Distribution of Constituents and Metabolites of Maritime Pine Bark Extract (Pycnogenol®) into Serum, Blood Cells, and Synovial Fluid of Patients with Severe Osteoarthritis: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Study Design
2.2. Chemicals, Reagents, and Special Materials
2.3. Standard Solutions
2.4. Human Specimen for Calibration Curves
2.5. Liquid Chromatography (LC)
2.6. Mass Spectrometry (MS/MS)
2.7. Preparation of Human Serum Samples
2.8. Preparation of Human Blood Cell Samples
2.9. Preparation of Human Synovial Fluid Samples
2.10. Quantification of the Samples of the Study Participants
2.11. Statistical Analysis
3. Results
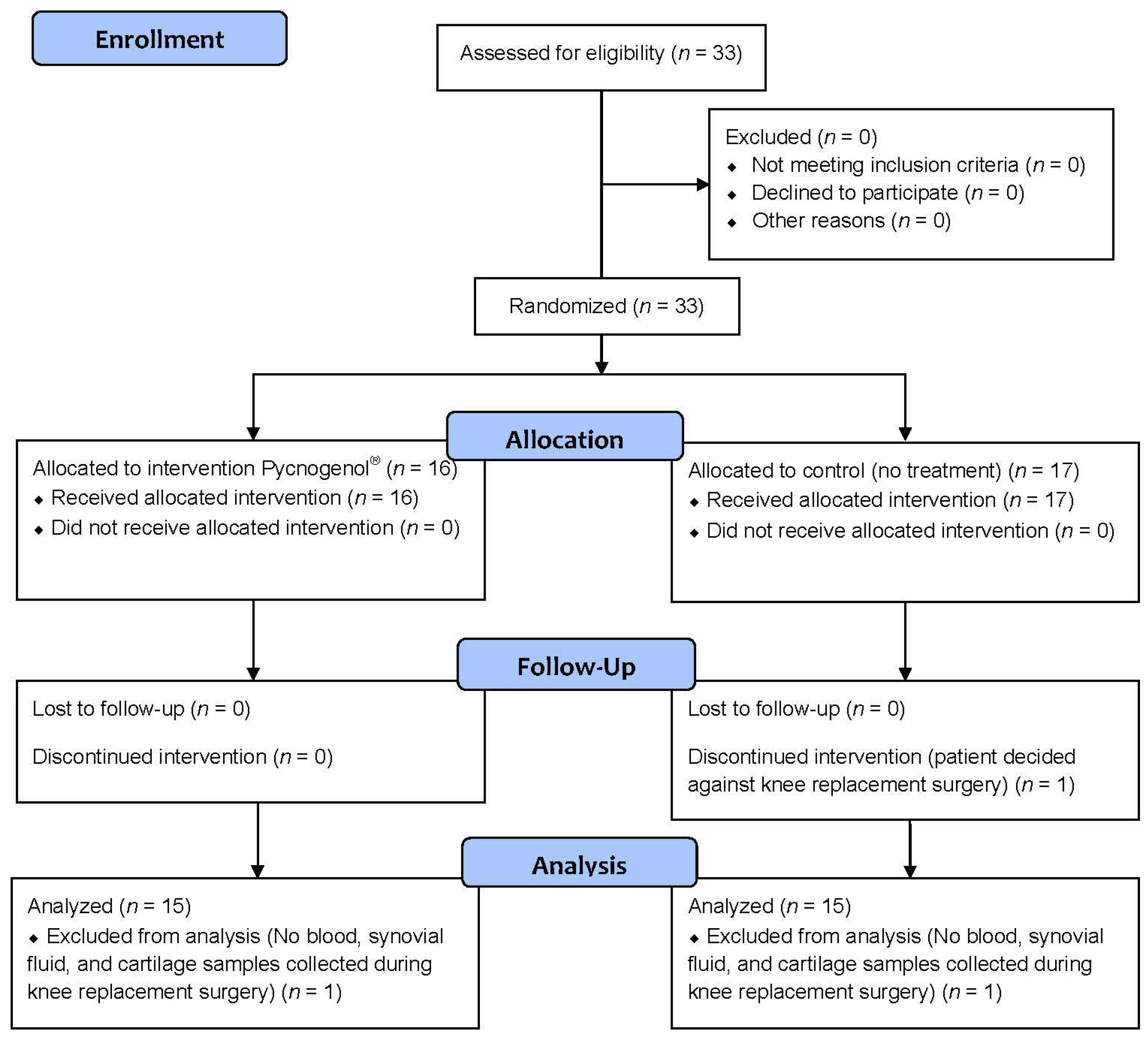
3.1. Patients and Protocol Adherence
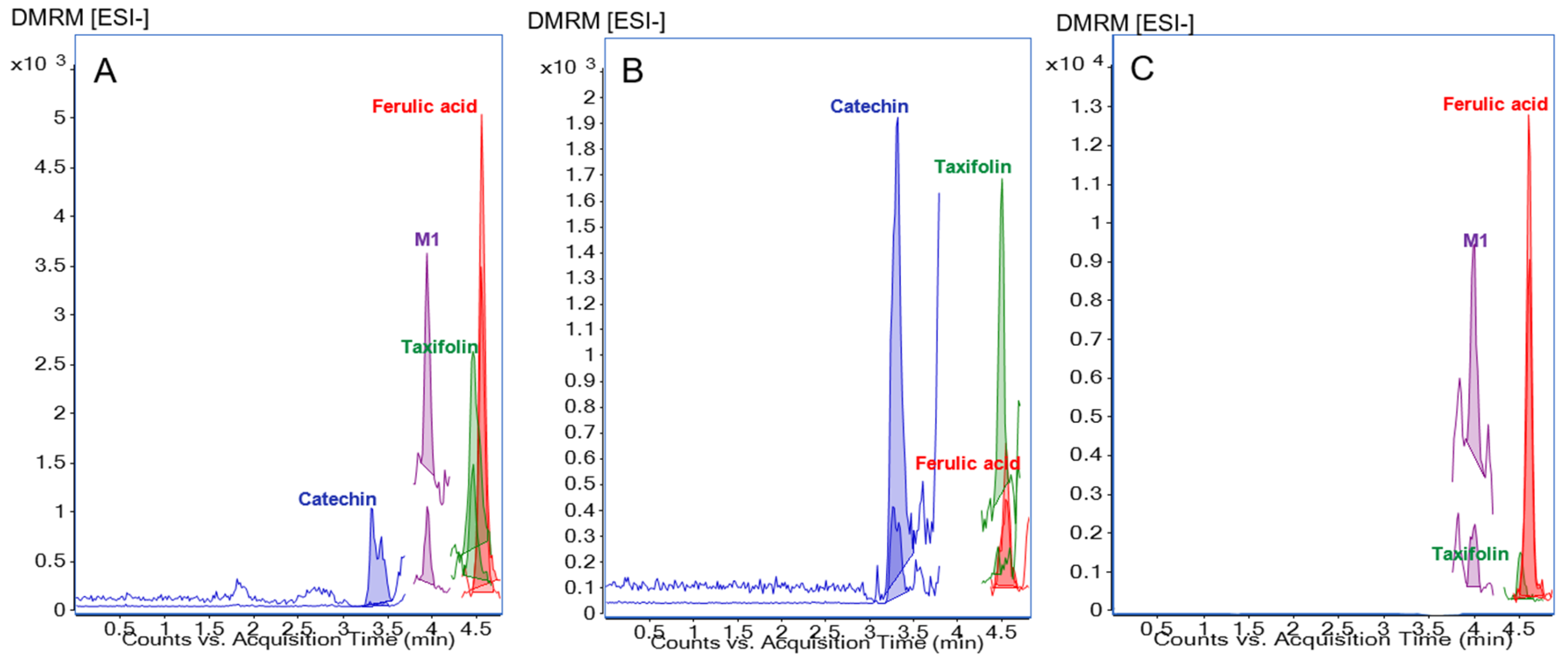
3.2. Method for Analysis of Polyphenols in Human Synovial Fluid Samples
3.3. Pycnogenol® Constituents and Metabolites in Serum Samples
3.4. Pycnogenol® Constituents and Metabolites in Blood Cell Samples
3.5. Pycnogenol® Constituents and Metabolites in Synovial Fluid Samples
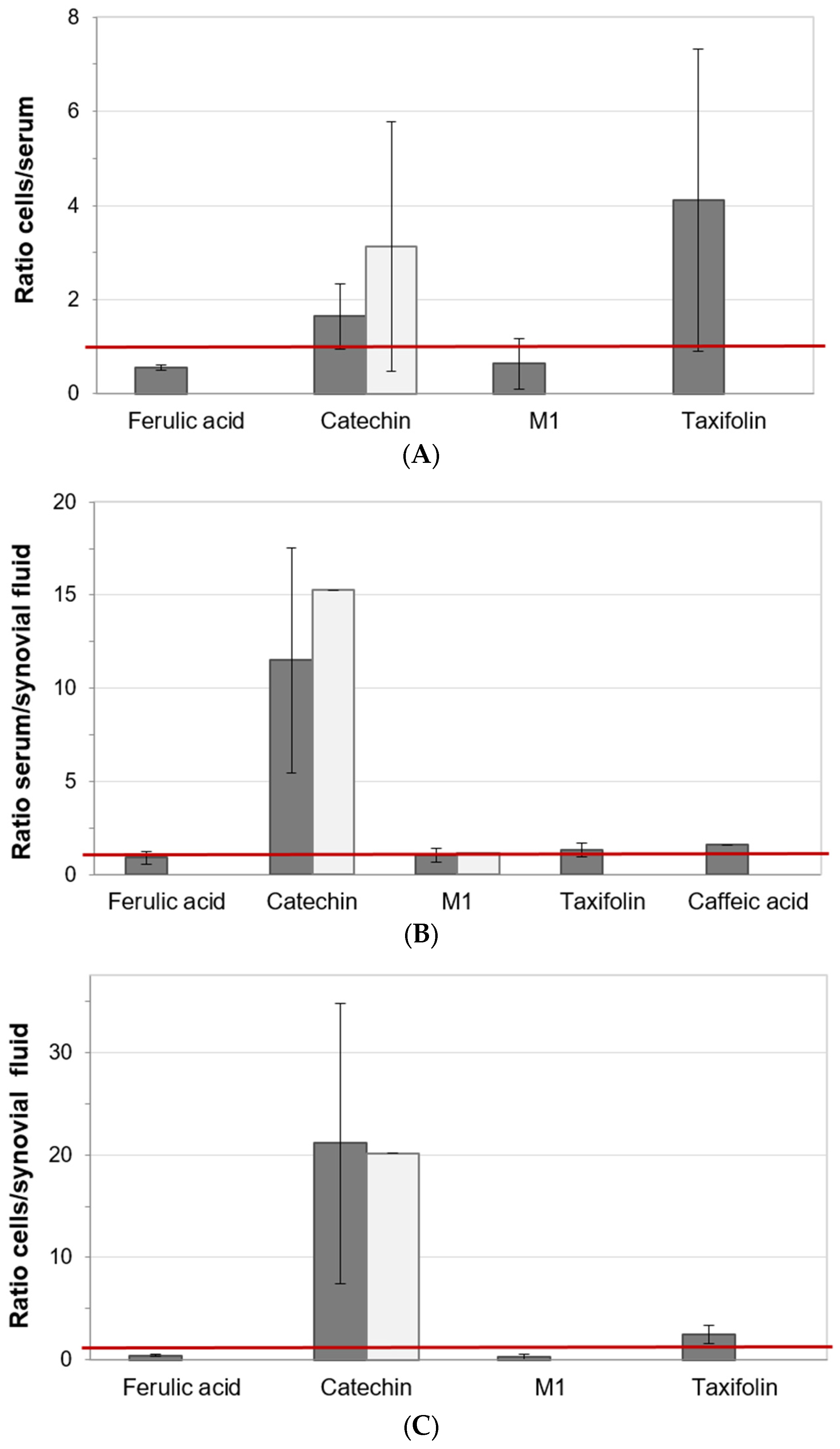
3.6. Distribution of Pycnogenol® Constituents and Metabolites between Specimen
4. Discussion
5. Conclusion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Högger, P. Nutrition-derived bioactive metabolites produced by gut microbiota and their potential impact on human health. Nutr. Med. (NUME) 2013, 1, 1. [Google Scholar]
- Düweler, K.G.; Rohdewald, P. Urinary metabolites of French maritime pine bark extract in humans. Pharmazie 2000, 55, 364–368. [Google Scholar] [PubMed]
- Grimm, T.; Skrabala, R.; Chovanova, Z.; Muchova, J.; Sumegova, K.; Liptakova, A.; Durackova, Z.; Högger, P. Single and multiple dose pharmacokinetics of maritime pine bark extract (Pycnogenol) after oral administration to healthy volunteers. BMC Clin. Pharmacol. 2006, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Jameel, K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011, 133, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. Oarsi guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Hirst-Jones, K.L.; Davidson, R.K.; Jupp, O.; Bao, Y.; MacGregor, A.J.; Donell, S.T.; Cassidy, A.; Clark, I.M. The potential for dietary factors to prevent or treat osteoarthritis. Proc. Nutr. Soc. 2014, 73, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; et al. Treatment of osteoarthritis with Pycnogenol. The SVOS (San Valentino Osteo-arthrosis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytother. Res. 2008, 22, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Cisar, P.; Jany, R.; Waczulikova, I.; Sumegova, K.; Muchova, J.; Vojtassak, J.; Durackova, Z.; Lisy, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T.; Schäfer, A.; Högger, P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2004, 36, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T.; Chovanova, Z.; Muchova, J.; Sumegova, K.; Liptakova, A.; Durackova, Z.; Högger, P. Inhibition of NF-κb activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J. Inflamm. (Lond.) 2006, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhut, K.; Högger, P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kurlbaum, M.; Mülek, M.; Högger, P. Facilitated uptake of a bioactive metabolite of maritime pine bark extract (Pycnogenol) into human erythrocytes. PLoS ONE 2013, 8, e63197. [Google Scholar] [CrossRef] [PubMed]
- Mülek, M.; Fekete, A.; Wiest, J.; Holzgrabe, U.; Mueller, M.J.; Högger, P. Profiling a gut microbiota-generated catechin metabolite’s fate in human blood cells using a metabolomic approach. J. Pharm. Biomed. Anal. 2015, 114, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mülek, M.; Högger, P. Highly sensitive analysis of polyphenols and their metabolites in human blood cells using dispersive SPE extraction and LC-MS/MS. Anal. Bioanal. Chem. 2015, 407, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Mülek, M. Distribution and Metabolism of Constituents and Metabolites of a Standardized Maritime Pine Bark Extract (Pycnogenol®) in Human Serum, Blood Cells and Synovial Fluid of Patients with Severe Osteoarthritis. Ph.D. Thesis, Julius Maximilians-Universität, Würzburg, Germany, 2015. [Google Scholar]
- Cavaliere, C.; Foglia, P.; Gubbiotti, R.; Sacchetti, P.; Samperi, R.; Lagana, A. Rapid-resolution liquid chromatography/mass spectrometry for determination and quantitation of polyphenols in grape berries. Rapid Commun. Mass Spectrom. 2008, 22, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Pagana, G.; Bourne, L.; Rimbach, G.; Natella, F.; Rice-Evans, C.; Packer, L. Ferulic acid excretion as a marker of consumption of a French maritime pine (Pinus maritima) bark extract. Free Radic. Biol. Med. 2000, 28, 1249–1256. [Google Scholar] [CrossRef]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chromatography with mass selective detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 161–172. [Google Scholar] [CrossRef]
- Highley, M.S.; de Bruijn, E.A. Erythrocytes and the transport of drugs and endogenous compounds. Pharm. Res. 1996, 13, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; Hgarbisa, S.; Zoratti, M.; Paradisi, C. Determination of quercetin and resveratrol in whole blood—Implications for bioavailability studies. Molecules 2010, 15, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Tanaka, H.; Murota, K.; Naito, M.; Terao, J. (−)-epicatechin gallate accumulates in foamy macrophages in human atherosclerotic aorta: Implication in the anti-atherosclerotic actions of tea catechins. Biochem. Biophys. Res. Commun. 2008, 374, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Mabey, T.; Honsawek, S. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Aronson, W.; Niu, Y.; Conde, F.; Lee, N.H.; Seeram, N.P.; Lee, R.P.; Lu, J.; Harris, D.M.; Moro, A.; et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J. Nutr. 2006, 136, 1839–1843. [Google Scholar] [PubMed]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104 (Suppl. 3), S67–S90. [Google Scholar] [CrossRef] [PubMed]
| Analyte | LLOQ Synovial Fluid (ng/mL) | LLOQ Serum (ng/mL) | LLOQ Blood Cells (ng/mL) |
|---|---|---|---|
| Catechin | 2.14 | 5.86 | 28.90 |
| M1 | 0.12 | 0.16 | 0.12 |
| Taxifolin | 0.08 | 0.06 | 0.12 |
| Caffeic Acid | 3.07 | 8.22 | 48.40 |
| Ferulic Acid | 1.53 | 2.74 | 0.97 |
| A | Catechin | M1 | Taxifolin | Ferulic Acid | Caffeic Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | |||||||
| Serum | P+ | 52.53 ± 18.40 | n = 15 | 0.54 ± 0.84 | n = 9 | 0.20 ± 0.12 | n = 5 | 3.02 ± 0.39 | n = 7 | 9.28 ± 0.51 | n = 3 |
| Co | 45.85 ± 39.59 | n = 15 | 1.07 ± 1.09 | n = 5 | n.d. | - | n.d. | - | 14.84 ± 1.92 | n = 3 | |
| Blood Cells | P+ | 71.18 ± 27.34 | n = 14 | 0.19 ± 0.07 | n = 12 | 0.52 ± 0.23 | n = 15 | 1.86 ± 0.36 | n = 10 | n.d. | - |
| Co | 70.48 ± 36.22 | n = 13 | 0.21 ± 0.05 | n = 5 | 0.48 ± 0.32 | n = 15 | 1.80 ± 0.85 | n = 7 | n.d. | - | |
| Synovial Fluid | P+ | 2.99 ± 0.43 | n = 4 | 0.62 ± 0.77 | n = 5 | 0.21 ± 0.03 | n = 2 | 4.29 ± 1.83 | n = 8 | 10.32 ± 3.96 | n = 7 |
| Co | 3.94 ± 1.83 | n = 2 | 0.78 ± 0.74 | n = 4 | n.d. | - | 3.04 ± 0.79 | n = 6 | 12.83 ± 8.95 | n = 10 | |
| B | Catechin | M1 | Taxifolin | Ferulic Acid | Caffeic Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | Conc. (ng/mL) ± SD | |||||||
| Serum | P+ | 48.41 ± 18.61 | n = 11 | 0.70 ± 1.02 | n = 6 | 0.20 ± 0.12 | n = 5 | 3.09 ± 0.46 | n = 5 | 9.78 | n = 1 |
| Co | 32.24 ± 17.38 | n = 8 | 0.25 ± 0.05 | n = 2 | n.d. | - | n.d. | n.d. | - | ||
| Blood Cells | P+ | 73.75 ± 29.25 | n = 11 | 0.20 ± 0.07 | n = 9 | 0.56 ± 0.19 | n = 11 | 1.85 ± 0.38 | n = 9 | n.d. | - |
| Co | 63.31 ± 31.28 | n = 7 | 0.18 ± 0.05 | n = 2 | 0.39 ± 0.16 | n = 8 | 1.69 ± 0.10 | n = 3 | n.d. | - | |
| Synovial Fluid | P+ | 3.00 ± 0.58 | n = 2 | 0.92 ± 0.93 | n = 3 | 0.21 ± 0.03 | n = 2 | 4.31 ± 2.10 | n = 6 | 10.63 ± 3.86 | n = 4 |
| Co | 2.65 | n = 1 | 0.17 ± 0.03 | n = 2 | n.d. | - | 3.16 ± 0.22 | n = 3 | 10.99 ± 5.79 | n = 4 | |
| Analytes | Conjugation Degree (%) | |||||
|---|---|---|---|---|---|---|
| Current Study | Former Investigation | |||||
| Mean | ± SD | Sample Size | Mean | ± SD | Sample Size | |
| Catechin | 54.29 | 26.77 | n = 51 | 56.50 | 27.90 | n = 5 |
| M1 | 98.34 | 4.40 | n = 30 | 100 * | ||
| Taxifolin | 96.75 | 7.23 | n = 11 | 100 * | ||
| Ferulic Acid | 90.32 | 16.58 | n = 24 | 100 * | ||
| Caffeic Acid | 80.95 | 17.95 | n = 10 | 69.40 | 11.80 | n = 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mülek, M.; Seefried, L.; Genest, F.; Högger, P. Distribution of Constituents and Metabolites of Maritime Pine Bark Extract (Pycnogenol®) into Serum, Blood Cells, and Synovial Fluid of Patients with Severe Osteoarthritis: A Randomized Controlled Trial. Nutrients 2017, 9, 443. https://doi.org/10.3390/nu9050443
Mülek M, Seefried L, Genest F, Högger P. Distribution of Constituents and Metabolites of Maritime Pine Bark Extract (Pycnogenol®) into Serum, Blood Cells, and Synovial Fluid of Patients with Severe Osteoarthritis: A Randomized Controlled Trial. Nutrients. 2017; 9(5):443. https://doi.org/10.3390/nu9050443
Chicago/Turabian StyleMülek, Melanie, Lothar Seefried, Franca Genest, and Petra Högger. 2017. "Distribution of Constituents and Metabolites of Maritime Pine Bark Extract (Pycnogenol®) into Serum, Blood Cells, and Synovial Fluid of Patients with Severe Osteoarthritis: A Randomized Controlled Trial" Nutrients 9, no. 5: 443. https://doi.org/10.3390/nu9050443
APA StyleMülek, M., Seefried, L., Genest, F., & Högger, P. (2017). Distribution of Constituents and Metabolites of Maritime Pine Bark Extract (Pycnogenol®) into Serum, Blood Cells, and Synovial Fluid of Patients with Severe Osteoarthritis: A Randomized Controlled Trial. Nutrients, 9(5), 443. https://doi.org/10.3390/nu9050443




