The Effect of Short-Term Dietary Fructose Supplementation on Gastric Emptying Rate and Gastrointestinal Hormone Responses in Healthy Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Gastric Emptying Measurement
2.4. Sample Analysis
2.5. Data and Statistical Analysis
3. Results
3.1. Body Mass, Hydration Status and Drink Osmolality
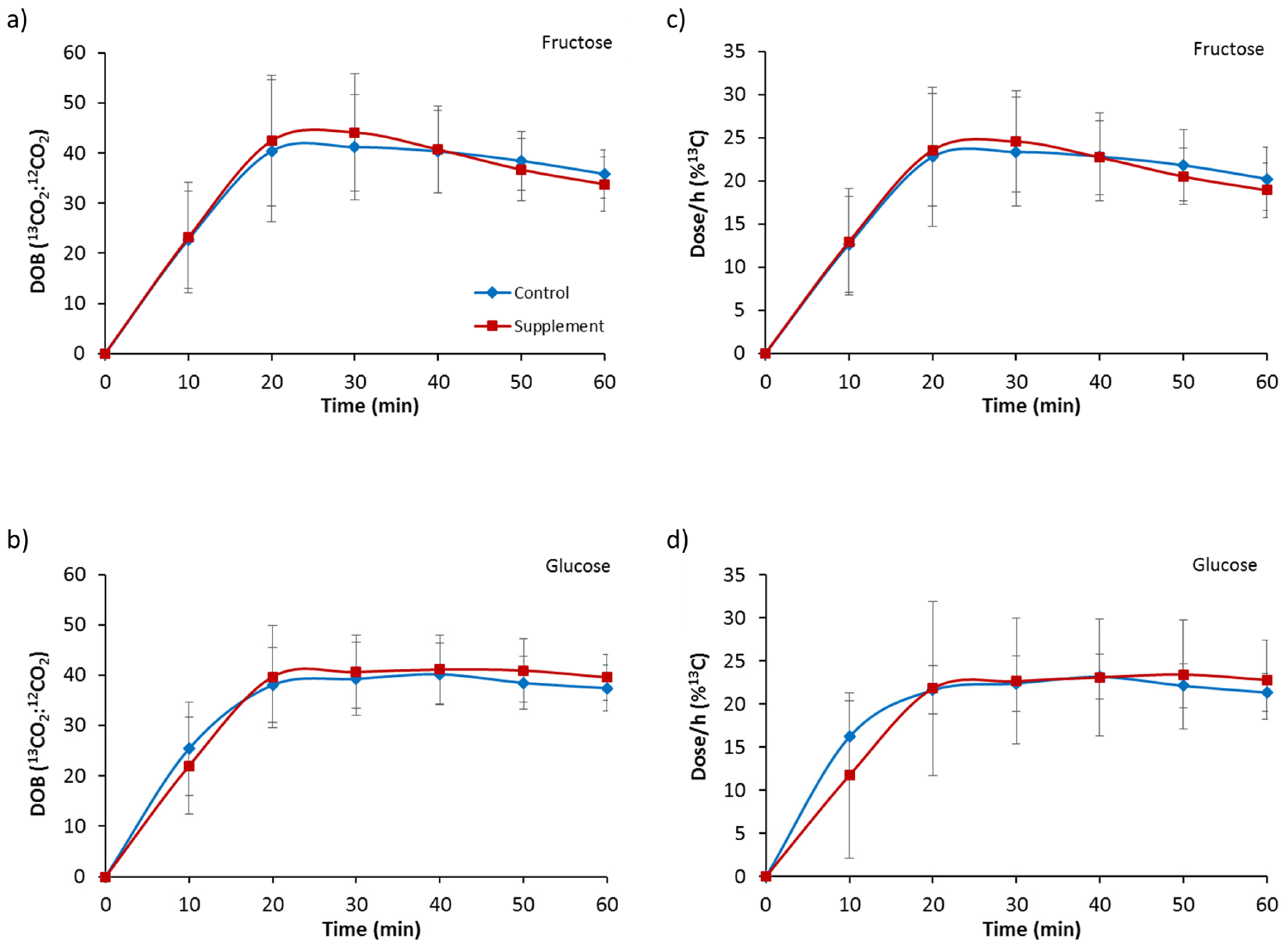
3.2. Gastric Emptying
3.3. Gut Hormones
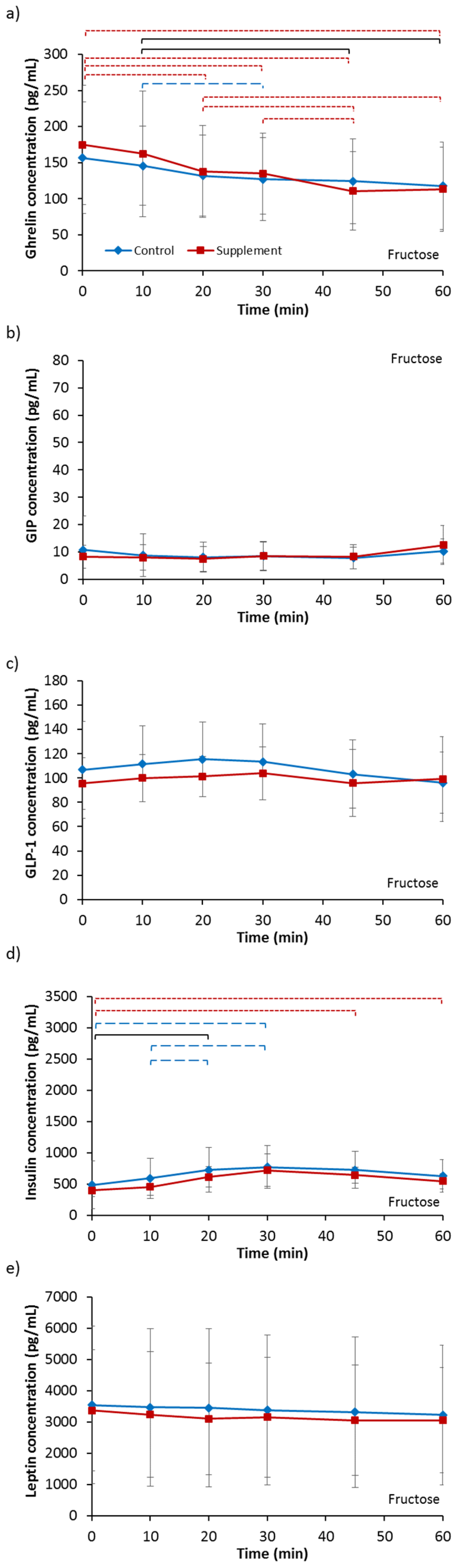
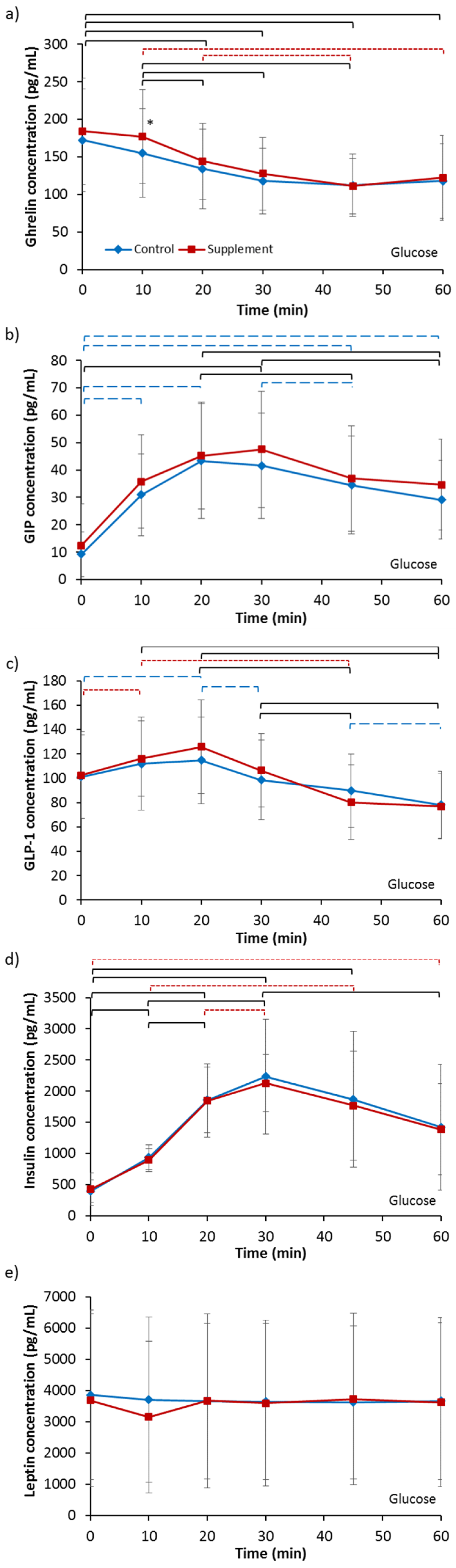
3.3.1. Ghrelin
3.3.2. GIP
3.3.3. GLP-1
3.3.4. Insulin
3.3.5. Leptin
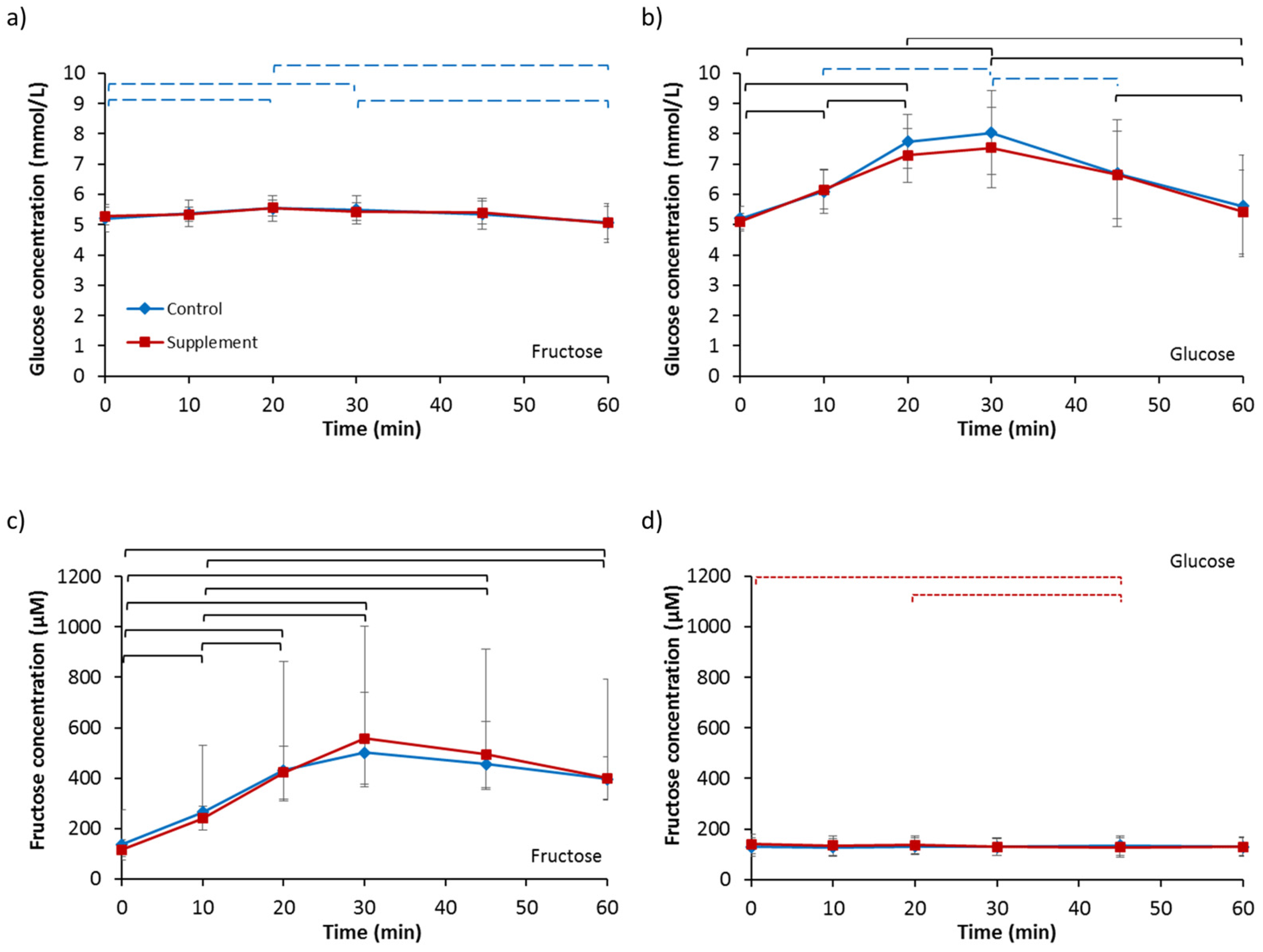
3.4. Blood Glucose and Fructose
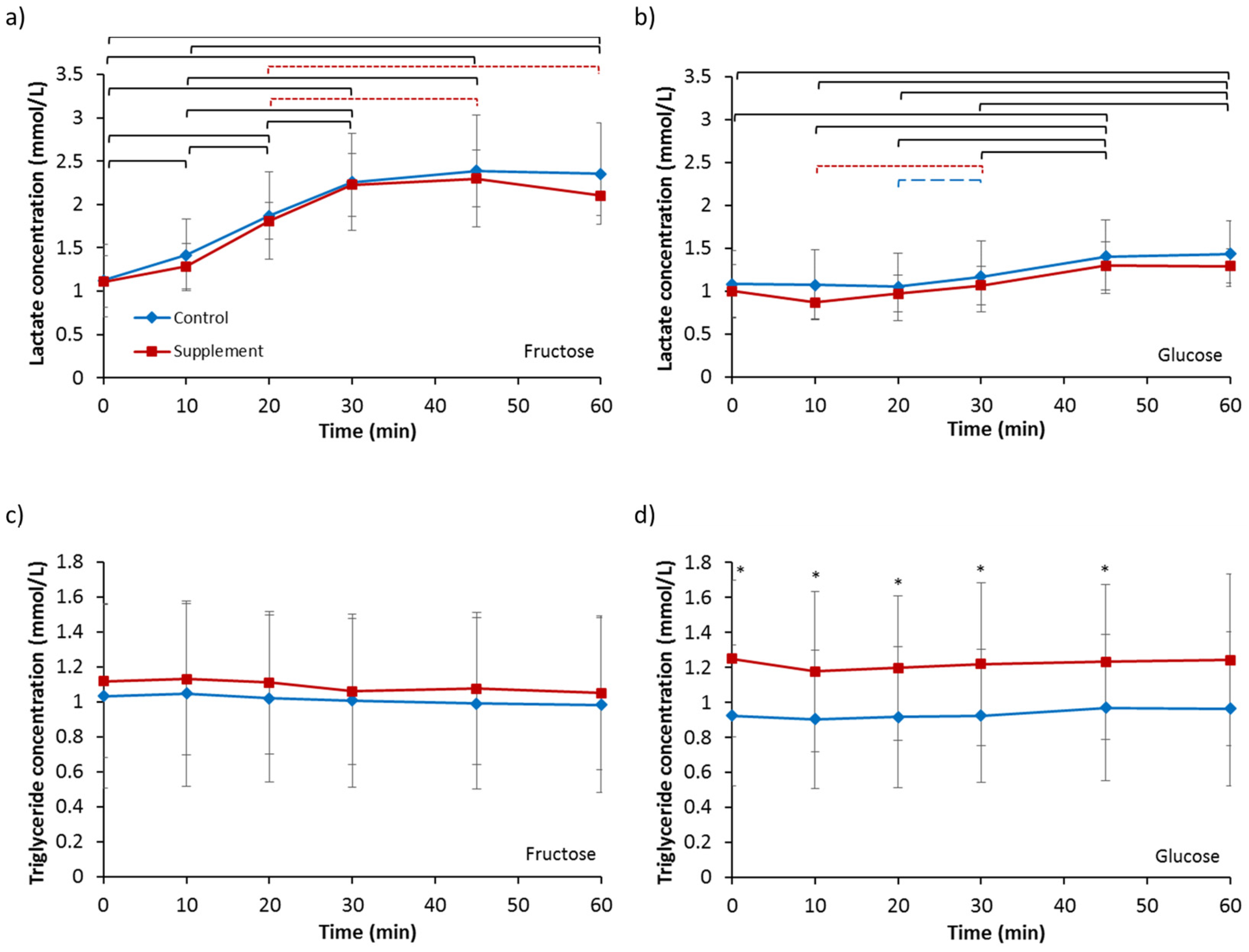
3.5. Lactate and Triglycerides
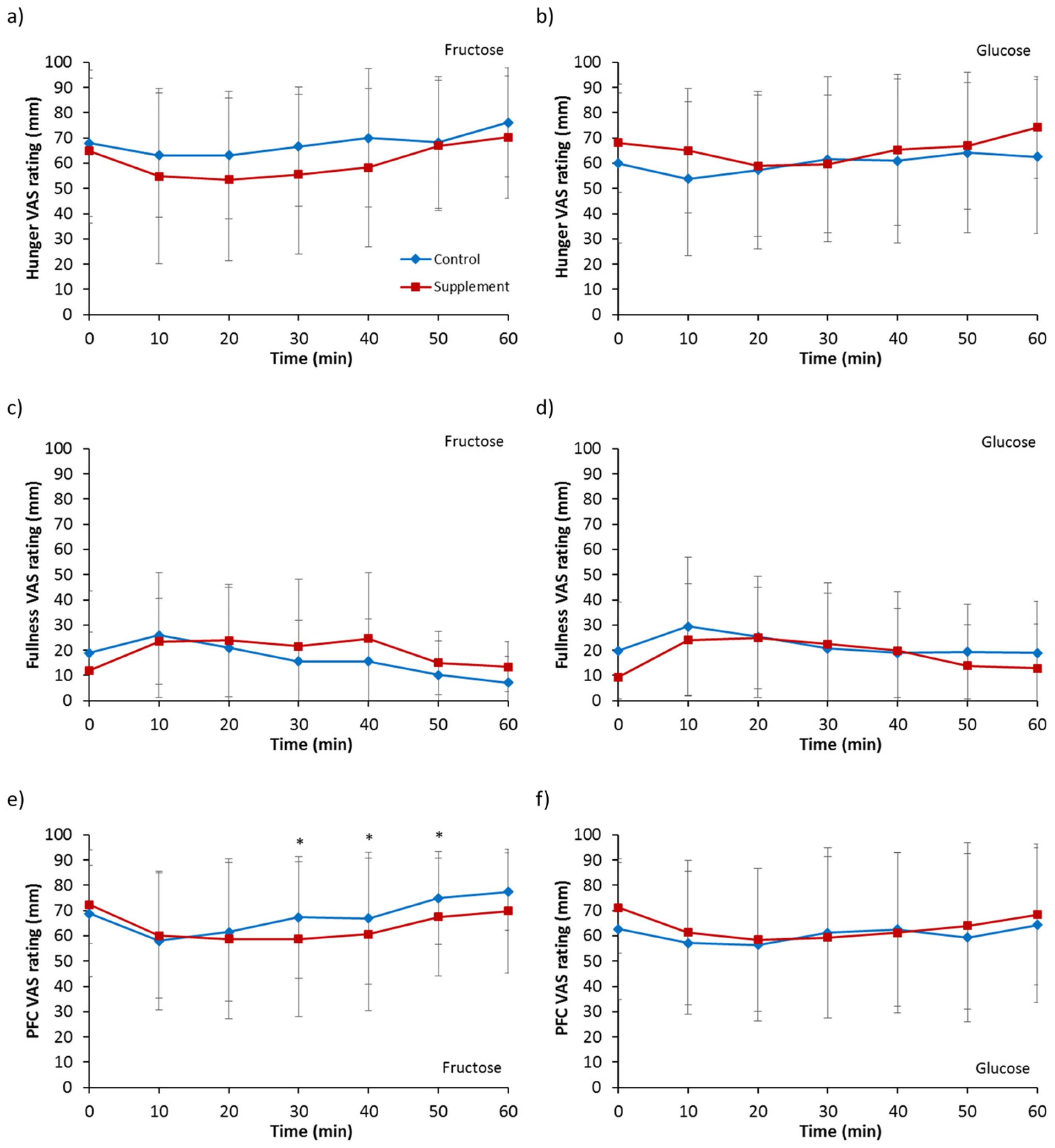
3.6. Appetite Ratings
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geliebter, A.; Westreich, S.; Gage, D. Gastric distension and gastric capacity in relation to food intake in humans. Physiol. Behav. 1988, 44, 665–668. [Google Scholar] [CrossRef]
- Falken, Y.; Webb, D.-L.; Abraham-Nordling, M.; Kressner, U.; Hellstrom, PM.; Naslund, E. Intravenous ghrelin accelerates postoperative gastric emptying and time to first bowel movement in humans. Neurogastroent. Motil. 2013, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Levin, F.; Edholm, T.; Schmidt, P.T.; Gryback, P.; Jacobsson, H.; Degerblad, M.; Hoybye, C.; Holst, J.J.; Rehfeld, J.F.; Hellstrom, P.M.; et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J. Clin. Endocrin. Metab. 2006, 91, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Degerblad, M.; Gryback, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.T.; Hellstrom, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroent. Motil. 2010, 22, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Wettergren, A.; Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Truncated glp-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Digest. Dis. Sci. 1993, 38, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.-B.; Gryback, P.; Holst, J.J.; Hilsted, L.; Hellstrom, P.M.; Jacobsson, H.; Schmidt, P.T. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul. Peptides 2009, 158, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Schwizer, W.; Borovicka, J.; Kunz, P.; Fraser, R.; Kreiss, C.; D’Amato, M.; Crelier, G.; Boesiger, P.; Fried, M. Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: Studies with the CCK antagonist loxiglumide. Gut 1997, 41, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A.; Morita, E.T.; Conrad, C.K.; Williams, J.A. Regulation of gastric emptying in humans by cholescystokinin. J. Clin. Investig. 1986, 77, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.E.; McKenna, P.; McClean, C.; Dabison, G.W.; Trinick, T.; Duly, E.; Shafat, A. Gastrointestinal transit, post-prandial lipaemia and satiety following 3 days high-fat diet in men. Eur. J. Clin. Nutr. 2011, 65, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.M.; Horowitz, M.; Read, N.W. The effect of short-term dietary supplementation with glucose on gastric-emptying in humans. Br. J. Nutr. 1991, 65, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Cunningham, K.M.; Wishart, J.M.; Jones, K.L.; Read, N.W. The effect of short-term dietary supplementation with glucose on gastric emptying of glucose and fructose and oral glucose tolerance in normal subjects. Diabetologia 1996, 39, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Yau, A.M.W.; McLaughlin, J.; Maughan, R.J.; Gilmore, W.; Evans, G.H. Short-term dietary supplementation with fructose accelerates gastric emptying of a fructose but not a glucose solution. Nutrition 2014, 30, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.M.; Daly, J.; Horowitz, M.; Read, N.W. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut 1991, 32, 483–486. [Google Scholar] [CrossRef] [PubMed]
- French, S.J.; Murray, B.; Rumsey, R.D.E.; Fadzlin, R.; Read, N.W. Adaptation to high-fat diets—Effects on eating behavior and plasma cholecystokinin. Br. J. Nutr. 1995, 73, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Feltrin, K.L.; Horowitz, M.; Meyer, J.H.; Wishart, J.; Chapman, I.M.; Feinle-Bisset, C. A high-fat diet raises fasting plasma CCK but does not affect upper gut motility, PYY, and ghrelin, or energy intake during CCK-8 infusion in lean men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 294, R45–R51. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Henderson, R.A.; Vist, G.E.; Rumsey, R.D. Plasma ghrelin response following a period of acute overfeeding in normal weight men. Int. J. Obes. 2004, 28, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.A.; O’Donovan, D.G.; Doran, S.; Wishart, J.; Chapman, I.M.; Horowitz, M.; Feinle, C. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am. J. Physiol. Gastrointest. Liver 2003, 284, G188–G196. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ghoos, Y.F.; Maes, B.D.; Geypens, B.J.; Mys, C.; Hiele, M.I.; Rutgeerts, P.J.; Vantrappen, G. Measurement of gastric-emptying rate of solids by means of a carbon-labeled octanoic-acid breath test. Gastroenterology 1993, 104, 1640–1647. [Google Scholar] [CrossRef]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Gribble, F.M.; Hartmann, B.; Reimann, F.; Windeløv, J.A.; Rehfeld, J.F.; Holst, J.J. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G622–G630. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Goetze, O.; Anstipp, J.; Hagemann, D.; Holst, J.J.; Schmidt, W.E.; Gallwitz, B.; Nauck, M.A. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E621–E625. [Google Scholar] [CrossRef] [PubMed]
- Wishart, J.M.; Horowitz, M.; Morris, H.A.; Jones, K.L.; Nauck, M.A. Relation between gastric emptying of glucose and plasma concentrations of glucagon-like peptide-1. Peptides 1998, 19, 1049–1053. [Google Scholar] [CrossRef]
- Lee, H.M.; Wang, G.Y.; Englander, E.W.; Kojima, M.; Greeley, G.H. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: Enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002, 143, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A.; Carter, J.D.; McDonald, A.R. Dietary regulation of rat intestinal cholecystokinin gene expression. J. Clin. Investig. 1988, 81, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Bezencon, C.; le Coutre, J.; Damak, S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 2007, 32, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Salmon, K.S.H.; Zibrik, L.; Shirazi-Beechey, S.P. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem. Soc. Trans. 2005, 33, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Gerspach, A.C.; Steinert, R.E.; Schonenberger, L.; Graber-Maier, A.; Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E317–E325. [Google Scholar] [CrossRef] [PubMed]
- Le, K.A.; Faeh, D.; Stettler, R.; Ith, M.; Kreis, R.; Vermathen, P.; Boesch, C.; Ravussin, E.; Tappy, L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006, 84, 1374–1379. [Google Scholar] [PubMed]
- Ngo Sock, E.T.; Le, K.-A.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Brit. J. Nutr. 2010, 103, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Bremer, A.A.; Medici, V.; Nakajima, K.; Ito, Y.; Nakano, T.; Chen, G.; Fong, T.H.; Lee, V.; Menorca, R.I.; et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J. Clin. Endocrinol. Metab. 2011, 96, E1596–E1605. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
| FC | FS | GC | GS | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body mass (kg) | 80.87 | 11.15 | 81.13 | 11.04 | 81.48 | 11.46 | 80.95 | 10.80 |
| Urine osmolality (mOsmol/kg) | 560 | 262 | 397 | 271 | 504 | 266 | 356 | 193 |
| FC | FS | GC | GS | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Ghrelin (pg/mL) | 156.65 | 77.25 | 174.64 | 82.47 | 172.06 | 68.19 | 184.05 | 71.00 |
| GIP (pg/mL) | 10.78 | 12.44 | 8.26 | 4.13 | 9.31 | 8.18 | 12.47 | 15.20 |
| GLP-1 (pg/mL) | 106.78 | 40.07 | 95.49 | 21.29 | 101.12 | 34.41 | 102.55 | 35.53 |
| Insulin (pg/mL) | 438.05 | 383.64 | 396.53 | 93.16 | 396.20 | 180.37 | 425.87 | 260.60 |
| Leptin (pg/mL) | 3542.36 | 2525.04 | 3371.53 | 1934.44 | 3857.64 | 2711.35 | 3687.42 | 2767.98 |
| Glucose (mmol/L) | 5.21 | 0.46 | 5.28 | 0.30 | 5.21 | 0.40 | 5.11 | 0.25 |
| Fructose (µM) | 137.0 | 48.8 | 115.8 | 39.6 | 129.8 | 36.6 | 139.4 | 38.4 |
| Lactate (mmol/L) | 1.12 | 0.41 | 1.11 | 0.30 | 1.08 | 0.39 | 1.00 | 0.30 |
| Triglycerides (mmol/L) | 1.03 | 0.53 | 1.12 | 0.44 | 0.92 | 0.40 | 1.25 | 0.45 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, A.M.W.; McLaughlin, J.; Maughan, R.J.; Gilmore, W.; Evans, G.H. The Effect of Short-Term Dietary Fructose Supplementation on Gastric Emptying Rate and Gastrointestinal Hormone Responses in Healthy Men. Nutrients 2017, 9, 258. https://doi.org/10.3390/nu9030258
Yau AMW, McLaughlin J, Maughan RJ, Gilmore W, Evans GH. The Effect of Short-Term Dietary Fructose Supplementation on Gastric Emptying Rate and Gastrointestinal Hormone Responses in Healthy Men. Nutrients. 2017; 9(3):258. https://doi.org/10.3390/nu9030258
Chicago/Turabian StyleYau, Adora M. W., John McLaughlin, Ronald J. Maughan, William Gilmore, and Gethin H. Evans. 2017. "The Effect of Short-Term Dietary Fructose Supplementation on Gastric Emptying Rate and Gastrointestinal Hormone Responses in Healthy Men" Nutrients 9, no. 3: 258. https://doi.org/10.3390/nu9030258
APA StyleYau, A. M. W., McLaughlin, J., Maughan, R. J., Gilmore, W., & Evans, G. H. (2017). The Effect of Short-Term Dietary Fructose Supplementation on Gastric Emptying Rate and Gastrointestinal Hormone Responses in Healthy Men. Nutrients, 9(3), 258. https://doi.org/10.3390/nu9030258





