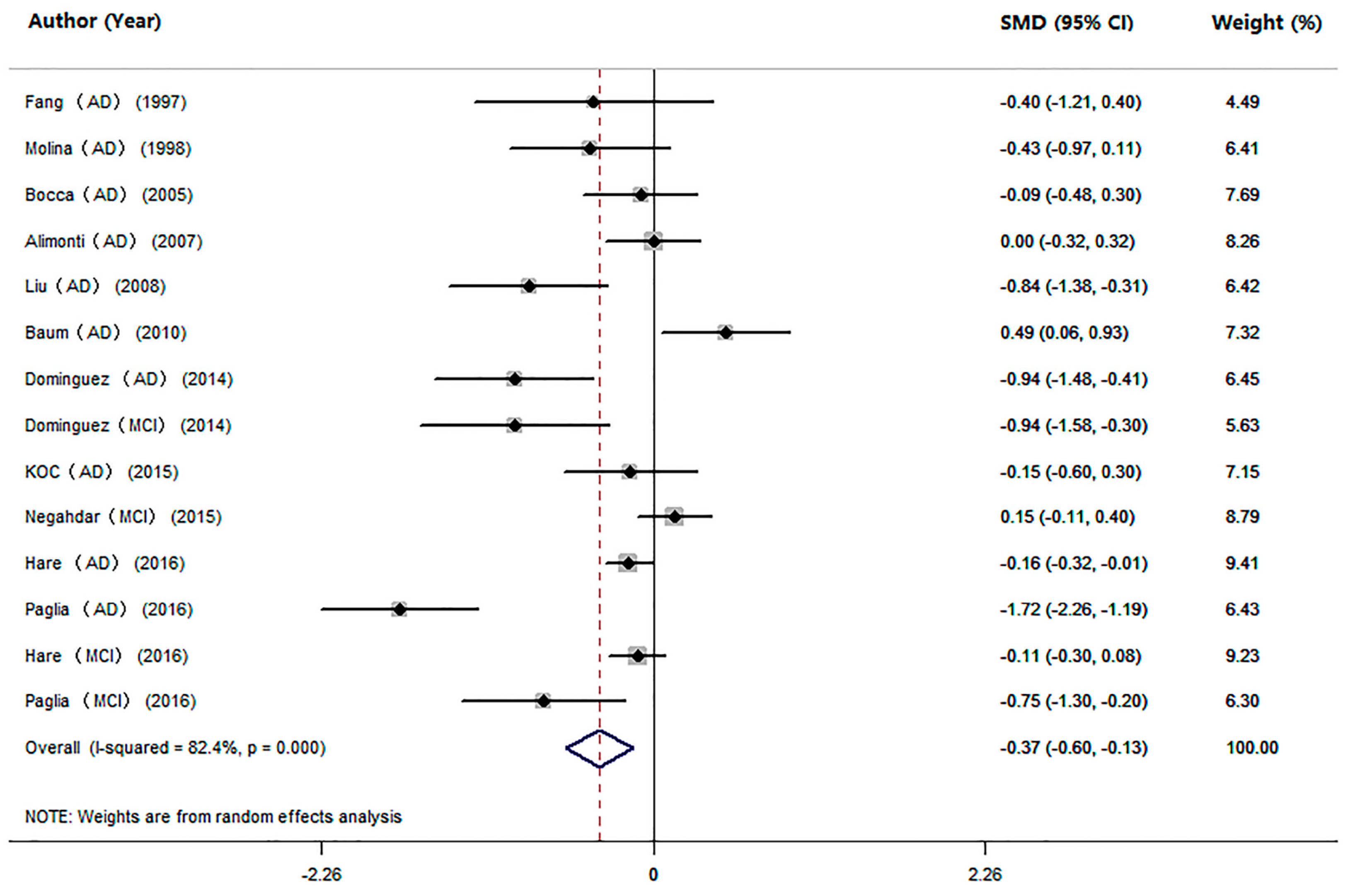
3.4. Studies on Mn Levels between Cognitive Impairment Individuals and HC
3.5. Studies on Mn Levels between Individuals with AD and MCI
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Silvestrelli, G.; Lanari, A.; Parnetti, L.; Tomassoni, D.; Amenta, F. Treatment of Alzheimer’s disease: From pharmacology to a better understanding of disease pathophysiology. Mech. Ageing Dev. 2006, 127, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nisha, C.M.; Silakari, C.; Sharma, I.; Anusha, K.; Gupta, N.; Nair, P.; Tripathi, T.; Kumar, A. Current and novel therapeutic molecules and targets in alzheimer’s disease. J. Formos. Med. Assoc. 2016, 115, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild cognitive impairment represents early-stage alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Janoutova, J.; Sery, O.; Hosak, L.; Janout, V. Is mild cognitive impairment a precursor of Alzheimer’s disease? Short review. Cent. Eur. J. Public Health 2015, 23, 365–367. [Google Scholar] [PubMed]
- Ma, Y.; Zhang, S.; Li, J.; Zheng, D.M.; Guo, Y.; Feng, J.; Ren, W.D. Predictive accuracy of amyloid imaging for progression from mild cognitive impairment to Alzheimer disease with different lengths of follow-up: A meta-analysis. Medicine 2014, 93, e150. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Kehoe, P.G.; Molloy, D.W. Renin angiotensin aldosterone system inhibition in controlling dementia-related cognitive decline. J. Alzheimer’s Dis. 2014, 42 (Suppl. S4), 575–586. [Google Scholar]
- Lista, S.; Dubois, B.; Hampel, H. Paths to Alzheimer’s disease prevention: From modifiable risk factors to biomarker enrichment strategies. J. Nutr. Health Aging 2015, 19, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Luo, Y.J.; Xiao, J.; Yu, N.W.; Yi, G. Impact of insulin sensitizers on the incidence of dementia: A meta-analysis. Dement. Geriatr. Cogn. Disord. 2016, 41, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dominguez, R.; Garcia-Barrera, T.; Gomez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Tan, L.; Wang, H.F.; Ma, J.; Liu, J.; Tan, M.; Sun, J.H.; Zhu, X.C.; Jiang, T.; Yu, J.T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimer’s Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, M.; Bucossi, S.; Panetta, V.; Squitti, R. Copper in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer’s Dis. 2012, 30, 981–984. [Google Scholar]
- Ventriglia, M.; Brewer, G.J.; Simonelli, I.; Mariani, S.; Siotto, M.; Bucossi, S.; Squitti, R. Zinc in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer’s Dis. 2015, 46, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chakraborty, S.; Mukhopadhyay, S.; Lee, E.; Paoliello, M.M.; Bowman, A.B.; Aschner, M. Manganese homeostasis in the nervous system. J. Neurochem. 2015, 134, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Sotogaku, N.; Oku, N. Influence of manganese on the release of neurotransmitters in rat striatum. Brain Res. 2003, 965, 279–282. [Google Scholar] [CrossRef]
- Takeda, A. Manganese action in brain function. Brain Res. Brain Res. Rev. 2003, 41, 79–87. [Google Scholar] [CrossRef]
- Benedetto, A.; Au, C.; Aschner, M. Manganese-induced dopaminergic neurodegeneration: Insights into mechanisms and genetics shared with Parkinson’s disease. Chem. Rev. 2009, 109, 4862–4884. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Jiang, L.; Fu, X.; Huang, S.; Huang, Y.N.; Li, X.R.; Chen, J.W.; Li, Y.; Luo, H.L.; Wang, F.; et al. Pallidal index as biomarker of manganese brain accumulation and associated with manganese levels in blood: A meta-analysis. PLoS ONE 2014, 9, e93900. [Google Scholar] [CrossRef] [PubMed]
- Grunecker, B.; Kaltwasser, S.F.; Zappe, A.C.; Bedenk, B.T.; Bicker, Y.; Spoormaker, V.I.; Wotjak, C.T.; Czisch, M. Regional specificity of manganese accumulation and clearance in the mouse brain: Implications for manganese-enhanced mri. NMR Biomed. 2013, 26, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology 2005, 64, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.P.; Olanow, C.W. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007, 66, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Takagi, H.; Hattori, H.; Matsuzaki, T. Localization of manganese superoxide dismutase in the cerebral cortex and hippocampus of alzheimer-type senile dementia. Osaka City Med. J. 1997, 43, 1–5. [Google Scholar] [PubMed]
- Omar, R.A.; Chyan, Y.J.; Andorn, A.C.; Poeggeler, B.; Robakis, N.K.; Pappolla, M.A. Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J. Alzheimer’s Dis. 1999, 1, 139–145. [Google Scholar]
- Aschner, M.; Aschner, J.L. Manganese transport across the blood-brain barrier: Relationship to iron homeostasis. Brain Res. Bull. 1990, 24, 857–860. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Rogers, J.T.; Wang, F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 42, 679–690. [Google Scholar]
- Zatta, P.; Drago, D.; Bolognin, S.; Sensi, S.L. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol. Sci. 2009, 30, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, B.; Zhao, G.; Jin, J.; Zhang, Y.; Hu, J.; Fang, S. Determination and discussion of seven trace elements in serum of the patients with senile dementia. Acta Nutr. Sin. 1997, 19, 88–90. [Google Scholar]
- Molina, J.A.; Jimenez-Jimenez, F.J.; Aguilar, M.V.; Meseguer, I.; Mateos-Vega, C.J.; Gonzalez-Munoz, M.J.; de Bustos, F.; Porta, J.; Orti-Pareja, M.; Zurdo, M.; et al. Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease. J. Neural Transm. 1998, 105, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Forte, G.; Petrucci, F.; Pino, A.; Marchione, F.; Bomboi, G.; Senofonte, O.; Giubilei, F.; Alimonti, A. Monitoring of chemical elements and oxidative damage in patients affected by Alzheimer’s disease. Ann. Ist. Super. Sanita 2005, 41, 197–203. [Google Scholar] [PubMed]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Sepe Monti, M.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, parkinson disease and multiple sclerosis. Neurotoxicology 2007, 28, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. The Study of the Association between Trace Element and Senile Dementia/Depressive Disorder. Master’s Thesis, Shandong University, Jinan, China, 2008. [Google Scholar]
- Baum, L.; Chan, I.H.; Cheung, S.K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 2010, 23, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Koc, E.R.; Ilhan, A.; Zubeyde, A.; Acar, B.; Gurler, M.; Altuntas, A.; Karapirli, M.; Bodur, A.S. A comparison of hair and serum trace elements in patients with Alzheimer disease and healthy participants. Turk. J. Med. Sci. 2015, 45, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive pattern of serum elements during the progression of Alzheimer’s disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.J.; Faux, N.G.; Roberts, B.R.; Volitakis, I.; Martins, R.N.; Bush, A.I. Lead and manganese levels in serum and erythrocytes in Alzheimer’s disease and mild cognitive impairment: Results from the australian imaging, biomarkers and lifestyle flagship study of ageing. Metallomics 2016, 8, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Negahdar, H.; Hosseini, S.R.; Parsian, H.; Kheirkhah, F.; Mosapour, A.; Khafri, S.; Haghighi, A.H. Homocysteine, trace elements and oxidant/antioxidant status in mild cognitively impaired elderly persons: A cross-sectional study. Rom. J. Intern. Med. 2015, 53, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of metal levels between postmortem brain and ventricular fluid in Alzheimer’s disease and nondemented elderly controls. Toxicol. Sci. 2016, 150, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Lundh, T.; Minthon, L.; Londos, E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Qian, Z.M.; He, X.; Gong, Q.; Wu, K.C.; Jiang, L.R.; Lu, L.N.; Zhu, Z.J.; Zhang, H.Y.; Yung, W.H.; et al. Reducing iron in the brain: A novel pharmacologic mechanism of huperzine a in the treatment of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.E.; White, J.K.; Howson, J.M.; Pask, R.; Smith, A.N.; Brayne, C.; Evans, J.G.; Xuereb, J.; Cairns, N.J.; Rubinsztein, D.C.; et al. Candidate gene association study of solute carrier family 11a members 1 (SLC11A1) and 2 (SLC11A2) genes in Alzheimer’s disease. Neurosci. Lett. 2005, 374, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Feher, A.; Juhasz, A.; Pakaski, M.; Kalman, J.; Janka, Z. Association between the 9 repeat allele of the dopamine transporter 40 bp variable tandem repeat polymorphism and Alzheimer’s disease. Psychiatry Res. 2014, 220, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Aksenov, M.; Aksenova, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: Creatine kinase bb, glutamine synthase, and ubiquitin carboxy-terminal hydrolase l-1. Free Radic. Biol. Med. 2002, 33, 562–571. [Google Scholar] [CrossRef]
- Kan, M.J.; Lee, J.E.; Wilson, J.G.; Everhart, A.L.; Brown, C.M.; Hoofnagle, A.N.; Jansen, M.; Vitek, M.P.; Gunn, M.D.; Colton, C.A. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J. Neurosci. 2015, 35, 5969–5982. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Patel, A.B. Pyruvate carboxylase and pentose phosphate fluxes are reduced in AbetaPP-PS1 mouse model of Alzheimer’s disease: A (1) (3)c nmr study. J. Alzheimer’s Dis. 2014, 41, 387–399. [Google Scholar]
- Li, F.; Calingasan, N.Y.; Yu, F.; Mauck, W.M.; Toidze, M.; Almeida, C.G.; Takahashi, R.H.; Carlson, G.A.; Flint Beal, M.; Lin, M.T.; et al. Increased plaque burden in brains of app mutant mnsod heterozygous knockout mice. J. Neurochem. 2004, 89, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.P.; Sankar, T.V.; Ganguly, S.; Mahanty, A.; Anandan, R.; Chakraborty, K.; Paul, B.N.; Sarma, D.; Dayal, J.S.; Mathew, S.; et al. Micronutrient composition of 35 food fishes from india and their significance in human nutrition. Biol. Trace Elem. Res. 2016, 174, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Platel, K. Effect of sulfur-containing spices on the bioaccessibility of trace minerals from selected cereals and pulses. J. Sci. Food Agric. 2016. [Google Scholar] [CrossRef] [PubMed]
| Studies on AD to HC | |||||||||||
| AD Patients | HC Subjects | ||||||||||
| Reference | Country | n | Gender | Age | Mn Concentration | Criteria for AD Diagnosis | n | Gender | Age | Mn Concentration | Method |
| (% Female) | Mean ± SD (Year) | Mean ± SD (µg/L) | (% Female) | Mean ± SD (Year) | Mean ± SD (µg/L) | ||||||
| Fang 1997 [32] | China | 24 | 33 | 61–87 | 42.85 + 17.03 | DSM-III | 8 | 38 | 58–72 | 50.00 + 19.78 | ICP-AES |
| Molina 1998 [33] | Spain | 26 | 46 | 73.1 ± 8.2 | 1.03 + 0.68 | DSM-IV, NINCDS-ADRDA criteria | 28 | 43 | 70.8 ± 7.3 | 1.31 + 0.63 | AAS |
| Bocca 2005 [34] | Italy | 60 | 67 | 74.6 ± 6.4 | 0.63 + 0.22 | NINCDS-ADRDA criteria | 44 | 25 | ≥45 | 0.65 + 0.24 | ICP-MS |
| Alimonti 2007 [35] | Italy | 53 | 68 | 74.5 ± 6.5 | 0.60 + 0.08 | NINCDS-ADRDA criteria | 124 | 35 | 44.8 ± 12.7 | 0.60 + 0.04 | ICP-MS |
| Liu 2008 [36] | China | 30 | 47 | 66.2 ± 9.9 | 15.00 ± 4.00 | DSM-IV, NINCDS-ADRDA criteria | 28 | 46 | 66.8 ± 8.3 | 18.00 ± 3.00 | ICP-AES |
| Baum 2010 [37] | Hong Kong | 44 | 66 | 74.3 ± 8.7 | 1.18 ± 1.15 | NINCDS-ADRDA criteria | 41 | 49 | 79.1 ± 6.0 | 0.73 ± 0.51 | ICP-MS |
| Dominguez 2014 [11] | Spain | 30 | 60 | 80.9 ± 4.5 | 0.62 ± 0.35 | NINCDS-ADRDA criteria | 30 | 57 | 74.0 ± 5.7 | 1.16 ± 0.73 | ICP-MS |
| KOC 2015 [38] | Turkey | 44 | 49 | 77.7 ± 9.3 | 9.00 ± 7.50 | DSM-IV, NINCDS-ADRDA criteria | 33 | 52 | 73.2 ± 10.6 | 10.00 ± 5.00 | ICP-MS |
| Paglia 2016 [39] | Italy | 34 | 74 | 72.4 ± 7.5 | 0.59 ± 0.32 | NINCDS-ADRDA criteria | 40 | 63 | 65.5 ± 6.4 | 1.24 ± 0.42 | ICP-MS |
| Hare 2016 [40] | Australia | 206 | 62 | 78.0 ± 8.6 | 0.82 ± 0.25 | - | 758 | 31 | 70.0 ± 7.0 | 0.92 ± 0.70 | ICP-MS |
| Studies on MCI to HC | |||||||||||
| MCI Individuals | HC Subjects | ||||||||||
| Reference | Country | n | Gender | Age | Mn Concentration | Criteria for AD Diagnosis | n | Gender | Age | Mn Concentration | Method |
| (% Female) | Mean ± SD (Year) | Mean ± SD (µg/L) | (% Female) | Mean ± SD (Year) | Mean ± SD (µg/L) | ||||||
| Dominguez 2014 [11] | Spain | 16 | 38 | 75.9 ± 5.7 | 0.57 ± 0.33 | - | 30 | 57 | 74.0 ± 5.7 | 1.16 ± 0.73 | ICP-MS |
| Negahdar 2015 [41] | Iran | 120 | 50 | 74.3 ± 7.8 | 14.30 + 5.18 | - | 120 | 50 | 67.7 ± 6.9 | 13.50 + 5.30 | AAS |
| Paglia 2016 [39] | Italy | 20 | 80 | 68.3 ± 7.8 | 0.91 ± 0.48 | - | 40 | 63 | 65.5 ± 6.4 | 1.24 ± 0.42 | ICP-MS |
| Hare 2016 [40] | Australia | 129 | 57 | 75.7 ± 7.6 | 0.85 ± 0.37 | - | 758 | 31 | 70.0 ± 7.0 | 0.92 ± 0.70 | ICP-MS |
| Studies on MCI to AD | |||||||||||
| AD patients | MCI Individuals | ||||||||||
| Reference | Country | n | Gender | Age | Mn Concentration | Criteria for AD Diagnosis | n | Gender | Age | Mn Concentration | Method |
| (% Female) | Mean ± SD (Year) | Mean ± SD (µg/L) | (% Female) | Mean ± SD (Year) | Mean ± SD (µg/L) | ||||||
| Dominguez 2014 [11] | Spain | 30 | 60 | 80.9 ± 4.5 | 0.62 ± 0.35 | NINCDS-ADRDA criteria | 16 | 38 | 75.9 ± 5.7 | 0.57 ± 0.33 | ICP-MS |
| Paglia 2016 [39] | Italy | 34 | 74 | 72.4 ± 7.5 | 0.59 ± 0.32 | NINCDS-ADRDA criteria | 20 | 80 | 68.3 ± 7.8 | 0.91 ± 0.48 | ICP-MS |
| Hare 2016 [40] | Australia | 206 | 62 | 78.0 ± 8.6 | 0.82 ± 0.25 | - | 129 | 57 | 75.7 ± 7.6 | 0.85 ± 0.37 | ICP-MS |
| Subgroups | n of Studies | SMD (95% CI) | I2 | p-Value |
|---|---|---|---|---|
| All studies | 10 | −0.39 (−0.71, −0.08) | 84.0% | 0.000 |
| Methods | ||||
| ICP-MS | 7 | −0.33 (−0.73, 0.06) | 88.1% | 0.000 |
| ICP-AES | 2 | −0.71 (−1.16, −0.26) | 0.0% | 0.373 |
| AAS | 1 | −0.43 (−0.97, 0.11) | - | - |
| Geographic locations | ||||
| Europe | 6 | −0.53 (−1.03, −0.04) | 86.4% | 0.000 |
| Asia | 3 | −0.23 (−1.14, 0.68) | 86.8% | 0.001 |
| Australia | 1 | −0.16 (−0.32, −0.01) | - | - |
| Subgroups | n of Studies | SMD (95% CI) | I2 | p-Value |
|---|---|---|---|---|
| All studies | 14 | −0.37 (−0.60, −0.13) | 82.4% | 0.000 |
| Methods | ||||
| ICP-MS | 10 | −0.38 (−0.66, −0.10) | 85.0% | 0.000 |
| ICP-AES | 2 | −0.71 (−1.16, −0.26) | 0.0% | 0.373 |
| AAS | 2 | −0.09 (−0.64, 0.46) | 71.8% | 0.060 |
| Geographic locations | ||||
| Europe | 8 | −0.60 (−1.01, −0.20) | 82.8% | 0.000 |
| Asia | 4 | −0.11 (−0.65, 0.43) | 81.7% | 0.001 |
| Australia | 2 | −0.14 (−0.26, −0.02) | 0.0% | 0.651 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, K.; Liu, M.; Pan, Y.; Zhong, X.; Wei, M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta‐Analysis. Nutrients 2017, 9, 231. https://doi.org/10.3390/nu9030231
Du K, Liu M, Pan Y, Zhong X, Wei M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta‐Analysis. Nutrients. 2017; 9(3):231. https://doi.org/10.3390/nu9030231
Chicago/Turabian StyleDu, Ke, Mingyan Liu, Yanzhu Pan, Xin Zhong, and Minjie Wei. 2017. "Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta‐Analysis" Nutrients 9, no. 3: 231. https://doi.org/10.3390/nu9030231





