Genetic Variations Associated with Vitamin A Status and Vitamin A Bioavailability
Abstract
:1. Introduction
- -
- 1 µg RAE = 1 μg RET
- -
- 1 µg RAE = 2 µg all-trans β-carotene (βC) from supplements
- -
- 1 µg RAE = 12 µg of all-trans βC from food
- -
- 1 µg RAE = 24 µg α-carotene or β-cryptoxanthin from food
- -
- 1 µg RAE = 3.33 IU RET
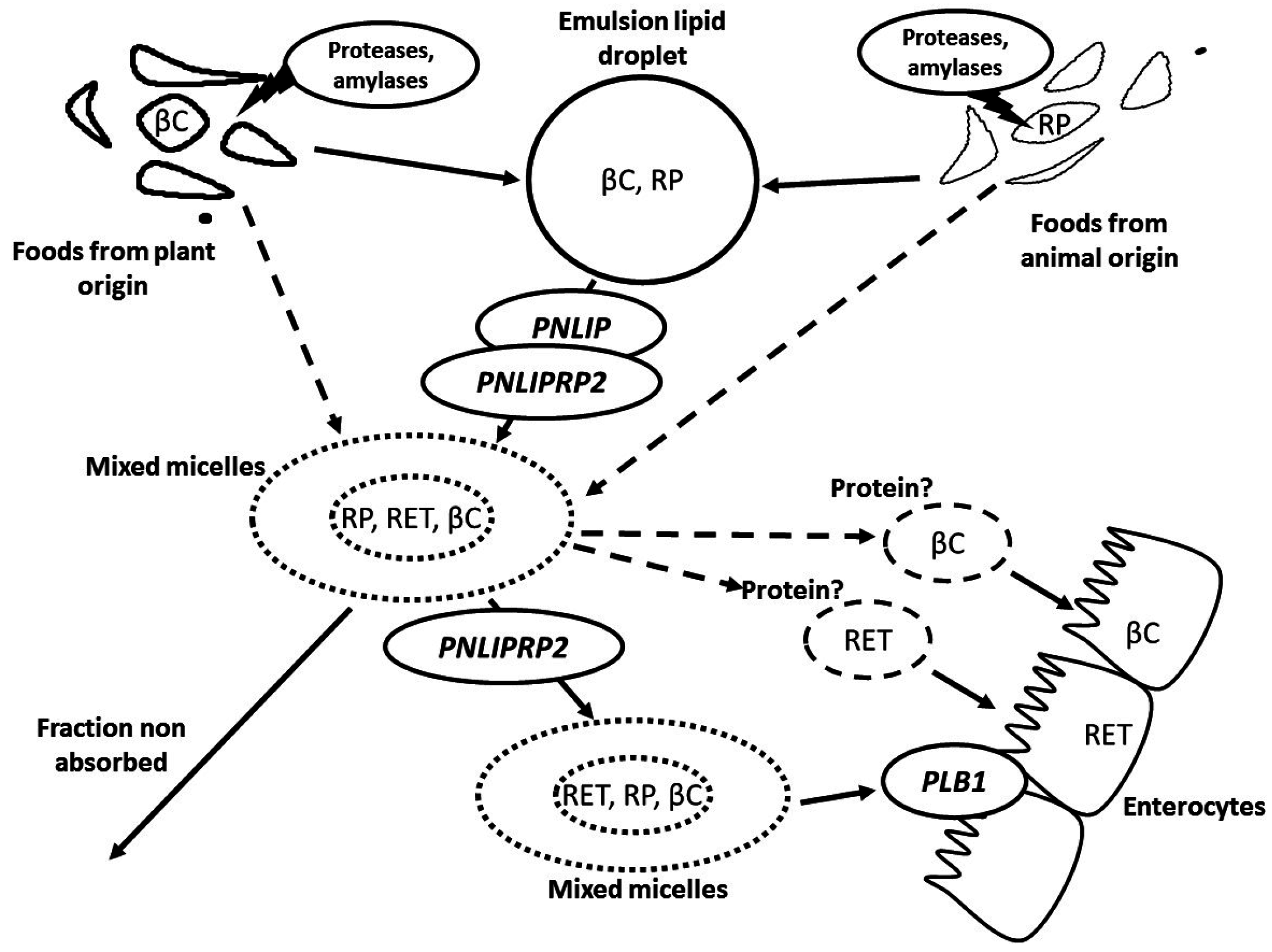
2. Metabolism of Vitamin A in the Gastrointestinal Lumen
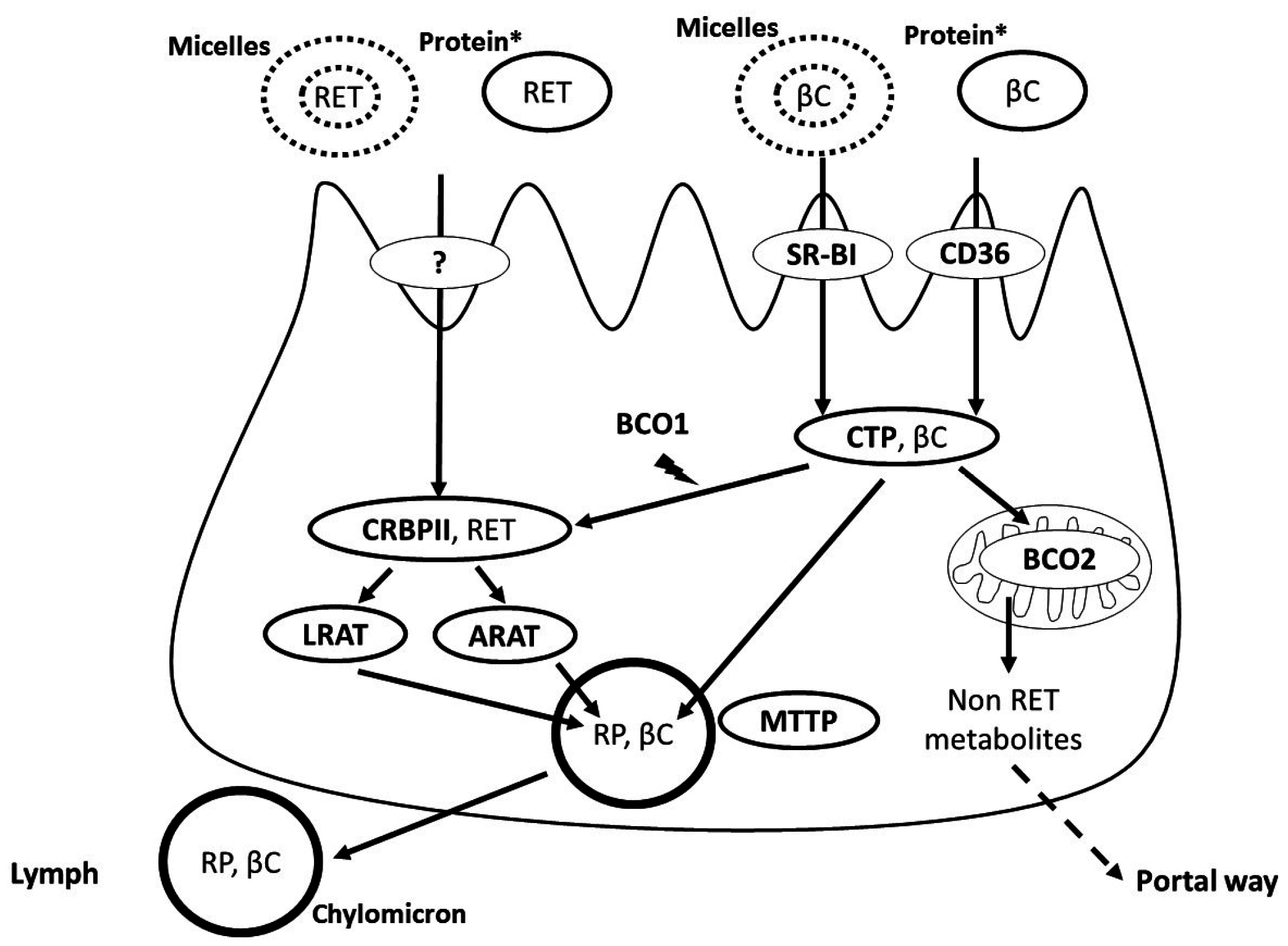
3. Apical Uptake, Intracellular Metabolism and Basolateral Secretion of Vitamin A by the Intestinal Cell
4. Regulation of Vitamin A Absorption
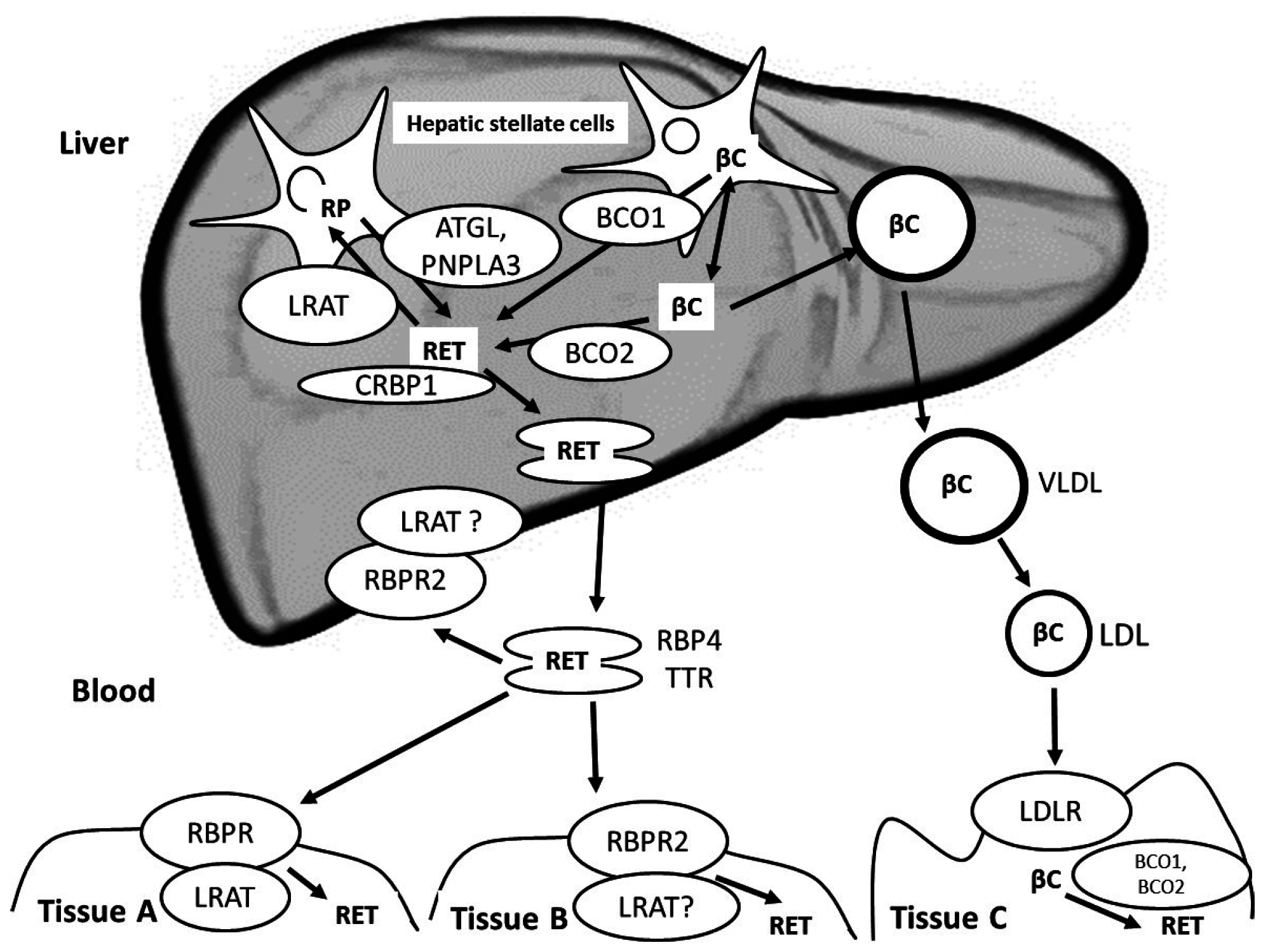
5. Postprandial Blood Transport of Newly-Absorbed Vitamin A from the Intestine to the Liver
6. Liver Metabolism of Vitamin A and Blood Transport of Vitamin A from the Liver to Extra-Hepatic Tissues
7. Vitamin A Metabolism in Extra-Hepatic Tissues
8. Physiological Regulation of Blood Vitamin A Concentrations
9. Genetic Variations that Have Been Suggested to Modulate Blood Vitamin A Concentrations
9.1. Genetic Variations Associated with Fasting Blood Vitamin A Concentrations
9.2. Genetic Variations Associated with Postprandial Blood Vitamin A Concentrations
10. Other Genetic Variations that Could Be Involved in the Blood Concentration of Vitamin A
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rucker, R.B.; Suttie, J.W.; McCormick, D.B.; Machlin, L.J. Handbook of Vitamins, 3rd ed.; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 2001. [Google Scholar]
- Dowling, J.E. George wald (1906-97)—Biologist who discovered the role of vitamin A in vision—Obituary. Nature 1997, 387, 356. [Google Scholar] [CrossRef] [PubMed]
- Viewpoint, A.P.; Maumenee, A.E. The history of vitamin A and its ophthalmic implications. Arch. Aphthalmol. 1993, 111, 547–550. [Google Scholar]
- Rando, R.R. The chemistry of vitamin A and vision. Angew. Chem. Int. Ed. Engl. 1990, 29, 461–480. [Google Scholar] [CrossRef]
- Wright, C.B.; Redmond, T.M.; Nickerson, J.M. A history of the classical visual cycle. Prog. Mol. Biol. Transl. Sci. 2015, 134, 433–448. [Google Scholar] [PubMed]
- Ross, A.C.; Gardner, E.M. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv. Exp. Med. Biol. 1994, 352, 187–200. [Google Scholar] [PubMed]
- Love, J.M.; Gudas, L.J. Vitamin A, differentiation and cancer. Curr. Opin. Cell Biol. 1994, 6, 825–831. [Google Scholar] [CrossRef]
- Zile, M.H. Function of vitamin A in vertebrate embryonic development. J. Nutr. 2001, 131, 705–708. [Google Scholar] [PubMed]
- Clagett-Dame, M.; de Luca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.S. Vitamin A and retinoids in health and disease. N. Engl. J. Med. 1984, 310, 1023–1031. [Google Scholar] [PubMed]
- Mason, J.; Greiner, T.; Shrimpton, R.; Sanders, D.; Yukich, J. Vitamin A policies need rethinking. Int. J. Epidemiol. 2015, 44, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Grune, T. The contribution of beta-carotene to Vitamin A supply of humans. Mol. Nutr. Food Res. 2012, 56, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Borel, P. Factors affecting intestinal absorption of highly lipophilic food microconstituents (fat-soluble vitamins, carotenoids and phytosterols). Clin. Chem. Lab. Med. 2003, 41, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Berton, A.; Moussa, M.; Kreuzer, C.; Crenon, I.; Borel, P. Pancreatic lipase and pancreatic lipase-related protein 2, but not pancreatic lipase-related protein 1, hydrolyze retinyl palmitate in physiological conditions. Biochim. Biophys. Acta 2006, 1761, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Tyssandier, V.; Reboul, E.; Dumas, J.F.; Bouteloup-Demange, C.; Armand, M.; Marcand, J.; Sallas, M.; Borel, P. Processing of vegetable-born carotenoids in the human stomach and duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G913–G923. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Pasquier, B.; Armand, M.; Tyssandier, V.; Grolier, P.; Alexandre-Gouabau, M.C.; Andre, M.; Senft, M.; Peyrot, J.; Jaussan, V.; et al. Processing of Vitamin A and E in the human gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G95–G103. [Google Scholar] [PubMed]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, in press. [Google Scholar]
- Mensi, A.; Borel, P.; Goncalves, A.; Nowicki, M.; Gleize, B.; Roi, S.; Chobert, J.M.; Haertle, T.; Reboul, E. Beta-lactoglobulin as a vector for beta-carotene food fortification. J. Agric. Food Chem. 2014, 62, 5916–5924. [Google Scholar] [CrossRef] [PubMed]
- Tyssandier, V.; Lyan, B.; Borel, P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim. Biophys. Acta 2001, 1533, 285–292. [Google Scholar] [CrossRef]
- Rigtrup, K.M.; Kakkad, B.; Ong, D.E. Purification and partial characterization of a retinyl ester hydrolase from the brush border of rat small intestine mucosa: Probable identity with brush border phospholipase b. Biochemistry (Mosc.) 1994, 33, 2661–2666. [Google Scholar] [CrossRef]
- Fernandez, E.; Borgstrom, B. Intestinal absorption of retinol and retinyl palmitate in the rat. Effects of tetrahydrolipstatin. Lipids 1990, 25, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.; Muralidhara, K.S. Vitamin a1 intestinal absorption in vivo: Influence of luminal factors on transport. Am. J. Physiol. Gastrointest. Liver Physiol. 1977, 232, E471–E477. [Google Scholar]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Soayfane, Z.; Goncalves, A.; Cantiello, M.; Bott, R.; Nauze, M.; Terce, F.; Collet, X.; Comera, C. Respective contributions of intestinal niemann-pick c1-like 1 and scavenger receptor class b type i to cholesterol and tocopherol uptake: In vivo v. In vitro studies. Br. J. Nutr. 2012, 107, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger receptor class b type i (sr-bi) is involved in vitamin e transport across the enterocyte. J. Biol. Chem. 2006, 281, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Margier, M.; Roi, S.; Collet, X.; Niot, I.; Goupy, P.; Caris-Veyrat, C.; Reboul, E. Intestinal scavenger receptors are involved in vitamin k1 absorption. J. Biol. Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. Cd36 and sr-bi are involved in cellular uptake of provitamin A carotenoids by caco-2 and hek cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters sr-bi, npc1l1, and abca1 is downregulated in caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [PubMed]
- Van Bennekum, A.; Werder, M.; Thuahnai, S.T.; Han, C.H.; Duong, P.; Williams, D.L.; Wettstein, P.; Schulthess, G.; Phillips, M.C.; Hauser, H. Class b scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry (Mosc.) 2005, 44, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a srbi-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.; Wang, H.P.; Chu, C.Y.T.; Badawi, M.A. Preleminary characterization of a small intestinal binding component for retinol and fatty acids in the rat. Life Sci. 1978, 23, 1011–1018. [Google Scholar] [CrossRef]
- Ong, D.E.; Page, D.L. Cellular retinol-binding protein (type two) is abundant in human small intestine. J. Lipid Res. 1987, 28, 739–745. [Google Scholar] [PubMed]
- Wongsiriroj, N.; Piantedosi, R.; Palczewski, K.; Goldberg, I.J.; Johnston, T.P.; Li, E.; Blaner, W.S. The molecular basis of retinoid absorption: A genetic dissection. J. Biol. Chem. 2008, 283, 13510–13519. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Wongsiriroj, N.; Libien, J.; Vogel, S.; Goldberg, I.J.; Baehr, W.; Palczewski, K.; Blaner, W.S. Retinoid absorption and storage is impaired in mice lacking lecithin:Retinol acyltransferase (lrat). J. Biol. Chem. 2005, 280, 35647–35657. [Google Scholar] [CrossRef] [PubMed]
- Ables, G.P.; Yang, K.J.; Vogel, S.; Hernandez-Ono, A.; Yu, S.; Yuen, J.J.; Birtles, S.; Buckett, L.K.; Turnbull, A.V.; Goldberg, I.J.; et al. Intestinal dgat1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J. Lipid Res. 2012, 53, 2364–2379. [Google Scholar] [CrossRef] [PubMed]
- Sauvant, P.; Mekki, N.; Charbonnier, M.; Portugal, H.; Lairon, D.; Borel, P. Amounts and types of fatty acids in meals affect the pattern of retinoids secreted in human chylomicrons after a high-dose preformed vitamin A intake. Metabolism 2003, 52, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Duszka, C.; Grolier, P.; Azim, E.M.; Alexandre-Gouabau, M.C.; Borel, P.; Azais-Braesco, V. Rat intestinal beta-carotene dioxygenase activity is located primarily in the cytosol of mature jejunal enterocytes. J. Nutr. 1996, 126, 2550–2556. [Google Scholar] [PubMed]
- Grolier, P.; Duszka, C.; Borel, P.; Alexandre-Gouabau, M.C.; Azais-Braesco, V. In vitro and in vivo inhibition of beta-carotene dioxygenase activity by canthaxanthin in rat intestine. Arch. Biochem. Biophys. 1997, 348, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Dela Sena, C.; Riedl, K.M.; Narayanasamy, S.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J. Biol. Chem. 2014, 289, 13661–13666. [Google Scholar] [CrossRef] [PubMed]
- Dela Sena, C.; Narayanasamy, S.; Riedl, K.M.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant human beta-carotene 15,15′-oxygenase (bco1). J. Biol. Chem. 2013, 288, 37094–37103. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Widjaja-Adhi, M.A.; Rodriguez-Santiago, S.; Hessel, S.; Golczak, M.; Palczewski, K.; von Lintig, J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 2013, 288, 34081–34096. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, S.; Reed, V.; Blaner, W.S.; Harrison, E.H. Cellular localization of beta-carotene 15,15′ oxygenase-1 (bco1) and beta-carotene 9′,10′ oxygenase-2 (bco2) in rat liver and intestine. Arch. Biochem. Biophys. 2015, 572, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gajda, A.M.; Storch, J. Enterocyte fatty acid-binding proteins (fabps): Different functions of liver and intestinal fabps in the intestine. Prostaglandins Leukot. Essent. Fatty Acids 2015, 93, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Qin, J.; Dolnikowski, G.G.; Russell, R.M. Short-term (intestinal) and long-term (postintestinal) conversion of beta-carotene to retinol in adults as assessed by a stable-isotope reference method. Am. J. Clin. Nutr. 2003, 78, 259–266. [Google Scholar] [PubMed]
- Borel, P.; Grolier, P.; Mekki, N.; Boirie, Y.; Rochette, Y.; le Roy, B.; Alexandre-Gouabau, M.C.; Lairon, D.; Azais-Braesco, V. Low and high responders to pharmacological doses of beta-carotene: Proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J. Lipid Res. 1998, 39, 2250–2260. [Google Scholar] [PubMed]
- Nayak, N.; Harrison, E.H.; Hussain, M.M. Retinyl ester secretion by intestinal cells: A specific and regulated process dependent on assembly and secretion of chylomicrons. J. Lipid Res. 2001, 42, 272–280. [Google Scholar] [PubMed]
- Lobo, G.P.; Amengual, J.; Baus, D.; Shivdasani, R.A.; Taylor, D.; von Lintig, J. Genetics and diet regulate vitamin A production via the homeobox transcription factor isx. J. Biol. Chem. 2013, 288, 9017–9027. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; von Lintig, J. Isx is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, S.; Gannon, B.M.; Davis, C.R.; Chileshe, J.; Kaliwile, C.; Masi, C.; Rios-Avila, L.; Gregory, J.F., 3rd; Tanumihardjo, S.A. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in zambian preschool children are consistent with the presence of high liver vitamin A stores. Am. J. Clin. Nutr. 2015, 102, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R.; Helgerud, P.; Dueland, S.; Berg, T.; Pedersen, J.I.; Norum, K.R.; Drevon, C.A. Lymphatic absorption and transport of retinol and vitamin d-3 from rat intestine. Evidence for different pathways. Biochim. Biophys. Acta 1984, 772, 109–116. [Google Scholar] [CrossRef]
- Tyssandier, V.; Choubert, G.; Grolier, P.; Borel, P. Carotenoids, mostly the xanthophylls, exchange between plasma lipoproteins. Int. J. Vitam. Nutr. Res. 2002, 72, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Dallinga-Thie, G.M.; Franssen, R.; Mooij, H.L.; Visser, M.E.; Hassing, H.C.; Peelman, F.; Kastelein, J.J.; Peterfy, M.; Nieuwdorp, M. The metabolism of triglyceride-rich lipoproteins revisited: New players, new insight. Atherosclerosis 2010, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R.; Helgerud, P.; Rasmussen, M.; Berg, T.; Norum, K.R. In vivo uptake of chylomicron [3h]retinyl ester by rat liver: Evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc. Natl. Acad. Sci. USA 1982, 79, 7326–7330. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.E. Purufication and partial characterization of cellular retinol-binding protein from human liver. Cancer Res. 1982, 42, 1033–1037. [Google Scholar] [PubMed]
- Ong, D.E.; MacDonald, P.N.; Gubitosi, A.M. Esterification of retinol in rat liver. J. Biol. Chem. 1988, 263, 5789–5796. [Google Scholar] [PubMed]
- Rose, A.C. Retinol esterification by rat liver microsomes. J. Biol. Chem. 1982, 257, 2453–2459. [Google Scholar]
- Nagatsuma, K.; Hayashi, Y.; Hano, H.; Sagara, H.; Murakami, K.; Saito, M.; Masaki, T.; Lu, T.; Tanaka, M.; Enzan, H.; et al. Lecithin: Retinol acyltransferase protein is distributed in both hepatic stellate cells and endothelial cells of normal rodent and human liver. Liver Int. 2009, 29, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wake, K. Development of vitamin A-rich lipid droplets in multivesicular bodies or rat liver stellate cells. J. Cell Biol. 1974, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wake, K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int. Rev. Cytol. 1980, 66, 303–353. [Google Scholar] [PubMed]
- Taschler, U.; Schreiber, R.; Chitraju, C.; Grabner, G.F.; Romauch, M.; Wolinski, H.; Haemmerle, G.; Breinbauer, R.; Zechner, R.; Lass, A.; et al. Adipose triglyceride lipase is involved in the mobilization of triglyceride and retinoid stores of hepatic stellate cells. Biochim. Biophys. Acta 2015, 1851, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Pirazzi, C.; Valenti, L.; Motta, B.M.; Pingitore, P.; Hedfalk, K.; Mancina, R.M.; Burza, M.A.; Indiveri, C.; Ferro, Y.; Montalcini, T.; et al. Pnpla3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum. Mol. Genet. 2014, 23, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Pirazzi, C.; Mancina, R.M.; Motta, B.M.; Indiveri, C.; Pujia, A.; Montalcini, T.; Hedfalk, K.; Romeo, S. Recombinant pnpla3 protein shows triglyceride hydrolase activity and its i148m mutation results in loss of function. Biochim. Biophys. Acta 2014, 1841, 574–580. [Google Scholar] [CrossRef] [PubMed]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Cohen, J.C.; Hobbs, H.H. A sequence variation (i148m) in pnpla3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef] [PubMed]
- Shmarakov, I.; Fleshman, M.K.; D’Ambrosio, D.N.; Piantedosi, R.; Riedl, K.M.; Schwartz, S.J.; Curley, R.W., Jr.; von Lintig, J.; Rubin, L.P.; Harrison, E.H.; et al. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch. Biochem. Biophys. 2010, 504, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, M.R.; Asher, K.A.; Attlesey, M.G.; Satchithanandam, S.; Mychkovsky, I.; Coutlakis, P.J. Absorption, storage, and distribution of beta-carotene in normal and beta-carotene-fed rats: Roles of parenchymal and stellate cells. J. Lipid Res. 1989, 30, 1545–1550. [Google Scholar] [PubMed]
- Zachman, R.D.; Singer, M.B.; Olson, J.A. Biliary secretion of metabolites of retinol and of retinoic acid in the guinea pig and chick. J. Nutr. 1966, 88, 137–142. [Google Scholar] [PubMed]
- Zachman, R.D.; Olson, J.A. Formation and enterohepatic circulation of water-soluble metabolites of retinol(vitamin A)in the rat. Nature 1964, 201, 1222–1223. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.A. Characteristics of a vitamin A transporting protein complex occurring in human serum. J. Biol. Chem. 1971, 246, 34–43. [Google Scholar] [PubMed]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Alapatt, P.; Guo, F.; Komanetsky, S.M.; Wang, S.; Cai, J.; Sargsyan, A.; Rodriguez Diaz, E.; Bacon, B.T.; Aryal, P.; Graham, T.E. Liver retinol transporter and receptor for serum retinol-binding protein (rbp4). J. Biol. Chem. 2013, 288, 1250–1265. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Golczak, M.; Palczewski, K.; von Lintig, J. Lecithin:Retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 2012, 287, 24216–24227. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Harrison, E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. The molecular and genetic dissection of the retinoid signalling pathway. Gene 1993, 135, 223–228. [Google Scholar] [CrossRef]
- Iskakova, M.; Karbyshev, M.; Piskunov, A.; Rochette-Egly, C. Nuclear and extranuclear effects of vitamin A. Can. J. Physiol. Pharmacol. 2015, 93, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Strom, K.; Gundersen, T.E.; Hansson, O.; Lucas, S.; Fernandez, C.; Blomhoff, R.; Holm, C. Hormone-sensitive lipase (hsl) is also a retinyl ester hydrolase: Evidence from mice lacking hsl. FASEB J. 2009, 23, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of nutrition for development (bond)-vitamin A review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef] [PubMed]
- Herbeth, B.; Zittoun, J.; Miravet, L.; Bourgeay-Causse, M.; Carreguery, G.; Delacoux, E.; le Devehat, C.; Lemoine, A.; Mareschi, J.P.; Martin, J.; et al. Reference intervals for vitamins B1, B2, E, D, retinol, beta-carotene, and folate in blood: Usefulness of dietary selection criteria. Clin. Chem. 1986, 32, 1756–1759. [Google Scholar] [PubMed]
- Muto, Y.; Smith, J.E.; Milch, P.O.; Goodman, D.S. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J. Biol. Chem. 1972, 247, 2542–2550. [Google Scholar] [PubMed]
- Loerch, J.D.; Underwood, B.A.; Lewis, K.C. Response of plasma levels of vitamin A to a dose of vitamin A as an indicator of hepatic vitamin A reserves in rats. J. Nutr. 1979, 109, 778–786. [Google Scholar] [PubMed]
- Olson, J.A. The reproducibility, sensitivity and specificity of the relative dose response (rdr) test for determining vitamin A status. J. Nutr. 1991, 121, 917–920. [Google Scholar] [PubMed]
- Wahed, M.A.; Alvarez, J.O.; Khaled, M.A.; Mahalanabis, D.; Rahman, M.M.; Habte, D. Comparison of the modified relative dose response (mrdr) and the relative dose response (rdr) in the assessment of vitamin A status in malnourished children. Am. J. Clin. Nutr. 1995, 61, 1253–1256. [Google Scholar] [PubMed]
- Tanumihardjo, S.A.; Olson, J.A. The reproducibility of the modified relative dose response (mrdr) assay in healthy individuals over time and its comparison with conjunctival impression cytology (cic). Eur. J. Clin. Nutr. 1991, 45, 407–411. [Google Scholar] [PubMed]
- Borel, P.; Grolier, P.; Armand, M.; Partier, A.; Lafont, H.; Lairon, D.; Azais-Braesco, V. Carotenoids in biological emulsions: Solubility, surface-to-core distribution, and release from lipid droplets. J. Lipid Res. 1996, 37, 250–261. [Google Scholar] [PubMed]
- Clevidence, B.A.; Bieri, J.G. Association of carotenoids with human plasma lipoproteins. Methods Enzymol. 1993, 214, 33–46. [Google Scholar] [PubMed]
- Cornwell, D.G.; Kruger, F.A.; Robinson, H.B. Studies on the absorption of beta-carotene and the distribution of total carotenoid in human serum lipoproteins after oral administration. J. Lipid Res. 1962, 3, 65–70. [Google Scholar]
- Berr, F.; Kern, F. Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J. Lipid Res. 1984, 25, 805–812. [Google Scholar] [PubMed]
- Perez-Martinez, P.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Update on genetics of postprandial lipemia. Atheroscler. Suppl. 2010, 11, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Martin, J.C.; Planells, R.; Gastaldi, M.; Nowicki, M.; Goncalves, A.; Valero, R.; Lairon, D.; Borel, P. The postprandial chylomicron triacylglycerol response to dietary fat in healthy male adults is significantly explained by a combination of single nucleotide polymorphisms in genes involved in triacylglycerol metabolism. J. Clin. Endocrinol. Metab. 2014, 99, E484–E488. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations. Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Swizerland, 2011. [Google Scholar]
- Furr, H.C.; Green, M.H.; Haskell, M.; Mokhtar, N.; Nestel, P.; Newton, S.; Ribaya-Mercado, J.D.; Tang, G.; Tanumihardjo, S.; Wasantwisut, E. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr. 2005, 8, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, M.; Konigsrainer, I.; Konigsrainer, A.; Machicao, F.; Haring, H.U.; Schleicher, E.; Peter, A. The genetic variant i148m in pnpla3 is associated with increased hepatic retinyl-palmitate storage in humans. J. Clin. Endocrinol. Metab. 2015, 100, E1568–E1574. [Google Scholar] [CrossRef] [PubMed]
- Genomes Project, C.; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [Green Version]
- Pirazzi, C.; Adiels, M.; Burza, M.A.; Mancina, R.M.; Levin, M.; Stahlman, M.; Taskinen, M.R.; Orho-Melander, M.; Perman, J.; Pujia, A.; et al. Patatin-like phospholipase domain-containing 3 (pnpla3) i148m (rs738409) affects hepatic vldl secretion in humans and in vitro. J. Hepatol. 2012, 57, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lefevre, C.; Morava, E.; Mussini, J.M.; Laforet, P.; Negre-Salvayre, A.; Lathrop, M.; Salvayre, R. The gene encoding adipose triglyceride lipase (pnpla2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 2007, 39, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Donati, B.; Fares, R.; Lombardi, R.; Mancina, R.M.; Romeo, S.; Valenti, L. Pnpla3 i148m polymorphism and progressive liver disease. World J. Gastroenterol. 2013, 19, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Trepo, E.; Romeo, S.; Zucman-Rossi, J.; Nahon, P. Pnpla3 gene in liver diseases. J. Hepatol. 2016, 65, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Waits, R.P.; Yamada, T.; Uemichi, T.; Benson, M.D. Low plasma concentrations of retinol-binding protein in individuals with mutations affecting position 84 of the transthyretin molecule. Clin. Chem. 1995, 41, 1288–1291. [Google Scholar] [PubMed]
- Biesalski, H.K.; Frank, J.; Beck, S.C.; Heinrich, F.; Illek, B.; Reifen, R.; Gollnick, H.; Seeliger, M.W.; Wissinger, B.; Zrenner, E. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am. J. Clin. Nutr. 1999, 69, 931–936. [Google Scholar] [PubMed]
- Folli, C.; Viglione, S.; Busconi, M.; Berni, R. Biochemical basis for retinol deficiency induced by the i41n and g75d mutations in human plasma retinol-binding protein. Biochem. Biophys. Res. Commun. 2005, 336, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Yu, K.; Wheeler, W.; Zhang, H.; Weinstein, S.J.; Major, J.M.; Cornelis, M.C.; Mannisto, S.; Hazra, A.; Hsing, A.W.; et al. Genome-wide association study of circulating retinol levels. Hum. Mol. Genet. 2011, 20, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, S.J.; Hazra, A.; Chen, C.; Eliassen, A.H.; Kraft, P.; Rosner, B.A.; Willett, W.C. Beta-carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of european descent. Am. J. Clin. Nutr. 2012, 96, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.; Mancina, R.M.; Merlo, A.; Dongiovanni, P.; Rametta, R.; Montalcini, T.; Valenti, L.; Albanes, D.; Romeo, S. Pnpla3 i148m variant influences circulating retinol in adults with nonalcoholic fatty liver disease or obesity. J. Nutr. 2015, 145, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Perry, J.R.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2009, 84, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, S.; Urata, M.; Wai Kun, R.Y.; Masaki, M.; Shidoji, Y. Common snp rs6564851 in the bco1 gene affects the circulating levels of beta-carotene and the daily intake of carotenoids in healthy Japanese women. PLoS ONE 2016, 11, e0168857. [Google Scholar] [CrossRef] [PubMed]
- Herbeth, B.; Gueguen, S.; Leroy, P.; Siest, G.; Visvikis-Siest, S. The lipoprotein lipase serine 447 stop polymorphism is associated with altered serum carotenoid concentrations in the stanislas family study. J. Am. Coll. Nutr. 2007, 26, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human fasting plasma concentrations of vitamin e and carotenoids, and their association with genetic variants in apo c-iii, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. Br. J. Nutr. 2009, 101, 680–687. [Google Scholar] [PubMed]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human plasma levels of vitamin e and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J. Nutr. 2007, 137, 2653–2659. [Google Scholar] [PubMed]
- Hoekstra, M. SR-BI as target in atherosclerosis and cardiovascular disease—A comprehensive appraisal of the cellular functions of SR-BI in physiology and disease. Atherosclerosis 2017. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary beta-carotene bioavailability in healthy men. J. Nutr. 2015, 145, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Lietz, G.; Oxley, A.; Leung, W.; Hesketh, J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J. Nutr. 2012, 142, 161S–165S. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.C.; Hessel, S.; Meplan, C.; Flint, J.; Oberhauser, V.; Tourniaire, F.; Hesketh, J.E.; von Lintig, J.; Lietz, G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009, 23, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Carss, K.; Raymond, F.L.; Islam, F.; Nihr BioResource-Rare Diseases, C.; Moore, A.T.; Michaelides, M.; Arno, G. Vitamin A deficiency due to bi-allelic mutation of rbp4: There’s more to it than meets the eye. Ophthalmic Genet. 2016, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.R.; Perry, J.R.; Tanaka, T.; Hernandez, D.G.; Zheng, H.F.; Melzer, D.; Gibbs, J.R.; Nalls, M.A.; Weedon, M.N.; Spector, T.D.; et al. Imputation of variants from the 1000 genomes project modestly improves known associations and can identify low-frequency variant—Phenotype associations undetected by hapmap based imputation. PLoS ONE 2013, 8, e64343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ommen, B.; El-Sohemy, A.; Hesketh, J.; Kaput, J.; Fenech, M.; Evelo, C.T.; McArdle, H.J.; Bouwman, J.; Lietz, G.; Mathers, J.C.; et al. The micronutrient genomics project: A community-driven knowledge base for micronutrient research. Genes Nutr. 2010, 5, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| SNP/Mutation | Global MAF 1 | Nearest Gene | Trait | Reference | Study Type |
|---|---|---|---|---|---|
| (Ile59Asn) (rs121918584) | - | RBP4 | FB-RET | [101,102] | CS |
| Gly75Asp (rs1218585) | 0.115 | RBP4 | FB-RET | [101,102] | CS |
| c.248 + 1G>A | - | RBP4 | FB-RET | [116] | CS |
| rs10882272 | 0.390 | RBP4 | FB-RET | [103] | GWAS |
| rs1667255 | 0.500 | TTR | FB-RET | [103] | GWAS |
| rs738409 | 0.2622 | PNPLA3 | FB-RET | [105] | CGAS |
| rs6564851 | 0.476 | BCO1 | FB-βC | [104,106,107,114,117] | GWAS and CGAS |
| rs12926540 | 0.493 | BCO1 | FB-βC | [117] | GWAS |
| rs7501331 | 0.213 | BCO1 | FB-βC | [104,115] | CGAS |
| rs12934922 | 0.357 | BCO1 | FB-βC | [104,115] | CGAS |
| rs1800588 | 0.292 | HL | FB-βC | [109] | CGAS |
| S447X | - | LPL | FB-βC | [108] | CGAS |
| SR-BI intron 5 | - | SCARB1 | FB-βC | [110] | CGAS |
| rs61932577 | 0.033 | SCARB1 | FB-βC/αC | [28] | CGAS |
| rs1984112 | 0.347 | CD36 | FB-βCryt/αC | [28] | CGAS |
| rs1761667 | 0.390 | CD36 | FB-βCryt/αC | [28] | CGAS |
| rs7755 | 0.388 | CD36 | FB-βCryt/αC | [28] | CGAS |
| rs10991408 * | 0.116 | ABCA1 | βC-B 2 | [113] | CGAS |
| rs2791952 * | 0.140 | ABCA1 | βC-B | [113] | CGAS |
| rs3887137 * | 0.123 | ABCA1 | βC-B | [113] | CGAS |
| rs2278357 | 0.247 | ABCG5 | βC-B | [113] | CGAS |
| rs1042031 * | 0.153 | APOB | βC-B | [113] | CGAS |
| rs35364714 * | 0.115 | APOB | βC-B | [113] | CGAS |
| rs4643493 * | 0.082 | APOB | βC-B | [113] | CGAS |
| rs7196470 | 0.278 | BCO1 | βC-B | [113] | CGAS |
| rs1247620 | 0.137 | CXCL8 | βC-B | [113] | CGAS |
| rs1358594 | 0.291 | CXCL8 | βC-B | [113] | CGAS |
| rs6834586 | 0.221 | CXCL8 | βC-B | [113] | CGAS |
| rs3798709 | 0.252 | ELOVL2 | βC-B | [113] | CGAS |
| rs911196 | 0.252 | ELOVL2 | βC-B | [113] | CGAS |
| rs9468304 | 0.302 | ELOVL2 | βC-B | [113] | CGAS |
| rs16994824 | 0.206 | ISX | βC-B | [113] | CGAS |
| rs202313 | 0.113 | ISX | βC-B | [113] | CGAS |
| rs5755368 | 0.250 | ISX | βC-B | [113] | CGAS |
| rs11857380 * | 0.157 | LIPC | βC-B | [113] | CGAS |
| rs12185072 * | 0.198 | LIPC | βC-B | [113] | CGAS |
| rs1869138 * | 0.117 | LIPC | βC-B | [113] | CGAS |
| rs8043708 | 0.237 | PKD1L2 | βC-B | [113] | CGAS |
| rs12139131 | 0.096 | RPE65 | βC-B | [113] | CGAS |
| rs4926340 | 0.093 | RPE65 | βC-B | [113] | CGAS |
| rs2501175 | 0.327 | SOD2 | βC-B | [113] | CGAS |
| rs946199 * | 0.192 | TCF7L2 | βC-B | [113] | CGAS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borel, P.; Desmarchelier, C. Genetic Variations Associated with Vitamin A Status and Vitamin A Bioavailability. Nutrients 2017, 9, 246. https://doi.org/10.3390/nu9030246
Borel P, Desmarchelier C. Genetic Variations Associated with Vitamin A Status and Vitamin A Bioavailability. Nutrients. 2017; 9(3):246. https://doi.org/10.3390/nu9030246
Chicago/Turabian StyleBorel, Patrick, and Charles Desmarchelier. 2017. "Genetic Variations Associated with Vitamin A Status and Vitamin A Bioavailability" Nutrients 9, no. 3: 246. https://doi.org/10.3390/nu9030246






