Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animals
2.4. Anterior Cruciate Ligament Transaction (ACLT)
2.5. Experimental Design
2.6. Quantitative Real-Time-Polymerase Chain Reaction (q-PCR)
2.7. Western Blotting
2.8. ELISA
2.9. Histology and Immunohistochemistry
2.10. Statistical Analysis
3. Results
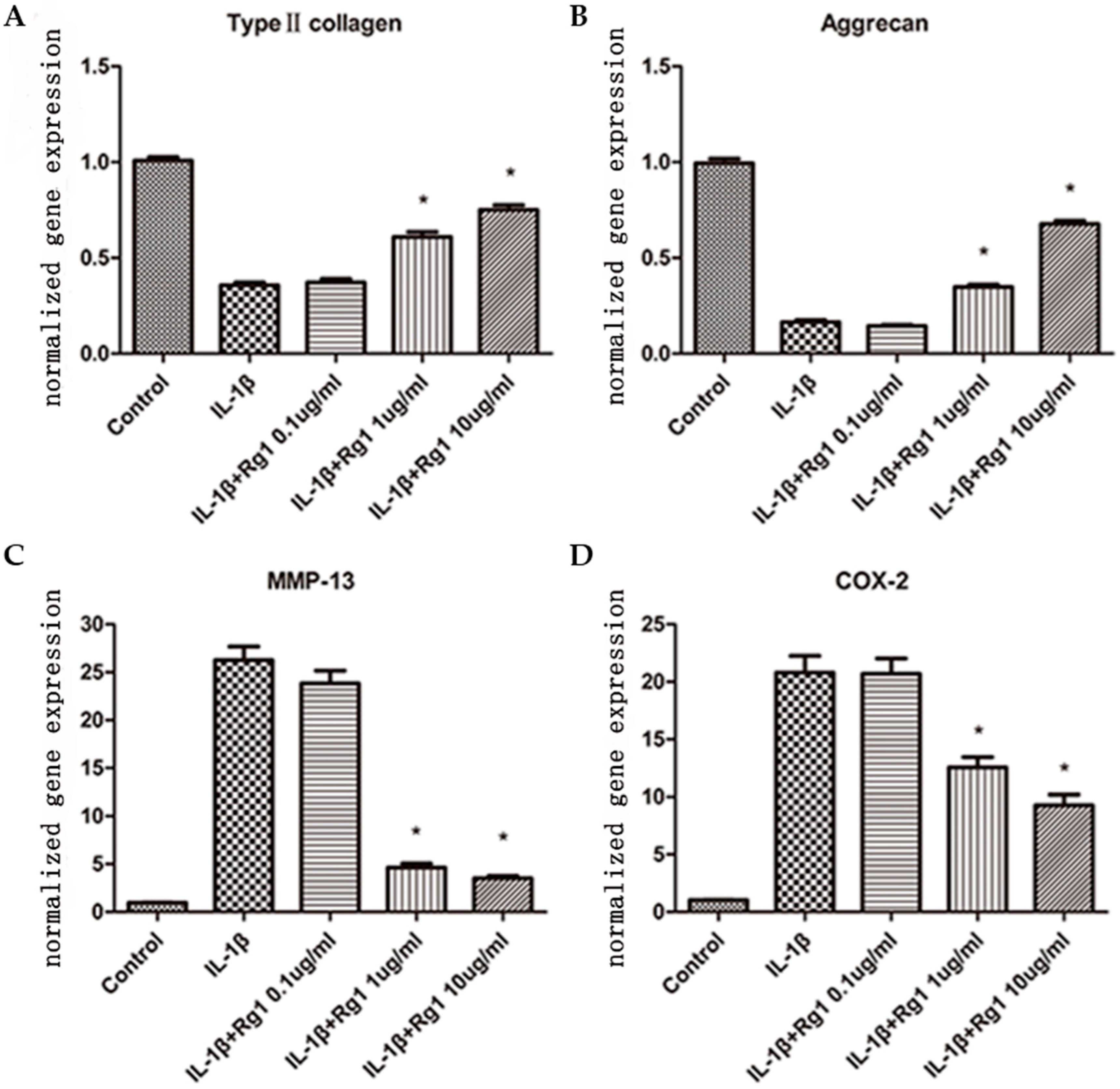
3.1. Effects of Rg1 on Gene Expression of Extracellular Matrix and Inflammatory Mediators after Induction by IL-1β
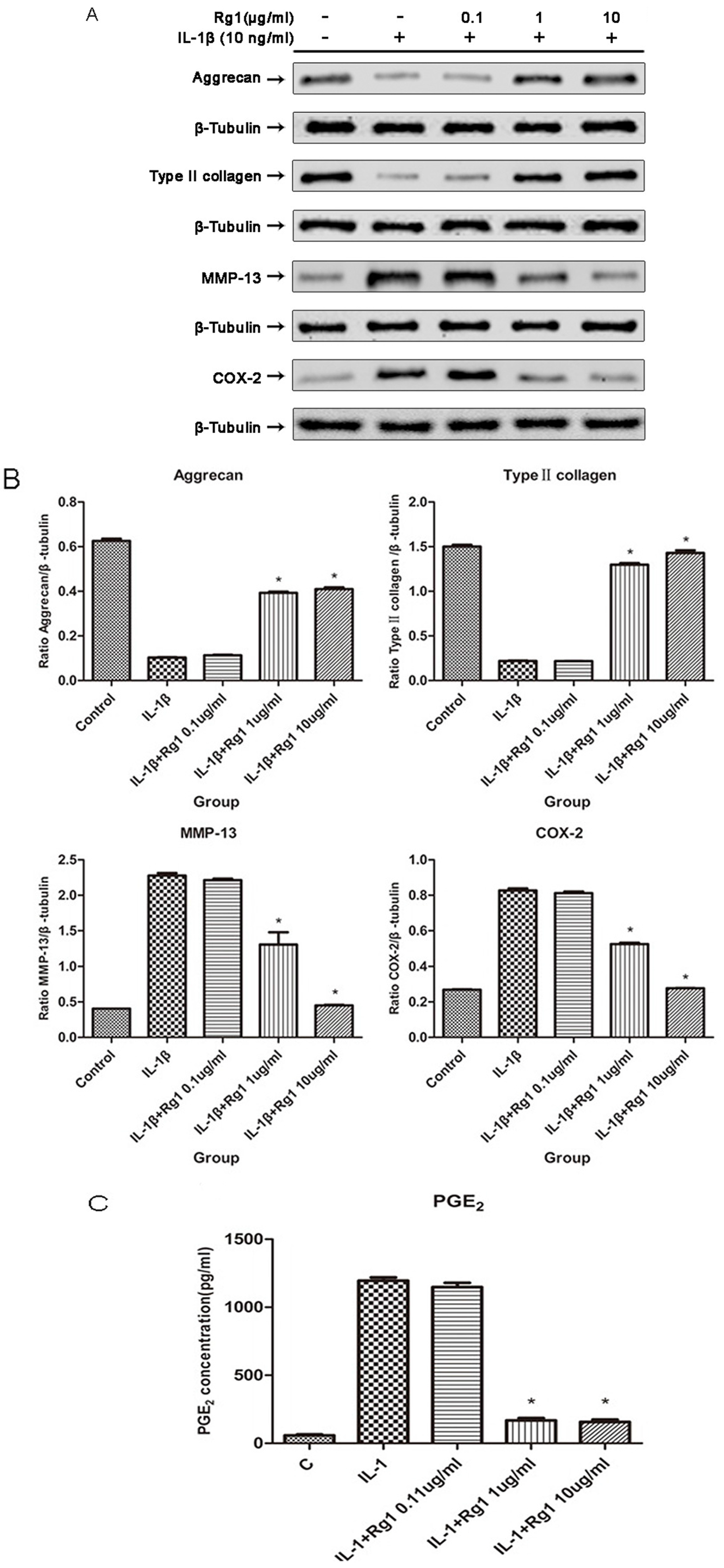
3.2. Effects of Rg1 on Protein Expression of Extracellular Matrix and Inflammatory Mediators after Induction by IL-1β
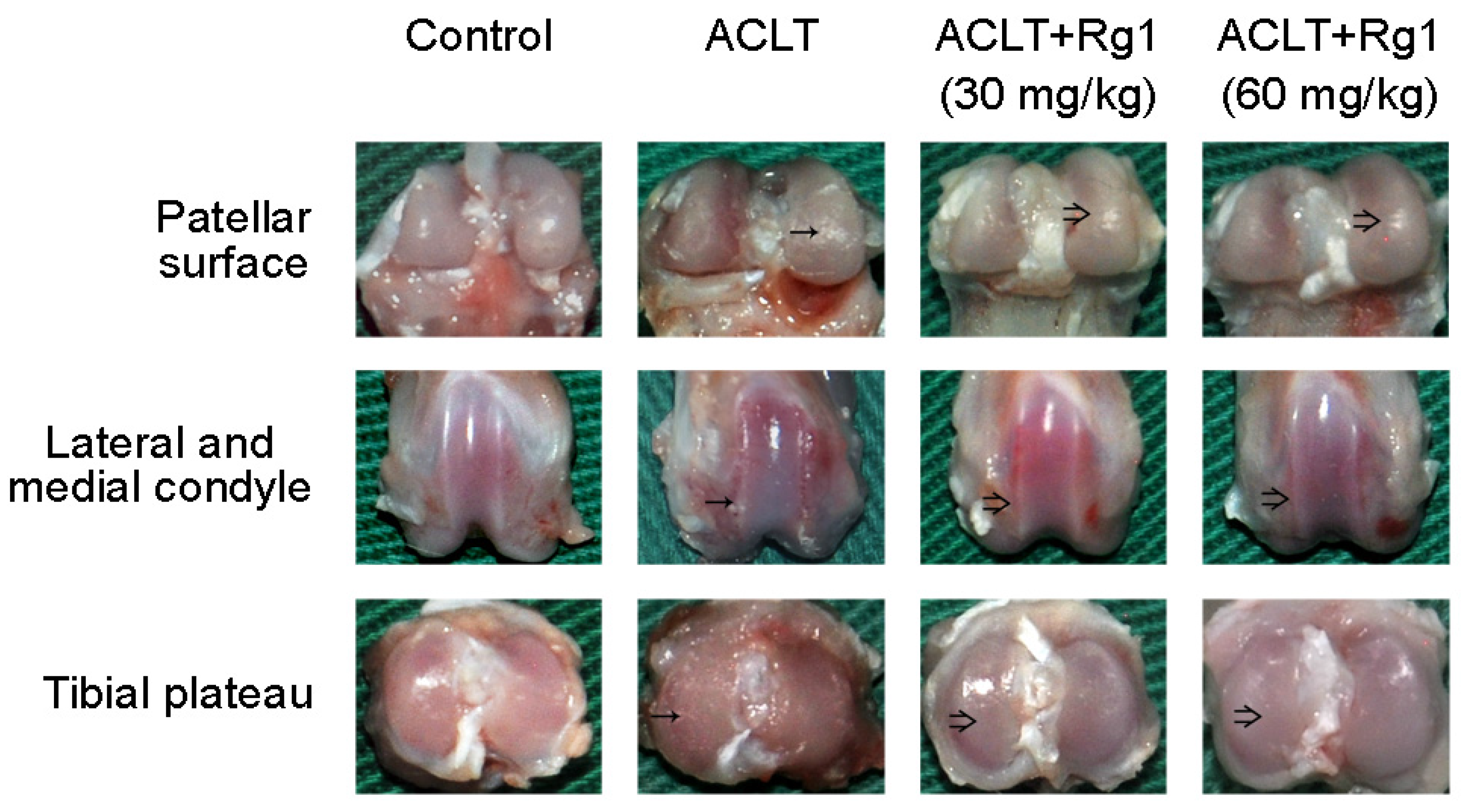
3.3. Rat OA and Gross Morphology
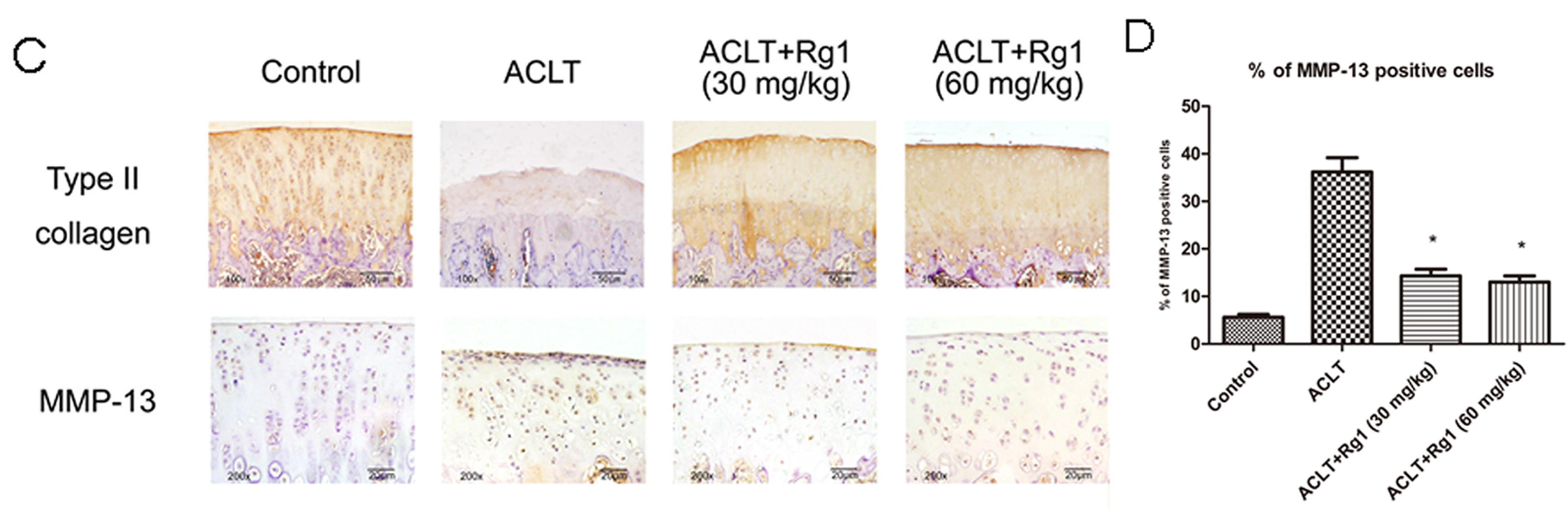
3.4. Histology and Immunohistochemistry Findings
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Cheng, W.; Wu, D.; Zuo, Q.; Wang, Z.; Fan, W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int. Orthop. 2013, 37, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collee, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef] [PubMed]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef]
- Attur, M.; Al-Mussawir, H.E.; Patel, J.; Kitay, A.; Dave, M.; Palmer, G.; Pillinger, M.H.; Abramson, S.B. Abramson, Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: Evidence for signaling via the EP4 receptor. J. Immunol. 2008, 181, 5082–5088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Xu, X.X.; Xu, T. Ginsenoside Ro suppresses interleukin-1beta-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-kappaB. Chin. J. Nat. Med. 2015, 13, 283–289. [Google Scholar] [PubMed]
- Martel-Pelletier, J.; Pelletier, J.P.; Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin. Arthritis Rheum. 2003, 33, 155–167. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.H.; Lee, K.W.; Kim, K.Y.; Kim, S.G.; Hong, S.H.; Kim, G.Y.; Park, C.; Kim, H.K.; Choi, Y.W.; et al. Mori folium inhibits interleukin-1beta-induced expression of matrix metalloproteinases and inflammatory mediators by suppressing the activation of NF-kappaB and p38 MAPK in SW1353 human chondrocytes. Int. J. Mol. Med. 2016, 37, 452–460. [Google Scholar] [PubMed]
- Du, J.; Cheng, B.; Zhu, X.; Ling, C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J. Immunol. 2011, 187, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Rausch, W.D. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004, 1021, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yao, J.P.; Wang, X.; Zheng, M.; Li, P.; He, C.; Wan, J.B.; Yao, X.; Su, H. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury. Mol. Med. Rep. 2016, 13, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.; Li, W.; Zhou, L.; Li, Q.; Wang, X.; He, P. A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer’s disease. J. Ginseng. Res. 2016, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, D.; Fan, W. Protection of ginsenoside Rg1 on chondrocyte from IL-1beta-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol. Cell. Biochem. 2014, 392, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.T.; McErlain, D.D.; Pitelka, V.; Schwartz, N.; Bernier, S.M.; Henry, J.L.; Holdsworth, D.W.; Beier, F. Forced mobilization accelerates pathogenesis: Characterization of a preclinical surgical model of osteoarthritis. Arthritis Res. Ther. 2007, 9, R13. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Woo, Y.J.; Jeong, J.H.; Park, M.K.; Oh, H.J.; Park, J.S.; Kim, E.K.; Cho, M.L.; Park, S.H.; Kim, H.Y.; et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthr. Cartil. 2012, 20, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Xiao, P.G. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992, 36, 27–38. [Google Scholar] [PubMed]
- Wang, A.; Wang, C.Z.; Wu, J.A.; Osinski, J.; Yuan, C.S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem. Anal. 2005, 16, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Sun, M.; Guo, J.; Huang, L.; Wang, S.; Meng, B.; Ping, Q. Active absorption of ginsenoside Rg1 in vitro and in vivo: The role of sodium-dependent glucose co-transporter 1. J. Pharm. Pharmacol. 2009, 61, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, J.J.; Eun, S.H.; Kim, D.H. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur. J. Pharmacol. 2015, 762, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Xiong, J.; Zhu, W.; Liu, Q.; Wang, D.; Liu, W.; Li, Z.; Wang, D. E2 regulates MMP-13 via targeting miR-140 in IL-1beta-induced extracellular matrix degradation in human chondrocytes. Arthritis Res. Ther. 2016, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.O.; Lee, M.; Kim, O.K.; Ha, Y.; Jun, W.; Lee, J. Effect of Hijikia fusiforme extracts on degenerative osteoarthritis in vitro and in vivo models. Nutr. Res. Pract. 2016, 10, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Price, J.S.; Waters, J.G.; Darrah, C.; Pennington, C.; Edwards, D.R.; Donell, S.T.; Clark, I.M. The role of chondrocyte senescence in osteoarthritis. Aging Cell 2002, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cai, S.Z.; Zhou, Y.; Zhang, X.P.; Liu, D.F.; Jiang, R.; Wang, Y.P. Senescence as a consequence of ginsenoside rg1 response on k562 human leukemia cell line. Asian Pac. J. Cancer Prev. 2012, 13, 6191–6196. [Google Scholar] [CrossRef] [PubMed]
- Sin, S.; Kim, S.Y.; Kim, S.S. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. Int. J. Oncol. 2012, 41, 1669–1674. [Google Scholar] [PubMed]
- Shi, A.W.; Gu, N.; Liu, X.M.; Wang, X.; Peng, Y.Z. Ginsenoside Rg1 enhances endothelial progenitor cell angiogenic potency and prevents senescence in vitro. J. Int. Med. Res. 2011, 39, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Fang, Y.; Zhao, C.; Zhu, Y. Ginsenoside Rg1 delays tert-butyl hydroperoxide-induced premature senescence in human WI-38 diploid fibroblast cells. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.W.; Wu, Z.H.; Weng, X.S. Celecoxib can suppress expression of genes associated with PGE2 pathway in chondrocytes under inflammatory conditions. Int. J. Clin. Exp. Med. 2015, 8, 10902–10910. [Google Scholar] [PubMed]
- Heinecke, L.F.; Grzanna, M.W.; Au, A.Y.; Mochal, C.A.; Rashmir-Raven, A.; Frondoza, C.G. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthr. Cartil. 2010, 18, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Shibakawa, A.; Tanaka, M.; Kato, T.; Nishioka, K. Effects of glucosamine hydrochloride on the production of prostaglandin E2, nitric oxide and metalloproteases by chondrocytes and synoviocytes in osteoarthritis. Clin. Exp. Rheumatol. 2004, 22, 293–299. [Google Scholar] [PubMed]
- Afara, I.; Prasadam, I.; Crawford, R.; Xiao, Y.; Oloyede, A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthr. Cartil. 2012, 20, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Hsieh, S.P.; Lee, C.H.; Huang, G.S.; Ng, H.F.; Neoh, C.A.; Hsieh, C.S.; et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: Association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthr. Cartil. 2010, 18, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Watari, T.; Furuhata, A.; Yomogida, S.; Sakamoto, K.; Kurosawa, H.; Kaneko, K.; Nagaoka, I. Evaluation of the effect of glucosamine on an experimental rat osteoarthritis model. Life Sci. 2010, 86, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006, 38, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R.; Buma, P.; van der Kraan, P.M.; Hollander, A.P.; Billinghurst, R.C.; Meijers, T.H.M; Poole, A.R.; van den Berg, W.B. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthr. Cartil. 2001, 9, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.S.; Chan, R.Y.; Guo, D.A.; Wong, M.S. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERalpha pathway. J Steroid Biochem. Mol. Biol. 2008, 108, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Li, Y.; Zhu, X.; Zhang, C.; Li, M. Ginsenosides may reverse the dexamethasone-induced down-regulation of glucocorticoid receptor. Gen. Comp. Endocrinol. 2005, 140, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.Y.; Chen, W.F.; Dong, A.; Guo, D.; Wong, M.S. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J. Clin. Endocrinol. Metab. 2002, 87, 3691–3695. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.; Jing, J.; Wang, Z.; Wu, D.; Huang, Y. Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection. Nutrients 2017, 9, 263. https://doi.org/10.3390/nu9030263
Cheng W, Jing J, Wang Z, Wu D, Huang Y. Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection. Nutrients. 2017; 9(3):263. https://doi.org/10.3390/nu9030263
Chicago/Turabian StyleCheng, Wendan, Juehua Jing, Zhen Wang, Dongying Wu, and Yumin Huang. 2017. "Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection" Nutrients 9, no. 3: 263. https://doi.org/10.3390/nu9030263



