Prevalence of Vitamin D Deficiency and Its Associations with Skin Color in Pregnant Women in the First Trimester in a Sample from Switzerland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Vitamin D Blood Samples
2.3. Skin Color
2.4. Covariates
2.5. Statistical Analyses
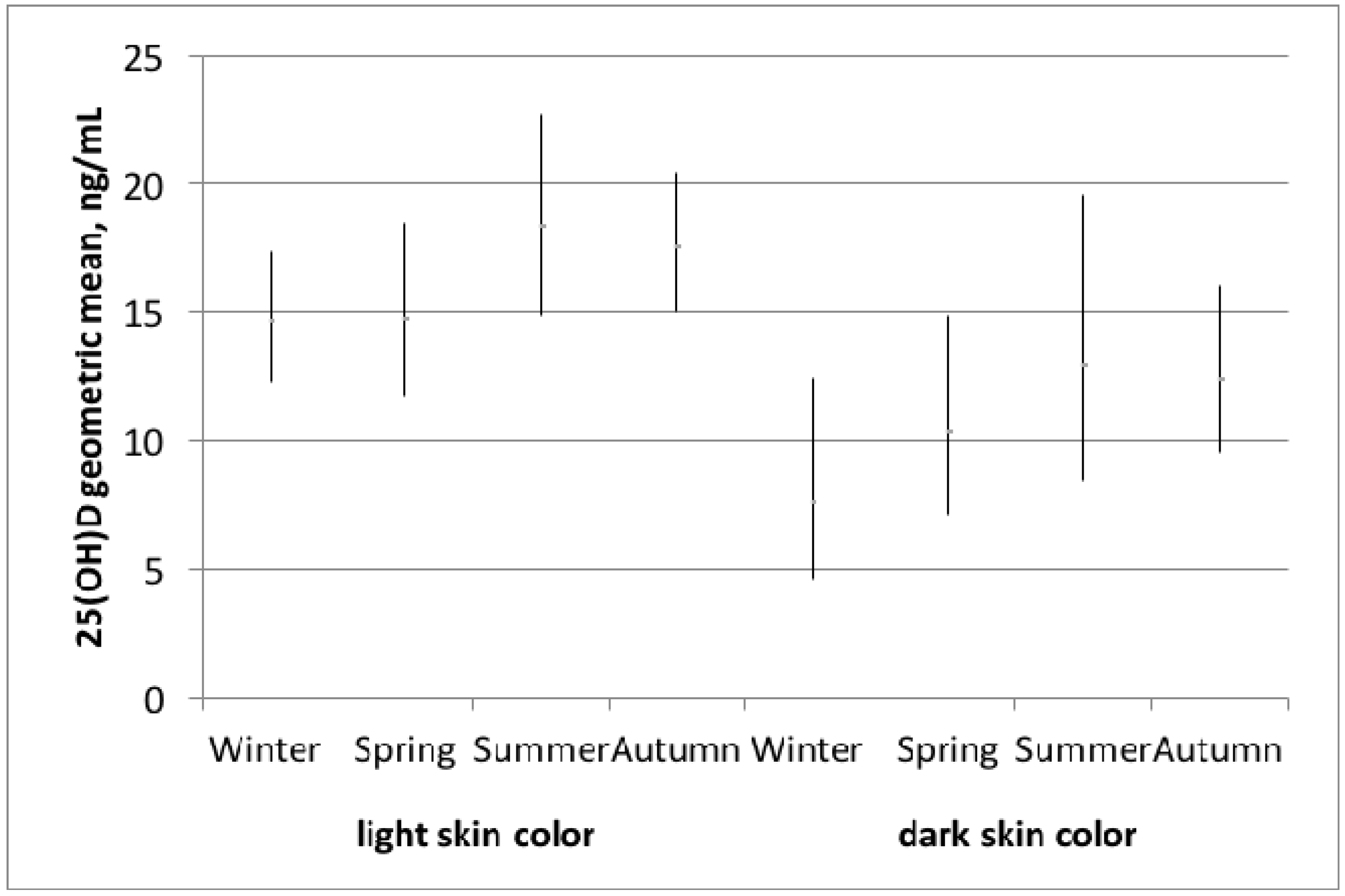
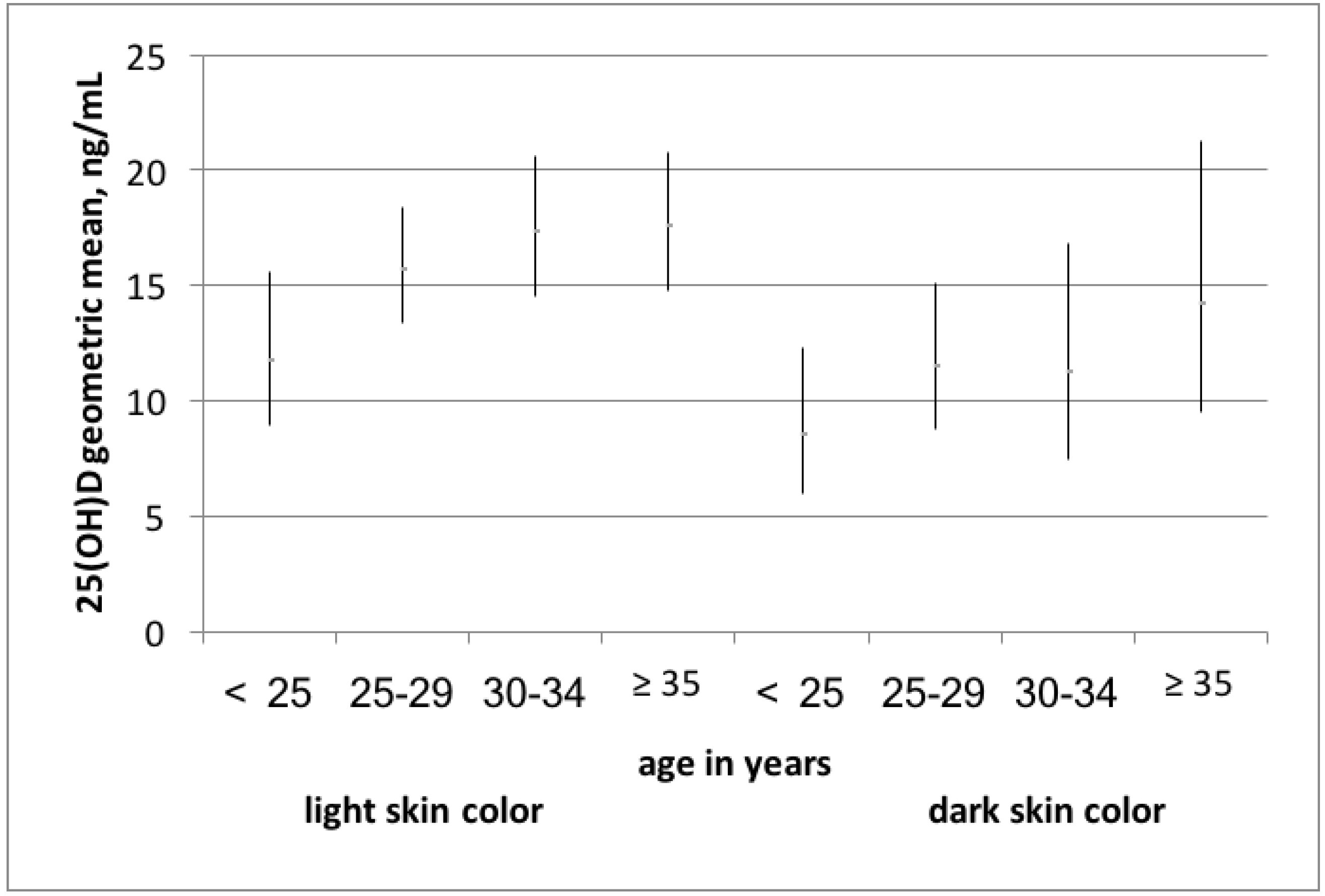
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davies, J.H.; Reed, J.M.; Blake, E.; Mrcpch, M.P.; Jackson, A.A.; Clarke, N.M. Epidemiology of vitamin d deficiency in children presenting to a pediatric orthopaedic service in the uk. J. Pediatr. Orthop. 2011, 31, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Merewood, A.; Mehta, S.D.; Grossman, X.; Chen, T.C.; Mathieu, J.S.; Holick, M.F.; Bauchner, H. Widespread vitamin d deficiency in urban massachusetts newborns and their mothers. Pediatrics 2010, 125, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Sullivan, A.F.; Mansbach, J.M.; Camargo, C.A., Jr. Vitamin d insufficiency in pregnant and nonpregnant women of childbearing age in the united states. Am. J. Obstet. Gynecol. 2010, 202, 436.e1–436.e8. [Google Scholar] [CrossRef] [PubMed]
- Ergur, A.T.; Berberoglu, M.; Atasay, B.; Siklar, Z.; Bilir, P.; Arsan, S.; Soylemez, F.; Ocal, G. Vitamin d deficiency in turkish mothers and their neonates and in women of reproductive age. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Viljakainen, H.T.; Karkkainen, M.U.; Saarnio, E.; Laitinen, K.; Lamberg-Allardt, C. Prevalence of vitamin d deficiency and secondary hyperparathyroidism during winter in pre-menopausal bangladeshi and somali immigrant and ethnic finnish women: Associations with forearm bone mineral density. Br. J. Nutr. 2012, 107, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, L.; Catling-Paull, C.; Diamond, T.; Homer, C.; Davis, G.; Craig, M.E. Vitamin d, pth and calcium levels in pregnant women and their neonates. Clin. Endocrinol. 2009, 70, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, P.; Hatzistamatiou, Z.; Papadopoulou, A.; Kaleyias, J.; Floropoulou, E.; Lagona, E.; Tsagris, V.; Costalos, C.; Antsaklis, A. Low vitamin d status in mother-newborn pairs in greece. Calcif. Tissue Int. 2006, 78, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin d deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, A.M.; Sayers, A.; Fraser, W.D.; Lewis, G.; Zammit, S.; Lawlor, D.A. The association of serum 25-hydroxyvitamin d3 and d2 with depressive symptoms in childhood—A prospective cohort study. J. Child Psychol. Psychiatry 2012, 53, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Akeson, P.K.; Lind, T.; Hernell, O.; Silfverdal, S.A.; Ohlund, I. Serum vitamin d depends less on latitude than on skin color and dietary intake during early winter in northern europe. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Cadario, F.; Savastio, S.; Pozzi, E.; Capelli, A.; Dondi, E.; Gatto, M.; Zaffaroni, M.; Bona, G. Vitamin d status in cord blood and newborns: Ethnic differences. Ital. J. Pediatr. 2013, 39, 35. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin d3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J.L. Vitamin d: An overview of vitamin d status and intake in europe. Nutr. Bull. BNF 2014, 39, 322–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qiu, C.; Hu, F.B.; David, R.M.; van Dam, R.M.; Bralley, A.; Williams, M.A. Maternal plasma 25-hydroxyvitamin d concentrations and the risk for gestational diabetes mellitus. PLoS ONE 2008, 3, e3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin d deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Halhali, A.; Diaz, L.; Avila, E.; Ariza, A.C.; Garabedian, M.; Larrea, F. Decreased fractional urinary calcium excretion and serum 1,25-dihydroxyvitamin d and igf-i levels in preeclampsia. J. Steroid Biochem. Mol. Biol. 2007, 103, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Halhali, A.; Tovar, A.R.; Torres, N.; Bourges, H.; Garabedian, M.; Larrea, F. Preeclampsia is associated with low circulating levels of insulin-like growth factor i and 1,25-dihydroxyvitamin d in maternal and umbilical cord compartments. J. Clin. Endocrinol. Metab. 2000, 85, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Brooke, O.G.; Brown, I.R.; Bone, C.D.; Carter, N.D.; Cleeve, H.J.; Maxwell, J.D.; Robinson, V.P.; Winder, S.M. Vitamin d supplements in pregnant asian women: Effects on calcium status and fetal growth. Br. Med. J. 1980, 280, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Quack Lötscher, K. Vitamin D and Pregnancy; Federal Office for Public Health: Zurich, Switzerland, 2012. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin d deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types i through vi. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.K.; Jovel, C.; Norton, H.L.; Parra, E.J.; Shriver, M.D. Comparing quantitative measures of erythema, pigmentation and skin response using reflectometry. Pigment Cell Res. Spons. Eur. Soc. Pigment Cell Res. Int. Pigment Cell Soc. 2002, 15, 379–384. [Google Scholar] [CrossRef]
- Guessous, I.; Dudler, V.; Glatz, N.; Theler, J.M.; Zoller, O.; Paccaud, F.; Burnier, M.; Bochud, M.; Swiss Survey on Salt, G. Vitamin d levels and associated factors: A population-based study in switzerland. Swiss Med. Wkly. 2012, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karras, S.; Paschou, S.A.; Kandaraki, E.; Anagnostis, P.; Annweiler, C.; Tarlatzis, B.C.; Hollis, B.W.; Grant, W.B.; Goulis, D.G. Hypovitaminosis d in pregnancy in the mediterranean region: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, F.R.; Fernandez-Alonso, A.M.; Ferrando-Marco, P.; Gonzalez-Salmeron, M.D.; Dionis-Sanchez, E.C.; Fiol-Ruiz, G.; Chedraui, P. First trimester serum 25-hydroxyvitamin d status and factors related to lower levels in gravids living in the spanish mediterranean coast. Reprod. Sci. 2011, 18, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ferre, N.; Torrejon, M.J.; Fuentes, M.; Fernandez, M.D.; Ramos, A.; Bordiu, E.; del Valle, L.; Rubio, M.A.; Bedia, A.R.; Montanez, C.; et al. Association of low serum 25-hydroxyvitamin d levels in pregnancy with glucose homeostasis and obstetric and newborn outcomes. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2012, 18, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alonso, A.M.; Dionis-Sanchez, E.C.; Chedraui, P.; Gonzalez-Salmeron, M.D.; Perez-Lopez, F.R.; The Spanish Vitamin D and Women’s Health Research Group. First-trimester maternal serum 25-hydroxyvitamin d(3) status and pregnancy outcome. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2012, 116, 6–9. [Google Scholar]
- Fernandez-Alonso, A.M.; Valdera-Simbron, C.J.; Fiol-Ruiz, G.; Rodriguez-Sanchez, F.; Chedraui, P.; Perez-Lopez, F.R. First trimester serum levels of 25-hydroxyvitamin d, free beta-human chorionic gonadotropin, and pregnancy-associated plasma protein a in spanish women. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2011, 27, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Vandevijvere, S.; Amsalkhir, S.; Van Oyen, H.; Moreno-Reyes, R. High prevalence of vitamin d deficiency in pregnant women: A national cross-sectional survey. PLoS ONE 2012, 7, e43868. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, I.M.; Karamali, N.S.; Boeke, A.J.; Lips, P.; Middelkoop, B.J.; Verhoeven, I.; Wuister, J.D. High prevalence of vitamin d deficiency in pregnant non-western women in The Hague, The Netherlands. Am. J. Clin. Nutr. 2006, 84, 350–353. [Google Scholar] [PubMed]
- Viljakainen, H.T.; Saarnio, E.; Hytinantti, T.; Miettinen, M.; Surcel, H.; Makitie, O.; Andersson, S.; Laitinen, K.; Lamberg-Allardt, C. Maternal vitamin d status determines bone variables in the newborn. J. Clin. Endocrinol. Metab. 2010, 95, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Simhan, H.N.; Powers, R.W.; Frank, M.P.; Cooperstein, E.; Roberts, J.M. High prevalence of vitamin d insufficiency in black and white pregnant women residing in the northern united states and their neonates. J. Nutr. 2007, 137, 447–452. [Google Scholar] [PubMed]
- Libon, F.; Cavalier, E.; Nikkels, A.F. Skin color is relevant to vitamin d synthesis. Dermatology 2013, 227, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Markestad, T.; Elzouki, A.; Legnain, M.; Ulstein, M.; Aksnes, L. Serum concentrations of vitamin d metabolites in maternal and umbilical cord blood of libyan and norwegian women. Hum. Nutr. Clin. Nutr. 1984, 38, 55–62. [Google Scholar] [PubMed]
- Prentice, A. Vitamin d deficiency: A global perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Haddow, J.E.; Neveux, L.M.; Palomaki, G.E.; Lambert-Messerlian, G.; Canick, J.A.; Grenache, D.G.; Lu, J. The relationship between pth and 25-hydroxy vitamin d early in pregnancy. Clin. Endocrinol. 2011, 75, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, T.; Rajan, S.; Keller, B. Probability of vitamin d deficiency by body weight and race/ethnicity. J. Am. Board Fam. Med. JABFM 2016, 29, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, P.; Winkvist, A.; Olausson, H. Determinants of vitamin d status in pregnant fair-skinned women in Sweden. Br. J. Nutr. 2013, 110, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Sandstrom, H.; Stenlund, H.; Johansson, I.; Hultdin, J. Vitamin d status during pregnancy: A longitudinal study in swedish women from early pregnancy to seven months postpartum. PLoS ONE 2016, 11, e0150385. [Google Scholar] [CrossRef] [PubMed]
- Eggemoen, A.R.; Falk, R.S.; Knutsen, K.V.; Lagerlov, P.; Sletner, L.; Birkeland, K.I.; Jenum, A.K. Vitamin d deficiency and supplementation in pregnancy in a multiethnic population-based cohort. BMC Pregnancy Childbirth 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; De-Regil, L.M.; Lombardo, L.K.; Pena-Rosas, J.P. Vitamin d supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 2016, 164, 148–155. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Pena-Rosas, J.P. Vitamin d supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2016, 1, CD008873. [Google Scholar]
- Freisling, H.; Fahey, M.T.; Moskal, A.; Ocke, M.C.; Ferrari, P.; Jenab, M.; Norat, T.; Naska, A.; Welch, A.A.; Navarro, C.; et al. Region-specific nutrient intake patterns exhibit a geographical gradient within and between european countries. J. Nutr. 2010, 140, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Bjorn Jensen, C.; Thorne-Lyman, A.L.; Vadgard Hansen, L.; Strom, M.; Odgaard Nielsen, N.; Cohen, A.; Olsen, S.F. Development and validation of a vitamin d status prediction model in danish pregnant women: A study of the danish national birth cohort. PLoS ONE 2013, 8, e53059. [Google Scholar]
- Rodriguez, A.; Santa Marina, L.; Jimenez, A.M.; Esplugues, A.; Ballester, F.; Espada, M.; Sunyer, J.; Morales, E. Vitamin d status in pregnancy and determinants in a southern european cohort study. Paediatr. Perinat. Epidemiol. 2016, 30, 217–228. [Google Scholar] [CrossRef] [PubMed]
| Variables of Interest | Vitamin D Sufficiency 1 | Vitamin D Deficiency 2 | p-Value 5 |
|---|---|---|---|
| n (%) | 75 (37) | 129 (63) | |
| 25(OH)D ng/mL, geometric mean (95% CI) | 26.1 (24.8–27.4) | 10.5 (9.7–11.5) | <0.001 |
| Light skin color 3, % | 88 | 67 | <0.05 |
| Melanin levels, median (Q1, Q3) | 32.9 (30.8, 37.2) | 34.3 (30.8, 41.8) | 0.07 |
| Age, mean (SD) | 31.1 (4.8) | 29.4 (4.8) | <0.05 |
| Week of pregnancy, median (Q1, Q3) | 9 (8, 10) | 9 (8, 10) | 0.39 |
| Parity, % nulliparous | 55 | 52 | 0.32 |
| Gravidity, % first pregnancy | 43 | 40 | 0.11 |
| BMI (kg/m2) before pregnancy, median (Q1, Q3) | 20.7 (19.7, 23.1) | 22.5 (20.4, 25.3) | <0.05 |
| BMI (kg/m2) current, median (Q1, Q3) | 21.5 (20.1, 23.9) | 22.8 (20.7, 26.2) | <0.05 |
| Country of origin, % | |||
| Switzerland and Germany | 35 | 14 | |
| North America, North Europe, Caucasus, Central Asia and New Zealand (without Switzerland and Germany) | 28 | 15 | |
| South Europe, Australia, Latin America and the Caribbean | 28 | 29 | |
| South-, East Asia and Pacific | 5 | 22 | |
| Africa and Middle East | 4 | 22 | <0.001 |
| Educational level achieved 4, % | |||
| less than compulsory education | 3 | 8 | |
| low education | 4 | 16 | |
| middle education | 35 | 33 | |
| high education | 59 | 44 | <0.05 |
| Educational level achieved of the partner 4, % | |||
| less than compulsory education | 4 | 8 | |
| low education | 3 | 14 | |
| middle education | 33 | 43 | |
| high education | 60 | 35 | 0.001 |
| Smoking status, % | |||
| Never smoker | 47 | 67 | |
| Ever smoker | 45 | 22 | |
| Current smoker | 8 | 12 | <0.05 |
| Season | |||
| Winter | 24 | 25 | |
| Spring | 19 | 23 | |
| Summer | 20 | 19 | |
| Fall | 37 | 33 | 0.90 |
| Days per week spent at least 1 h outdoor in the past half year, median (Q1, Q3) | 2 (2, 5) | 3 (2, 7) | 0.44 |
| Using sun protection in summer, % | |||
| Never | 13 | 31 | |
| Sometimes | 51 | 31 | |
| Always | 36 | 38 | <0.05 |
| Fish consumption at least once per week, % | 51 | 45 | 0.41 |
| Vitamin D supplement intake, % | 9 | 9 | 0.97 |
| Dark Skin Color | OR | 95% CI | AIC |
|---|---|---|---|
| age adjusted model | 3.25 | (1.46, 7.24) | 259 |
| age and season adjusted | 3.29 | (1.47, 7.36) | 264 |
| multivariable adjusted model 1 | 2.56 | (1.08, 6.11) | 266 |
| multivariable adjusted model 2 | 2.60 | (1.08, 6.22) | 268 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richard, A.; Rohrmann, S.; Quack Lötscher, K.C. Prevalence of Vitamin D Deficiency and Its Associations with Skin Color in Pregnant Women in the First Trimester in a Sample from Switzerland. Nutrients 2017, 9, 260. https://doi.org/10.3390/nu9030260
Richard A, Rohrmann S, Quack Lötscher KC. Prevalence of Vitamin D Deficiency and Its Associations with Skin Color in Pregnant Women in the First Trimester in a Sample from Switzerland. Nutrients. 2017; 9(3):260. https://doi.org/10.3390/nu9030260
Chicago/Turabian StyleRichard, Aline, Sabine Rohrmann, and Katharina C. Quack Lötscher. 2017. "Prevalence of Vitamin D Deficiency and Its Associations with Skin Color in Pregnant Women in the First Trimester in a Sample from Switzerland" Nutrients 9, no. 3: 260. https://doi.org/10.3390/nu9030260




