Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction
Abstract
:1. Introduction
2. Materials and Methods
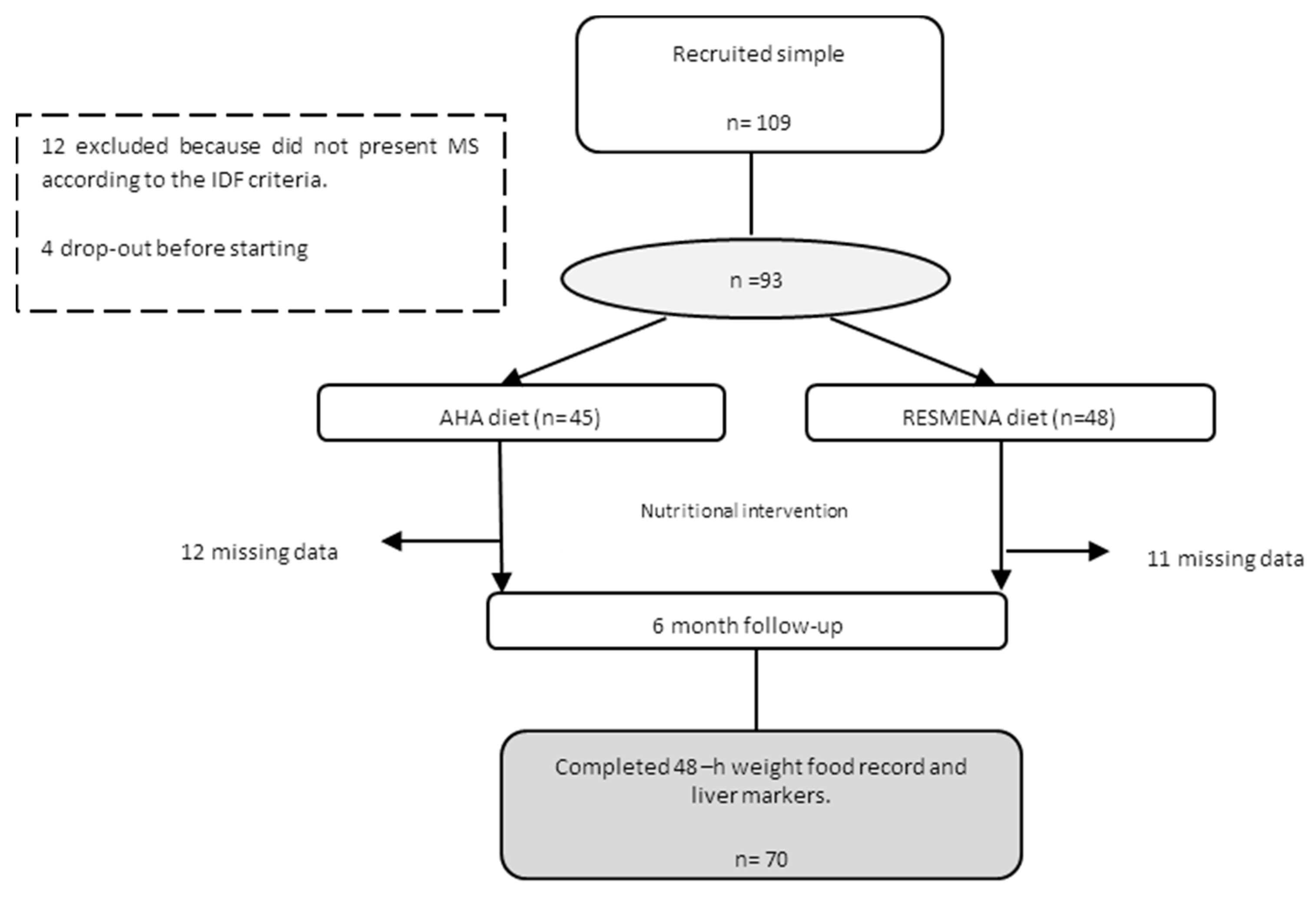
2.1. Study Design
2.2. Nutritional Intervention
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V.; LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tatsumi, H.; Hattori, M.; Sugiyama, H.; Wada, S.; Kuwahata, M.; Tanaka, S.; Kanemasa, K.; Sumida, Y.; Naito, Y.; et al. Comparisons of dietary intake in japanese with non-alcoholic fatty liver disease and type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 2016, 59, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism 2017, 68, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of dash diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Africa, J.A.; Newton, K.P.; Schwimmer, J.B. Lifestyle interventions including nutrition, exercise, and supplements for nonalcoholic fatty liver disease in children. Dig. Dis. Sci. 2016, 61, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Yang, B.; Tang, J.; Li, D. Fatty acid and non-alcoholic fatty liver disease: Meta-analyses of case-control and randomized controlled trials. Clin. Nutr. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Lopez-Legarrea, P.; de la Iglesia, R.; Lahortiga, F.; Martinez, J.A.; Zulet, M.A. Longitudinal relationship of diet and oxidative stress with depressive symptoms in patients with metabolic syndrome after following a weight loss treatment: The resmena project. Clin. Nutr. 2014, 33, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Zulet, M.A.; Bondia-Pons, I.; Abete, I.; de la Iglesia, R.; López-Legarrea, P.; Forga, L.; Navas-Carretero, S.; Martínez, J.A. The reduction of the metabolyc syndrome in navarra-spain (resmena-s) study: A multidisciplinary strategy based on chrononutrition and nutritional education, together with dietetic and psychological control. Nutr. Hosp. 2011, 26, 16–26. [Google Scholar] [PubMed]
- Kissebah, A.H.; Krakower, G.R. Regional adiposity and morbidity. Physiol. Rev. 1994, 74, 761–811. [Google Scholar] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Gastaldelli, A.; Pihan-Le Bars, F.; Natali, A.; Roussel, R.; Petrie, J.; Tichet, J.; Marre, M.; Fromenty, B.; Balkau, B.; et al. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. J. Hypertens. 2017, 35, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Hung, H.F.; Lu, C.W.; Chang, H.H.; Lee, L.T.; Huang, K.C. Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.A.; Silaghi, H.; Colosi, H.A.; Craciun, A.E.; Farcas, A.; Cosma, D.T.; Hancu, N.; Pais, R.; Georgescu, C.E. Prevalence and predictors of non-alcoholic fatty liver disease as defined by the fatty liver index in a type 2 diabetes population. Clujul Med. 2016, 89, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Giral, P.; Charlotte, F.; Bruckert, E.; Thibault, V.; Theodorou, I.; Khalil, L.; Turpin, G.; Opolon, P.; Poynard, T. Liver fibrosis in overweight patients. Gastroenterology 2000, 118, 1117–1123. [Google Scholar] [CrossRef]
- Dai, D.; Chang, Y.; Chen, Y.; Chen, S.; Yu, S.; Guo, X.; Sun, Y. Visceral adiposity index and lipid accumulation product index: Two alternate body indices to identify chronic kidney disease among the rural population in northeast china. Int. J. Environ. Res. Public Health 2016, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Ramirez, M.J.; Zulet, M.A.; Martinez, J.A. Effect of dietary restriction on peripheral monoamines and anxiety symptoms in obese subjects with metabolic syndrome. Psychoneuroendocrinology 2014, 47, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Mansego, M.L.; Zulet, M.A.; Martinez, J.A. DNA hypermethylation of the serotonin receptor type-2a gene is associated with a worse response to a weight loss intervention in subjects with metabolic syndrome. Nutrients 2014, 6, 2387–2403. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Legarrea, P.; de la Iglesia, R.; Abete, I.; Bondia-Pons, I.; Navas-Carretero, S.; Forga, L.; Martinez, J.A.; Zulet, M.A. Short-term role of the dietary total antioxidant capacity in two hypocaloric regimes on obese with metabolic syndrome symptoms: The resmena randomized controlled trial. Nutr. Metab. 2013, 10, 1743–7075. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, L.; Carson, J.A.; Appel, L.J.; Burke, L.E.; Economos, C.; Karmally, W.; Lancaster, K.; Lichtenstein, A.H.; Johnson, R.K.; Thomas, R.J.; et al. Recommended dietary pattern to achieve adherence to the american heart association/american college of cardiology (aha/acc) guidelines: A scientific statement from the american heart association. Circulation 2016, 134, e505–e529. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Banerjee, R.; Rial, B.; Arlt, W.; Adiels, M.; Boren, J.; Marinou, K.; Fisher, C.; Mostad, I.L.; Stratton, I.M.; et al. Menopausal status and abdominal obesity are significant determinants of hepatic lipid metabolism in women. J. Am. Heart. Assoc. 2015, 4, 002258. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Rettig, I.; Staiger, H.; Machann, J.; Schick, F.; Scheja, L.; Gastaldelli, A.; Bugianesi, E.; Peter, A.; Schulze, M.B.; Fritsche, A.; Häring, H.U.; Stefan, N. An extended fatty liver index to predict non-alcoholic fatty liver disease. Diabetes Metab. 2017, 12, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Lattuada, G.; Ragogna, F.; Garancini, M.P.; Crosignani, P.; Villa, M.; Bosi, E.; Ruotolo, G.; Piemonti, L.; Perseghin, G. Fatty liver index and mortality: The cremona study in the 15th year of follow-up. Hepatology 2011, 54, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Louis, S.; Schnitzer, A.; Volynets, V.; Rings, A.; Basrai, M.; Bischoff, S.C. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017, 105, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Caviglia, G.P.; Abate, M.L.; Vanni, E.; Mezzabotta, L.; Touscos, G.A.; Olivero, A.; Marengo, A. Cytokeratin 18-aspartate396 apoptotic fragment for fibrosis detection in patients with non-alcoholic fatty liver disease and chronic viral hepatitis. Dig. Liver. Dis. 2016, 48, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The triglyceride and glucose index (tyg) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 017–0409. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Patidar, K.R.; Boyett, S.; Luketic, V.A.; Puri, P.; Sanyal, A.J. Performance of non-invasive models of fibrosis in predicting mild to moderate fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2016, 36, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.F.; Lai, M. Nutrition interventions for chronic liver diseases and nonalcoholic fatty liver disease. Med. Clin. N. Am. 2016, 100, 1303–1327. [Google Scholar] [CrossRef] [PubMed]
- Raszeja-Wyszomirska, J.; Szymanik, B.; Ławniczak, M.; Kajor, M.; Chwist, A.; Milkiewicz, P.; Hartleb, M. Validation of the bard scoring system in polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol. 2010, 10, 10–67. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the american association for the study of liver diseases, american college of gastroenterology, and the american gastroenterological association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 2, 13435. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of nafld with diet, physical activity and exercise. J. Hepatol. 2017, 22, 32052–32054. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; van der, A.D.; Boshuizen, H.C.; Forouhi, N.G.; Wareham, N.J.; Halkjaer, J.; Tjonneland, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; et al. Dietary fiber and subsequent changes in body weight and waist circumference in european men and women. Am. J. Clin. Nutr. 2010, 91, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ratziu, V.; Oren, R. Nutrition and physical activity in nafld: An overview of the epidemiological evidence. World J. Gastroenterol. 2011, 17, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.A.; Horsmans, Y.; Lambert, P.; Danse, E.; Delzenne, N.M. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: Results of a pilot study. Eur. J. Clin. Nutr. 2005, 59, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Cotrim, H.P.; Siqueira, A.C.; Floriano, S. Non alcoholic fatty liver disease: Treatment with soluble fibres. Arq. Gastroenterol. 2007, 44, 350–352. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Okuma, K. Determination of insoluble, soluble, and total dietary fiber (codex definition) by enzymatic-gravimetric method and liquid chromatography: Collaborative study. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary fiber and the human gut microbiota: Application of evidence mapping methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 6, 1290756. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.D.; Laaksonen, D.E. Dietary fibers: Current trends and health benefits in the metabolic syndrome and type 2 diabetes. Arq. Bras. Endocrinol. Metabol. 2009, 53, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, H.; Askari, G.; Siassi, F.; Feizi, A.; Sotoudeh, G. Intake of nutrients, fiber, and sugar in patients with nonalcoholic fatty liver disease in comparison to healthy individuals. Int. J. Prev. Med. 2016, 7, 2008–7802. [Google Scholar]

| n = 70 | AHA (n = 33) | RESMENA (n = 37) | Δp-Value | ||
|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | ||
| Body composition | |||||
| Weight (kg) | 99.2 (19) | 91.8 (17) ** | 100.4 (16) | 92.6 (16) ** | 0.155 |
| BMI | 35.8 (4) | 32.9 (4) ** | 35.7 (4) | 32.2 (4) ** | 0.153 |
| Conicity Index | 1.3 | 1.2 ** | 1.3 | 1.2 ** | 0.083 |
| WC (cm) | 110.2 (13) | 103.3 (13) ** | 111.8 (12) | 102.4 (2) ** | 0.441 |
| Total Fat Mass (%) | 41.7 (6) | 38.5 (7) ** | 42.3 (6) | 38.2 (7) ** | 0.191 |
| Android FM (kg) | 4.5 (1.3) | 3.9 (1.8) | 4.8 (1.2) | 3.6 (1) ** | 0.151 |
| IDF criteria | |||||
| Glucose (mg/dL) | 121.5 (33) | 115.4 (24) ** | 123.8 (37) | 111.7 (29) ** | 0.559 |
| TG (mg/dL) | 175.3 (90) | 139.6 (87) * | 194.2(122) | 145.4 (83) ** | 0.863 |
| SBP (mmHg) | 150 (17) | 137 (13) ** | 147 (21) | 135 (15) ** | 0.654 |
| DBP (mmHg) | 85 (9) | 78 (10) * | 84 (9) | 78 (10) ** | 0.436 |
| HDL-c (mg/dL) | 46.3 (9) | 51.5 (11) ** | 43.3 (9) | 46.2 (10) | 0.042 |
| LDL-c (mg/dL) | 140.4 (36) | 140.1(36) ** | 136.7 (41) | 136.3 (41) * | 0.570 |
| TC (mg/dL) | 221.8 (39) | 226.5 (40) | 218.5 (47) | 213.3 (39) | 0.332 |
| Homa-index | 4.6 (3.7) | 2.6 (2.9) ** | 4.4 (3.0) | 2.3 (1.7) ** | 0.820 |
| Cardiometabolic risk factors | |||||
| TyG index | 9.1 (0.5) | 8.8 (0.5) * | 9.1 (0.7) | 8.8 (0.6) ** | 0.487 |
| Waist to height ratio | 0.6 (0.0) | 0.6 (0.1) ** | 0.6 (0.1) | 0.6 (0.1) ** | 0.095 |
| LDL-c/HDL-c | 3.0 (1.0) | 3.5 (0.7) | 3.1(0.9) | 3.7 (0.8) | 0.432 |
| Diabetes % | 74.4 | 65.7 | 74.4 | 52.7 * | 0.389 |
| Hypertension % | 93 | 43 | 82.9 | 52 * | 0.323 |
| Macronutrient intake | |||||
| Total Energy (kcal) | 2102 (450) | 1529 (316) ** | 2276 (565) | 1548 (381) ** | 0.527 |
| Carbohydrate (g) | 186.5 (58) | 140.4 (45) ** | 201.3 (65) | 138.2 (37) ** | 0.098 |
| Proteins (g) | 93.7 (21) | 66.6 (18) ** | 95.7 (20) | 78.2 (17) ** | 0.198 |
| Lipids (g) | 97.1 (27) | 69.4(17) ** | 101.3 (29) | 66.5 (20) ** | 0.231 |
| Fiber consumption (g/1000 kcal) | |||||
| Total fiber | 18.7 (10) | 20.7 (8) * | 21.8 (7) | 26.0 (7) * | 0.585 |
| Soluble fiber | 2.1 (0.9) | 2.1 (0.8) | 2.0 (0.6) | 2.5 (0.9) | 0.657 |
| Insoluble fiber | 4.3 (3.2) | 4.5 (2.1) | 3.2 (1) | 5.6 (1.5) * | 0.190 |
| n = 70 | AHA (n = 33) | RESMENA (n = 37) | Δp-Value | ||
|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | ||
| Hepatic measurements | |||||
| ALT (U/L) | 37.4 (21) | 25.2 (8) * | 29.4 (11) | 22.8 (8) ** | 0.642 # |
| AST (U/L) | 25.1 (10) | 23.3 (6) | 21.7 (6) | 20.2 (4) ** | 0.126 # |
| GGT (U/L) | 40.2 (24) | 30.3 (17) * | 41.4 (26) | 27.1 (14) ** | 0.135 |
| M65 (U/L) | 307.8 (198) | 217.6 (108) ** | 259.4 (135) | 230.9 (91) | 0.125 |
| M30 (U/L) | 200.3 (125) | 128.7 (51) * | 156.1 (99) | 103.5 (35) * | 0.304 |
| NAFLD_LFS | 2.2 (2.6) | 0.4 (2.2) ** | 1.9 (1.9) | −0.09 (1.9) ** | 0.429 |
| FLI | 84.6 (17) | 68.4 (25) ** | 85.2 (16) | 69.4 (25) ** | 0.793 |
| HSI | 49.2 (6) | 44.1 (5) ** | 48.4 (5) | 43.1 (5) ** | 0.759 |
| VAI | 2.8 (1.9) | 2.0 (1.7) ** | 3.6 (2.9) | 2.3 (15) ** | 0.920 |
| BARD | 2.5 (0.9) | 2.8 (1.1) | 2.6 (1.2) | 2.8 (1) | 0.713 |
| BAAT | 2.1 (0.7) | 1.6 (0.7) ** | 2.0 (0.6) | 1.7 (0.7) ** | 0.265 |
| n = 70 | Total Fiber (g/Day) | Insoluble Fiber (g/Day) | Soluble Fiber (g/Day) | Fruit Fiber (g/Day) | ||||
|---|---|---|---|---|---|---|---|---|
| <39.1 | ≥39.1 | <7.5 | ≥7.5 | <3.2 | ≥3.2 | <8.8 | ≥8.8 | |
| ALT (U/L) | 26.3 (9) | 23.3 (8) | 26.2 (9) | 23.4 (8) | 26.4 (9) | 23.5 (7) | 27.2 (8) | 22.4 (8) # |
| AST (U/L) | 21.5 (6) | 21. 6(6) | 22.8 (5) | 21.6 (6) | 22.6 (5) | 21.4 (6) | 23.8 (5) | 19.3 (5) # |
| GGT (U/L) | 29.4 (17) | 28.2 (15) | 28.1 (14) | 29.5 (18) | 28.1 (16) | 28.2 (17) | 34.1 (18) | 23.5 (11) * |
| M65 (U/L) | 218.6 (83) | 232.1 (118) | 223.1 (14) | 227.4 (20) | 231.4 (15) | 220.6 (20) | 236.5 (22) | 213.4 (12) |
| M30 (U/L) | 120.2 (50) | 109.4 (45) | 123.2 (9) | 107.5 (7) | 124.3 (9) | 107.4 (6) | 121.2 (8) | 113.6 (7) |
| FLI | 75.3 (17) | 57.2 (29) | 74. 4 (21) | 60.6 (27) * | 71.1 (25) | 62.2 (25) | 72.4 (23) | 65.8 (26) |
| HSI | 45.2 (5) | 42.4 (6) | 4.27 (3) | 41.2 (4) * | 44.6 (5) | 42.7 (6) | 43.6 (6) | 44.1 (5) |
| VAI | 0.5 (2) | −0.1 (1) | 2.4 (1) | 1.7 (1) | 2.1 (1.8) | 1.9 (1.4) | 2.4 (2.1) | 1.9 (1.1) |
| NAFLD_LFS | 2.6 (1) | 2.8 (1) | 0.9 (2) | −0.5 (1) * | 0.2 (2.4) | 0.1 (1.7) | 0.8 (2.2) | −0.3 (1.7) |
| Δ Fatty Liver Index | β | p | Pmodel | R-Adjusted |
|---|---|---|---|---|
| Δ Fiber Fruits | 0.5769 | 0.025 | 0.0265 | 0.1168 |
| Age | −0.4860 | 0.03 | - | - |
| Δ Total energy (kcal) | −0.0691 | 0.149 | - | - |
| Δ MET | 0.0003 | 0.461 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantero, I.; Abete, I.; Monreal, J.I.; Martinez, J.A.; Zulet, M.A. Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction. Nutrients 2017, 9, 667. https://doi.org/10.3390/nu9070667
Cantero I, Abete I, Monreal JI, Martinez JA, Zulet MA. Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction. Nutrients. 2017; 9(7):667. https://doi.org/10.3390/nu9070667
Chicago/Turabian StyleCantero, Irene, Itziar Abete, J. Ignacio Monreal, J. Alfredo Martinez, and M. Angeles Zulet. 2017. "Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction" Nutrients 9, no. 7: 667. https://doi.org/10.3390/nu9070667






