Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Data Collection and Quality Evaluation
2.4. Statistical Analyses
3. Results
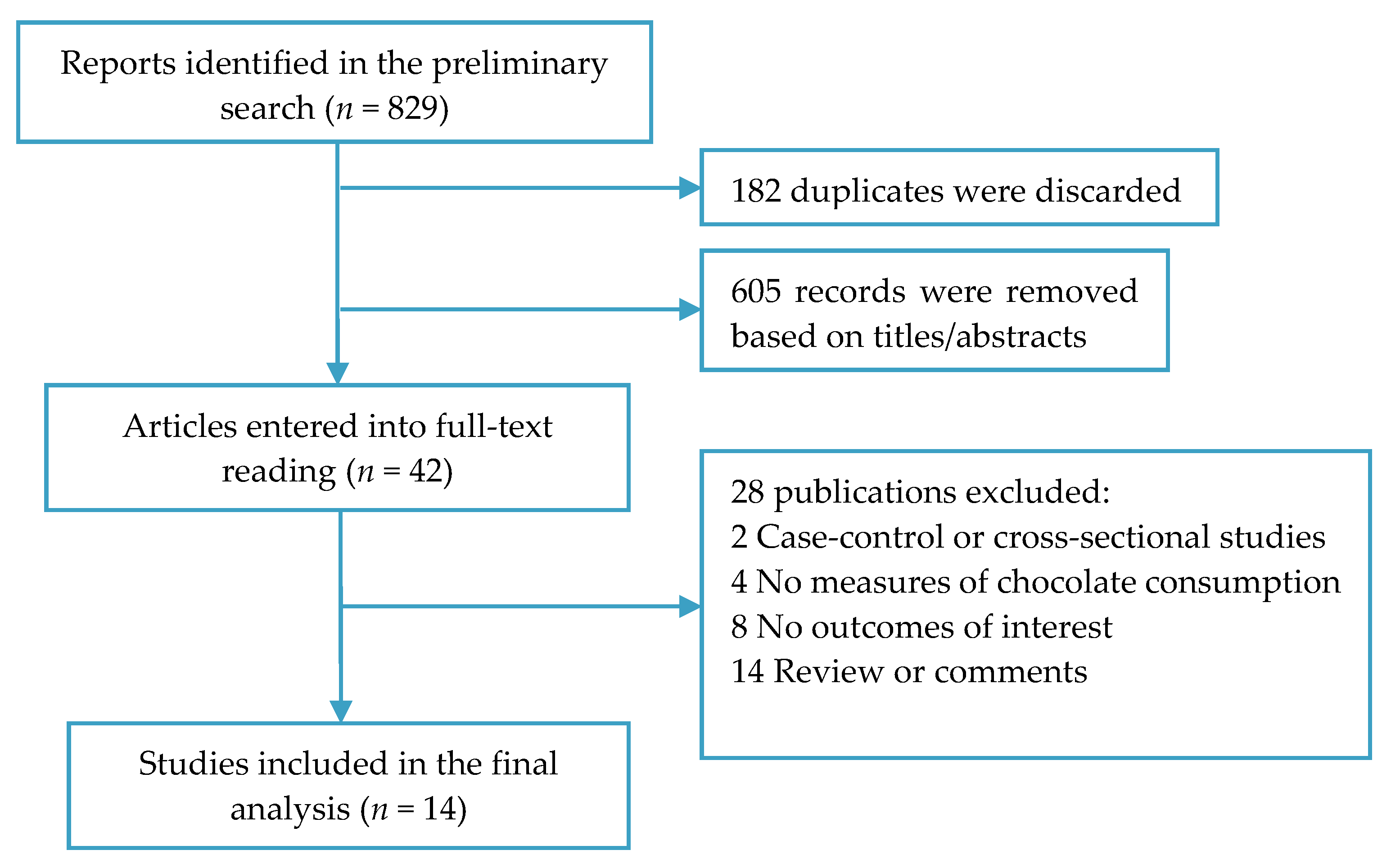
3.1. Study Search
3.2. Characteristics of Studies
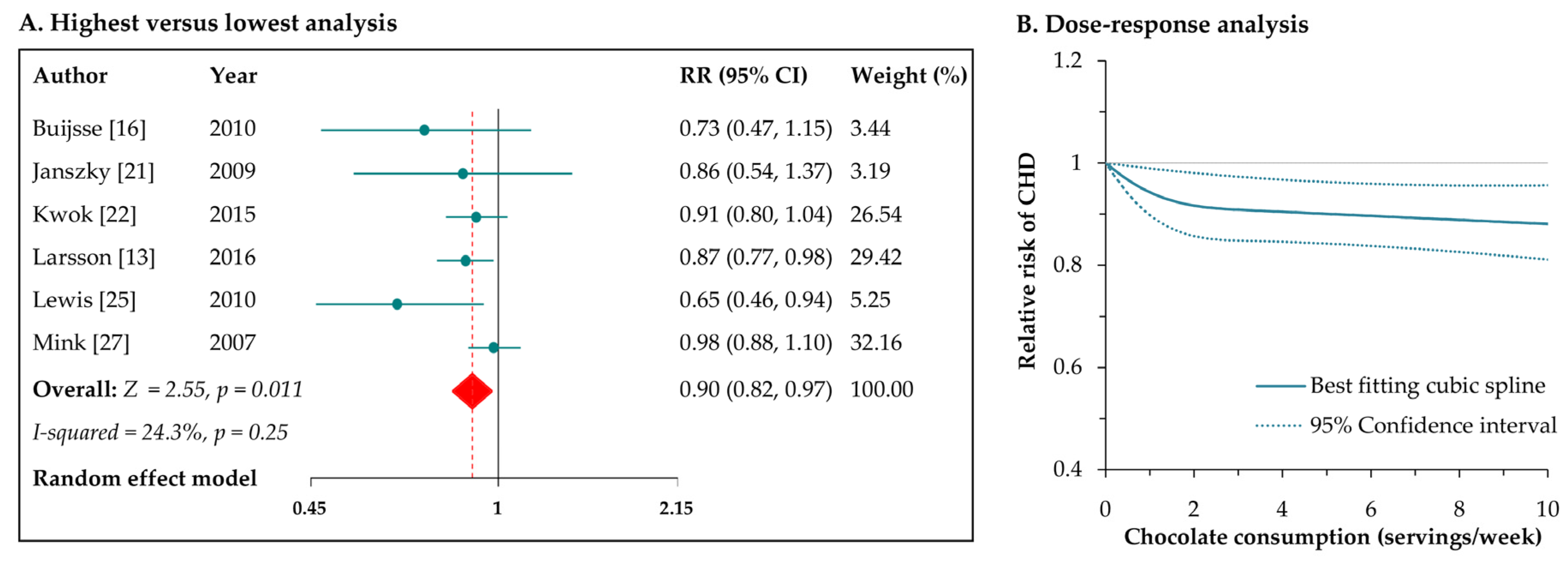
3.3. Chocolate Consumption and Risk of CHD
3.4. Chocolate Consumption and Risk of Stroke
3.5. Chocolate Consumption and Risk of Diabetes
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar]
- Bansilal, S.; Castellano, J.M.; Fuster, V. Global burden of CVD: Focus on secondary prevention of cardiovascular disease. Int. J. Cardiol. 2015, 201, S1–S7. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Murga, L.; Tarin, J.J.; Garcia-Perez, M.A.; Cano, A. The impact of chocolate on cardiovascular health. Maturitas 2011, 69, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, I.; Li, A.; Manson, J.E.; Sesso, H.D.; Wang, L.; Liu, S. Cocoa flavanol intake and biomarkers for cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2016, 146, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Berends, L.M.; van der Velpen, V.; Cassidy, A. Flavan-3-ols, theobromine, and the effects of cocoa and chocolate on cardiometabolic risk factors. Curr. Opin. Lipidol. 2015, 26, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 April 2017).
- Larsson, S.C.; Crippa, A.; Orsini, N.; Wolk, A.; Michaelsson, K. Milk consumption and mortality from all causes, cardiovascular disease, and cancer: A systematic review and meta-analysis. Nutrients 2015, 7, 7749–7763. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Larsson, S.C.; Akesson, A.; Gigante, B.; Wolk, A. Chocolate consumption and risk of myocardial infarction: A prospective study and meta-analysis. Heart 2016, 102, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; White, I.R.; Thompson, S.G. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat. Med. 2010, 29, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Weikert, C.; Drogan, D.; Bergmann, M.; Boeing, H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur. Heart J. 2010, 31, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Dearborn, P.; Robbins, M. Habitual chocolate intake and type 2 diabetes mellitus in the Maine-Syracuse Longitudinal Study: (1975–2010): Prospective observations. Appetite 2017, 108, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Iso, H.; Yamagishi, K.; Sawada, N.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Chocolate consumption and risk of stroke among men and women: A large population-based, prospective cohort study. Atherosclerosis 2017, 260, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A. Chocolate intake and diabetes risk. Clin. Nutr. 2015, 34, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Manson, J.E.; Tinker, L.; Neuhouser, M.L.; Garcia, L.; Vitolins, M.Z.; Phillips, L.S. Chocolate intake and diabetes risk in postmenopausal American women. Eur. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Janszky, I.; Mukamal, K.J.; Ljung, R.; Ahnve, S.; Ahlbom, A.; Hallqvist, J. Chocolate consumption and mortality following a first acute myocardial infarction: The Stockholm Heart Epidemiology Program. J. Intern. Med. 2009, 266, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Boekholdt, S.M.; Lentjes, M.A.; Loke, Y.K.; Luben, R.N.; Yeong, J.K.; Wareham, N.J.; Myint, P.K.; Khaw, K.T. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart 2015, 101, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Chocolate consumption and risk of stroke in women. J. Am. Coll. Cardiol. 2011, 58, 1828–1829. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Chocolate consumption and risk of stroke: A prospective cohort of men and meta-analysis. Neurology 2012, 79, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Prince, R.L.; Zhu, K.; Devine, A.; Thompson, P.L.; Hodgson, J.M. Habitual chocolate intake and vascular disease: A prospective study of clinical outcomes in older women. Arch. Intern. Med. 2010, 170, 1857–1858. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Petrone, A.B.; Sesso, H.D.; Gaziano, J.M.; Djousse, L. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am. J. Clin. Nutr. 2015, 101, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [PubMed]
- Oba, S.; Nagata, C.; Nakamura, K.; Fujii, K.; Kawachi, T.; Takatsuka, N.; Shimizu, H. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br. J. Nutr. 2010, 103, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Castell, M.; Saldana-Ruiz, S.; Rodriguez-Lagunas, M.J.; Franch, A.; Perez-Cano, F.J. Second International Congress on Chocolate and Cocoa in Medicine Held in Barcelona, Spain, 25–26th September 2015. Nutrients 2015, 7, 9785–9803. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Luscher, T.F. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Fakler, P.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2017, 4, CD008893. [Google Scholar] [PubMed]
- Rull, G.; Mohd-Zain, Z.N.; Shiel, J.; Lundberg, M.H.; Collier, D.J.; Johnston, A.; Warner, T.D.; Corder, R. Effects of high flavanol dark chocolate on cardiovascular function and platelet aggregation. Vascul. Pharmacol. 2015, 71, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Fernandez-Millan, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Yao, S.; Wan, J.; Gan, X. Chocolate Consumption and Risk of Heart Failure: A Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]


| Study | Population | N | Age | Ascertainments | Country | FU (Years) | Included Outcomes | Adjusted Factors | |
|---|---|---|---|---|---|---|---|---|---|
| Exposure | Outcome | ||||||||
| Buijsse 2010 [16] | General population | 19,357 | 35–65 | FFQ | ICD-10 code | Germany | 8.1 | CHD and stroke | Age, sex, smoking, drinking, BMI, diabetes, waist circumstance, employment status, physical activity, education, dietary energy, and food groups |
| Crichton 2017 [17] | Subjects without psychiatric illness and alcoholism | 590 | 62 (mean) | FFQ | Standard assay methods | US | 4.7 | Diabetes | Age, sex, race, education, BMI, cholesterol level, hypertension, C-reactive protein, physical activity, grains, coffee, and red wine |
| Dong 2017 [18] | Subjects without CVD, diabetes, and cancer | 84,597 | 44–76 | FFQ | Predefined diagnostic criteria | Japan | 12.9 | Stroke | Age, area, BMI, dietary energy, smoking, drinking, sports, occupation, medication use, and food groups |
| Greenberg 2015 [19] | Community-based adults | 7802 | 45–64 | FFQ | Medical records of diabetic medication | US | 13.3 | Diabetes | Age, sex, race, smoking, drinking, physical activity, dietary energy, Keys Index of Dietary Quality, family history of diabetes, and educational and occupational levels |
| Greenberg 2017 [20] | Postmenopausal women | 92,678 | 50–79 | FFQ | Self-report of diabetic medication usage | US | 13.1 | Diabetes | Age, race, WHI Studyarm, physical activity, smoking, family history of diabetes, coffee, non-chocolate energy intake, Alternative Modified Health Eating Index, education, family income, and physical functional ability |
| Janszky 2009 [21] | Non-diabetic patients with post MI | 1169 | 45–70 | Self-report | ICD-9 and 10 codes | Sweden | 8.7 | CHD and stroke | Age, sex, smoking, drinking, BMI, physical activity, coffee intake, education, and sweet score |
| Kwok 2015 [22] | General population | 20,951 | 59 (mean) | FFQ | ICD-10 code | UK | 11.9 | CHD and stroke | Age, sex, smoking, drinking, physical activity, dietary energy, diabetes, BMI, systolic BP, and cholesterol level |
| Larsson 2011 [23] | Women with no history of CVDs, diabetes, and cancer | 33,372 | 49–83 | FFQ | ICD-10 code | Sweden | 10.4 | Stroke | Age, smoking, drinking, BMI, education, physical activity, aspirin use, dietary energy, food groups, and history of hypertension, MI, and AF |
| Larsson 2012 [24] | General male population | 37,103 | 45–79 | FFQ | ICD-10 code | Sweden | 10.2 | Stroke | Age, smoking, drinking, BMI, education, physical activity, aspirin use, dietary energy, food groups, and history of hypertension, MI, and AF |
| Larsson 2016 [13] | Subjects without CVDs | 67,640 | 45–83 | FFQ | ICD-10 code | Sweden | 13 | CHD | Age, smoking, drinking, BMI, education, physical activity, exercise, aspirin use, dietary energy, food groups, and history of hypertension, MI, and AF |
| Lewis 2010 [25] | Older women | 1216 | NA | FFQ | ICD-10 code | Australia | 9.5 | CHD | Age, socioeconomic status, dietary energy, and BMI |
| Matsumoto 2015 [26] | Male physicians | 18,235 | 40–84 | FFQ | Self-report validated by medical records | US | 9.2 | Diabetes | Age, cohort status, smoking, drinking, exercise, BMI, dietary energy, and food groups |
| Mink 2007 [27] | Women without heart disease | 34,489 | 55–69 | FFQ | ICD-9 code | US | 16 | CHD and stroke | Age, smoking, dietary energy, marital status, education, BP, diabetes, BMI, waist-to-hip ratio, physical activity, and estrogen use |
| Oba 2010 [28] | Men and women without CVDs and cancer | 13,540 | ≤70 | FFQ | Self-reports | Japan | 10 | Diabetes | Age, smoking, drinking, dietary energy, BMI, physical activity, education, fat intake, women’s menopausal status |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Li, X.; Jin, Y.; Lu, J. Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 688. https://doi.org/10.3390/nu9070688
Yuan S, Li X, Jin Y, Lu J. Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies. Nutrients. 2017; 9(7):688. https://doi.org/10.3390/nu9070688
Chicago/Turabian StyleYuan, Sheng, Xia Li, Yalei Jin, and Jinping Lu. 2017. "Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies" Nutrients 9, no. 7: 688. https://doi.org/10.3390/nu9070688
APA StyleYuan, S., Li, X., Jin, Y., & Lu, J. (2017). Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies. Nutrients, 9(7), 688. https://doi.org/10.3390/nu9070688




