Anti-Obesity Effect of the Above-Ground Part of Valeriana dageletiana Nakai ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of the Extract
2.2. 3T3-L1 Cell Culture and Treatment
2.3. Oil Red O Staining and Determination of Lipid Content
2.4. Cell Viability Assay
2.5. Study of Animals and Their Diets
2.6. Collection of Serum and Tissue Samples
2.7. Biochemical Analysis
2.8. Histological Analysis
2.9. RNA Extraction, cDNA Synthesis and Real-Time PCR
2.10. NMR-Based Hepatic Metabolomics
2.11. Statistical Analysis
3. Results
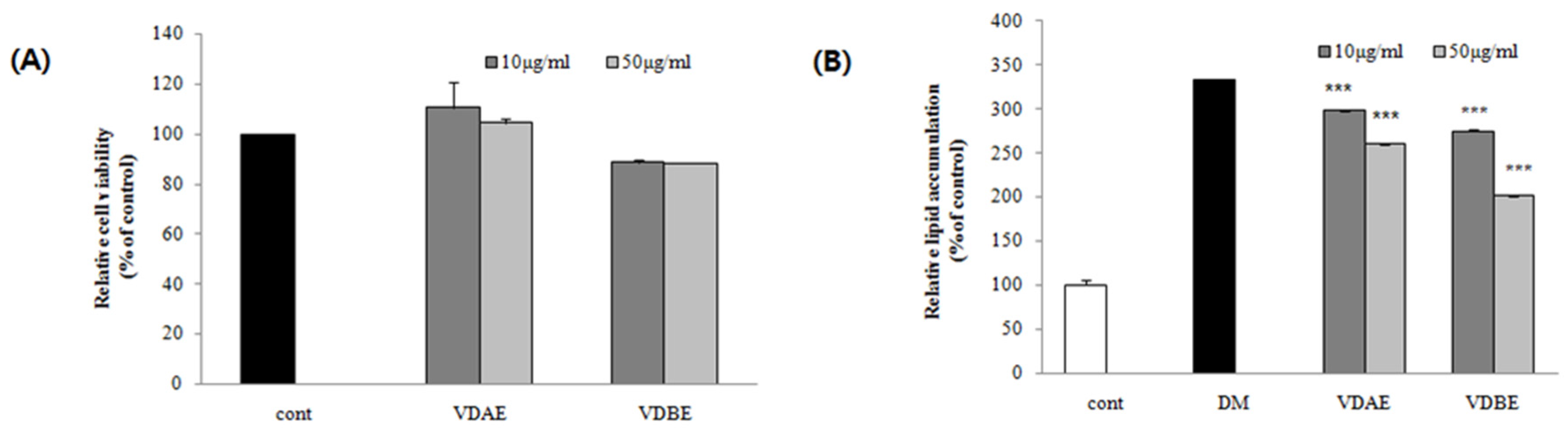
3.1. Effect of VD Extracts on Cell Viability in Pre-Adipocytes
3.2. Inhibitory Effects of VD Extracts on Lipid Accumulation in 3T3-L1 Cells
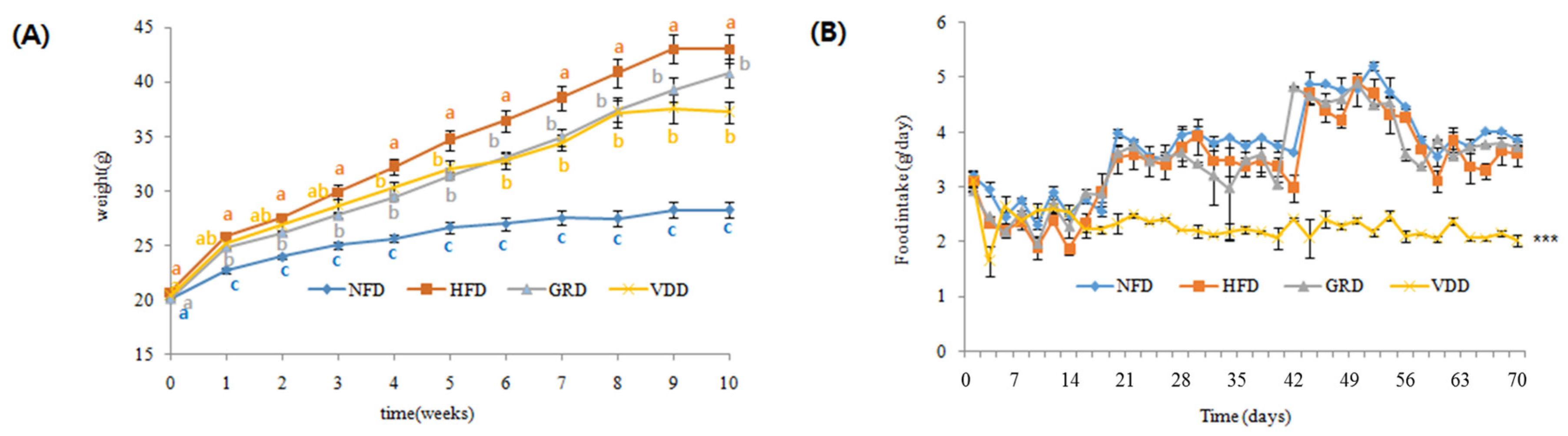
3.3. Changes in Body Weight, Food Intake, and Food Efficiency Ratio (FER)
3.4. Measurement of Lipid Profiles and Potential Toxicity in Serum
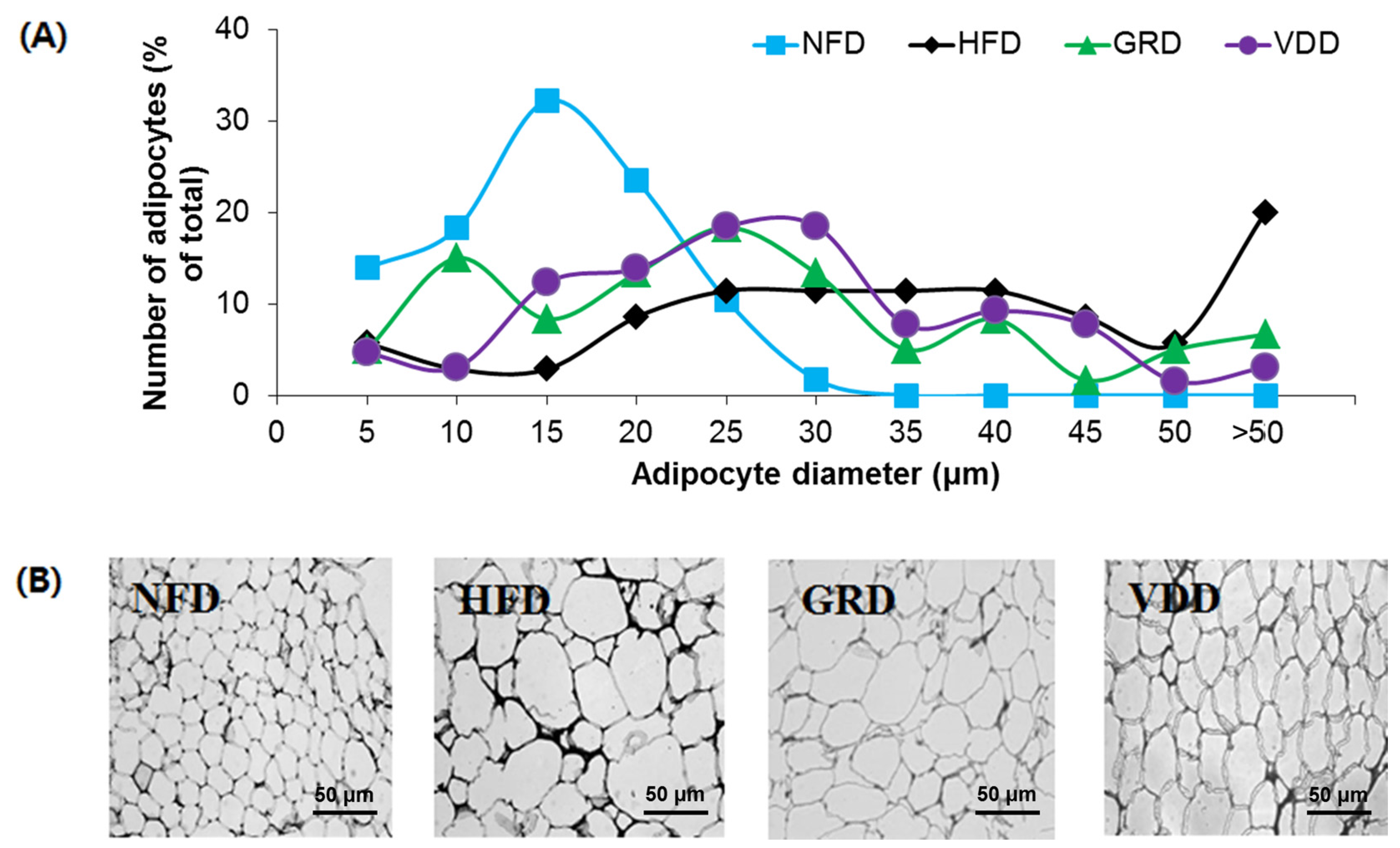
3.5. Changes in Weight and Morphology of Adipose Tissue
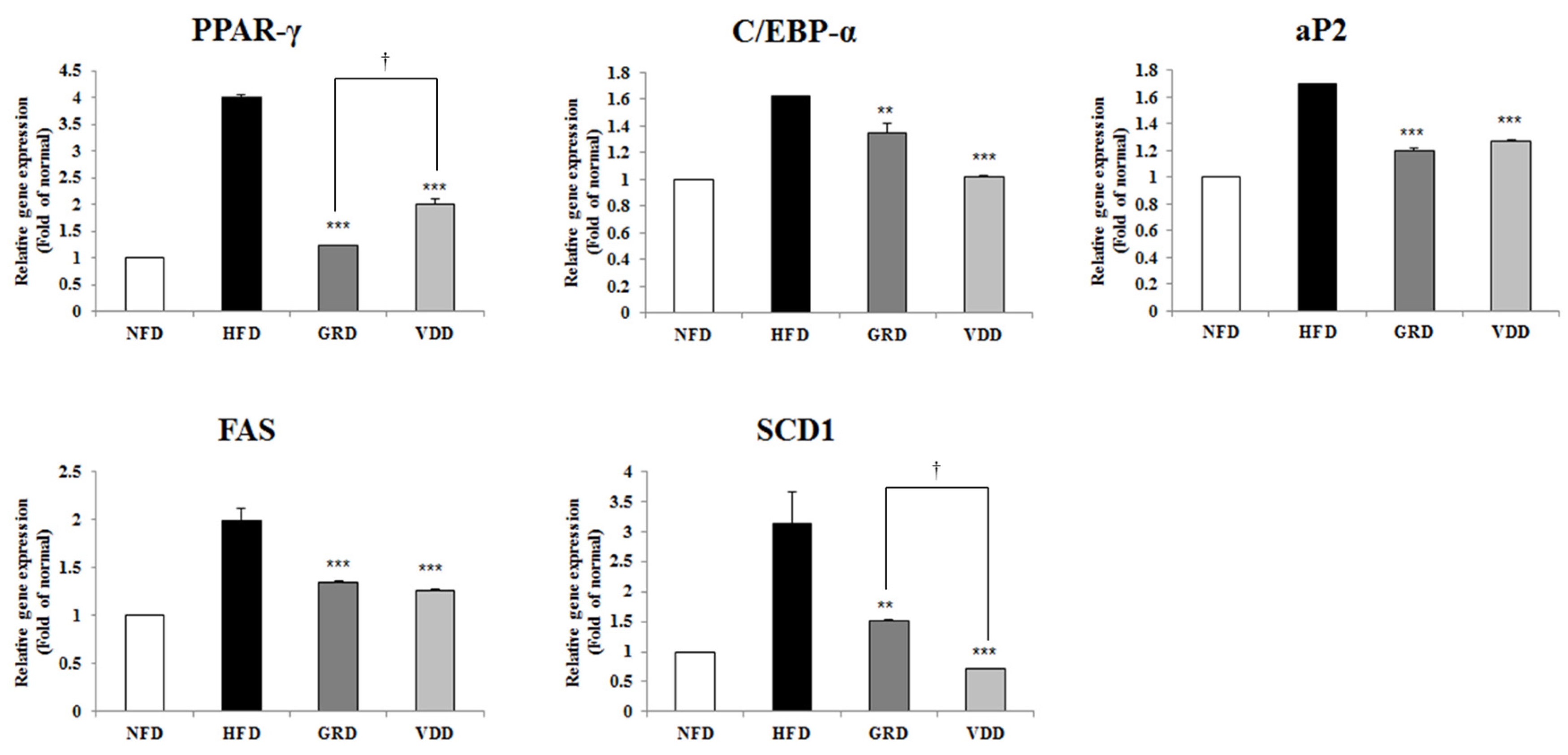
3.6. Effect of VOD on the Expression of Genes Related to Adipogenesis and Lipogenesis in White Adipose Tissue
3.7. Changes in Liver Weight and Hepatic Lipid Metabolites
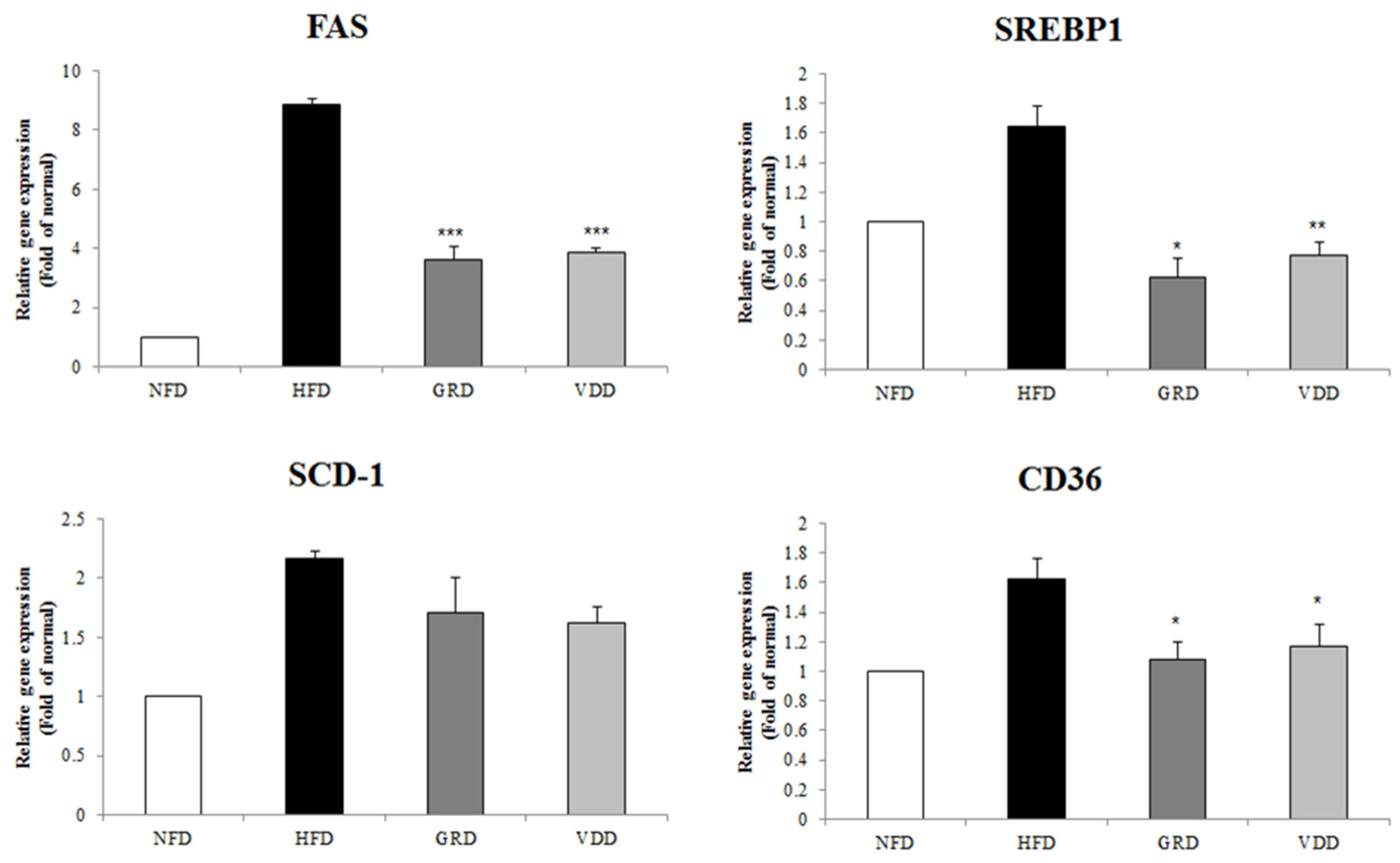
3.8. Effect of VOD on the Expression of Genes Related to Lipogenesis in the Liver
4. Discussion
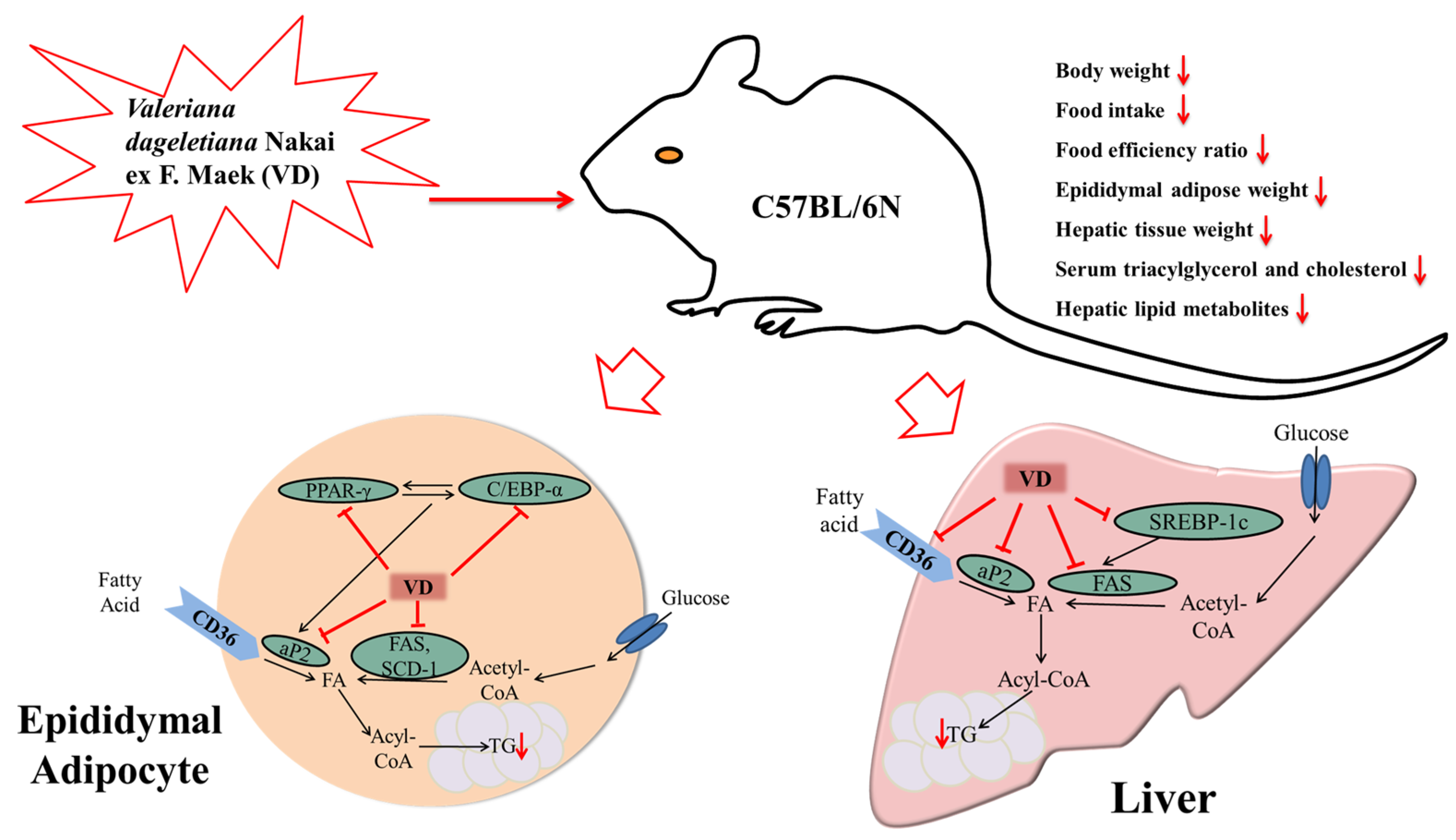
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stevens, G.A.; Singh, G.M.; Lu, Y.; Danaei, G.; Lin, J.K.; Finucane, M.M.; Bahalim, A.N.; McIntire, R.K.; Gutierrez, H.R.; Cowan, M.; et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. Obesity wars: Molecular progress confronts an expanding epidemic. Cell 2004, 116, 337–350. [Google Scholar] [CrossRef]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signaling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. What Are Overweight and Obesity, Facts About Overweight and Obesity. In Report of a WHO Consultation on Overweight and Obesity; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Girard, J.; Perdereau, D.; Foufelle, F.; Prip-Buss, C.; Ferré, P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994, 8, 36–42. [Google Scholar] [PubMed]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [PubMed]
- Morrison, R.F.; Farmer, S.R. Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 2000, 130, 3116S–3121S. [Google Scholar] [PubMed]
- Abdelmalek, M.F.; Lazo, M.; Horska, A.; Bonekamp, S.; Lipkin, E.W.; Balasubramanyam, A.; Bantle, J.P.; Johnson, R.J.; Diehl, A.M.; Clark, J.M.; et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012, 56, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Dwyer, B.J.; Forbes, S.; van Thiel, D.H.; Starkey Lewis, P.J.; Ramadori, G. Insulin production and resistance in different models of diet-induced obesity and metabolic syndrome. Int. J. Mol. Sci. 2017, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Tartaglione, E.V.; Kuver, R.; Geoffrey Haigh, W.; Farrell, G.C.; Subramanian, S.; Chait, A.; Yeh, M.M.; Quinn, L.S.; Ioannou, G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013, 57, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Schultze, F.C.; Wilting, J.; Mihm, S.; Raddatz, D.; Ramadori, G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS ONE 2014, 9, e104220. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.Y.; Choi, W.H.; Lee, D.H.; Ahn, J.Y.; Jung, C.H.; Moon, B.K.; Ha, T.Y. Shikonin protects against obesity through the modulation of adipogenesis, lipogenesis, and β-oxidation in vivo. J. Funct. Foods 2015, 16, 484–493. [Google Scholar] [CrossRef]
- Baby, R.; Cabezas, M.; Castro, E.; Filip, R.; Walsöe de Reca, N.E. Quality control of medicinal plants with an electronic nose. Sens. Actuators B 2005, 106, 24–28. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Liu, H.; Zhou, L.; Liu, Z.; Wang, J.; Han, J.; Yu, Z.; Yang, F. Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules 2010, 15, 6411–6422. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M. The Complete German Commission E monographs. In Therapeutic Guide to Herbal Medicines; American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- Kim, K.Y.; Lee, H.N.; Kim, Y.J.; Park, T. Garcinia cambogia extract ameliorates visceral adiposity in C57BL/6J mice fed on a high-fat diet. Biosci. Biotechnol. Biochem. 2008, 72, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, H.D.; Jeong, J.H.; Lee, M.K.; Jeong, Y.K.; Shim, K.H.; Seo, K.I. New vinegar produced by tomato suppresses adipocyte differentiation and fat accumulation in 3T3-L1 cells and obese rat model. Food Chem. 2013, 141, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, O.K.; Yoon, H.G.; Park, J.; You, Y.; Kim, K.; Lee, Y.H.; Choi, K.C.; Lee, J.; Jun, W. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr. Res. 2016, 60, 30428. [Google Scholar] [CrossRef] [PubMed]
- Zira, A.; Kostidis, S.; Theocharis, S.; Sigala, F.; Engelsen, S.B.; Andreadou, I.; Mikros, E. 1H NMR-based metabonomics approach in a rat model of acute liver injury and regeneration induced by CCl4 administration. Toxicology 2013, 303C, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, C.; Wu, H.; Sheng, L.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Tian, Y.; et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J. Gastroenterol. 2007, 13, 4669–4672. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Nöthlings, U.; Boeing, H. Obesity and cancer. Proc. Nutr. Soc. 2008, 67, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Antiobesity effect of Nelumbo nuciferaleaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Choe, S.S.; Jeong, H.W.; Park, S.W.; Shin, H.J.; Choi, S.M.; Park, J.Y.; Choi, E.W.; Kim, J.B.; Seen, D.S.; et al. Anti-obesity effects of Lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp. Mol. Med. 2011, 43, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Ackroff, K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C. The control of food intake: Behavioral versus molecular perspectives. Cell Metab. 2009, 9, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Schwartz, G.J. Gut-brain nutrient sensing in food reward. Appetite 2016, S0195-6663, 30900-X. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Bencivenga, D.; Caldarelli, I.; Tramontano, A.; Borgia, A.; Zappia, V.; Della, R.F. Resveratrol: From basic studies to bedside. Cancer Treat. Res. 2014, 159, 167–184. [Google Scholar] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Wu, J.C.; Pan, M.H. Molecular mechanism on functional food bioactivities for anti-obesity. Curr. Opin. Food Sci. 2015, 2, 9–13. [Google Scholar] [CrossRef]
- Wong, C.P.; Kaneda, T.; Morita, H. Plant natural products as an anti-lipid droplets accumulation agent. J. Nat. Med. 2014, 68, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty acid-binding protein 4 (FABP4): Pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin. Med. Insights Cardiol. 2014, 8, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Byrne, C.D. Modulation of sterol regulatory element binding proteins (SREBPS) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug Discov. Today 2007, 12, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-COA desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Amy, C.M.; Williams-Ahlf, B.; Naggert, J.; Smith, S. Molecular cloning of the mammalian fatty acid synthase gene and identification of the promoter region. Biochem. J. 1990, 271, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Pascucci, M.; Ahmad, S.; Malik, I.A.; Bianchi, A.; Ramadori, P.; Ahmad, G.; Ramadori, G. LIPOCALIN-2 is a majore acute-phase protein in a rat and mouse model of sterile abscess. Shock 2012, 37, 191–196. [Google Scholar] [PubMed]
- Asimakopoulou, A.; Fülöp, A.; Borkham-Kamphorst, E.; van de Leur, E.; Gassler, N.; Berger, T.; Beine, B.; Meyer, H.E.; Mak, T.W.; Hopf, C.; et al. Altered mitochondrial and peroxisomal integrity in llipocalin-2-deficient mice with hepatic steatosis. Biochim. Biophys. Acta 2017. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Seyhan, H.A.; Ahmad, S.; Mihm, S.; Ramadori, G.; Schultze, F.C. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J. Gastroenterol. 2014, 20, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Finelli, C.; Tarantino, G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J. Gastrointestin. Liver Dis. 2012, 21, 293–302. [Google Scholar] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; Mc Call, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Chin. Nutr. 2004, 79, 537–543. [Google Scholar]
- Poutahidis, T.; Kleinewietfeld, M.; Smillie, C.; Levkovich, T.; Perrotta, A.; Bhela, S.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Kearney, S.M.; et al. Microbial reprogramming inhibits western diet-associated obesity. PLoS ONE 2013, 8, e68596. [Google Scholar] [CrossRef] [PubMed]
- Traversy, G.; Chaput, J.P. Alcohol consumption and obesity: An update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Ramadori, G. How does bariatric surgery improve type II diabetes? The “Neglected” importance of the liver in clearing glucose and insulin from the portal blood. J. Obes. Weight Loss Ther. 2015, 5, 1000280. [Google Scholar] [CrossRef]
- Dib, N.; Kiciak, A.; Pietrzak, P.; Ferenc, K.; Jaworski, P.; Kapica, M.; Tarnowski, W.; Zabielski, R. Early-effect of bariatric surgery (Scopinaro method) on intestinal hormones and adipokines in insulin resistant Wistar rat. J. Physiol. Pharmacol. 2013, 64, 571–577. [Google Scholar] [PubMed]
- Spruss, A.; Kanuri, G.; Stahl, C.; Bischoff, S.C.; Bergheirm, I. Metformin protects against the development of fructose-induced steatosis in mice: Role of the intestinal barrier function. Lab. Investig. 2012, 92, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
| Groups 1 | NFD | HFD | GRD | VDD |
|---|---|---|---|---|
| Casein | 210 | 265 | 265 | 265 |
| L-cystine | 3 | 4 | 4 | 4 |
| Corn starch | 280 | - | - | - |
| Maltodextrin | 50 | 160 | 150 | 150 |
| Sucrose | 325 | 90 | 90 | 90 |
| Lard | 20 | 310 | 310 | 310 |
| Soybean Oil | 20 | 30 | 30 | 30 |
| Cellulose | 37.15 | 65.5 | 65.5 | 65.5 |
| Mineral Mixure 2 | 35 | 48 | 48 | 48 |
| Vitamin Mixture 3 | 15 | 21 | 21 | 21 |
| Calcium Phosphate, Diabasic | 2 | 3.4 | 3.4 | 3.4 |
| Choline Bitartrate | 2.75 | 3 | 3 | 3 |
| Yellow Food Color | 0.1 | - | - | - |
| Blue Food Color | - | 0.1 | 0.1 | 0.1 |
| Garcinia cambogia extract of 60% (-)-hydroxycitric acid | - | - | 10 | - |
| Valeriana dageletiana Nakai ex F. Maek extract from above-ground part | - | - | - | 10 |
| Total (g) | 1000 | 1000 | 1000 | 1000 |
| Gene | Primer Sequence (5’→3’) | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| β-Actin | GTCGTACCACTGGCATTGTG | GCCATCTCCTGCTCAAAGTC |
| C/EBP-α | AGACATCAAGCGCCTACATCG | TGTAGGTGCATGGTGGTCTG |
| PPAR-γ | CCCTGGCAAACGATTTGTAT | AATCCTTGGCCCTCTGAGAT |
| SREBP-1c | GCGCTACCGGTCTTCTATCA | TGCTGCCAAAAGACAAGGG |
| CD36 | TCCTCTGACATTTGCAGGTCTATC | GTGAATCCAGTTATGGGTTCCAC |
| SCD-1 | CGAGGGTTGGTTGTTGATCTGT | ATAGCACTGTTGGCCCTGGA |
| FAS | GATCCTGGAACGAGAACAC | AGACTGTGGAACACGGTGGT |
| aP2 | AACACCGAGATTTCCTTCAA | TCACGCCTTTCATAACACAT |
| Groups 1 | NFD | HFD | GRD | VDD |
|---|---|---|---|---|
| Body weight gain (g/10 weeks) | 8.29 ± 0.74 a,2 | 24.05 ± 1.28 b | 19.27 ± 1.28 c | 12.06 ± 0.81 d |
| Food intake (g/day) | 3.74 ± 0.13 a | 3.41 ± 0.13 a | 3.51 ± 0.13 a | 2.28 ± 0.04 b |
| FER 3 | 2.22 ± 0.2 a | 7.06 ± 5.49 b | 5.49 ± 0.36 c | 5.27 ± 0.36 c |
| Epididymal white adipose tissue weight (g) | 1.00 ± 0.16 a | 2.31 ± 0.16 b | 2.18 ± 0.16 b | 2.09 ± 0.09 b |
| Liver weight (g) | 1.27 ± 0.19 a | 1.65 ± 0.38 a | 1.19 ± 0.07 a | 1.09 ± 0.07 a |
| Groups 1 | NFD | HFD | GRD | VDD |
|---|---|---|---|---|
| Serum TG (mg/dL) | 115.5 ± 11.6 a,2 | 120.9 ± 13.7 a | 120.3 ± 8.4 a | 55.92 ± 4.92 b |
| Serum total-cholesterol (mg/dL) | 53.52 ± 4.23 b | 118.1 ± 5.67 a | 127.12 ± 8.30 a | 54.57 ± 2.8 b |
| Serum HDL-cholesterol (mg/dL) | 43.17 ± 2.79 b | 78.81 ± 3.16 a | 79.45 ± 2.38 a | 38.63 ± 1.46 b |
| HTR 3 | 0.81 ± 0.02 a | 0.70 ± 0.01 c | 0.66 ± 0.02 b | 0.71 ± 0.01 c |
| AST (U/L) | 66.54 ± 15.29 b,c | 79.61 ± 15.78 a,b | 113.69 ± 15.75 a | 33.93 ± 2.3 c |
| ALT (U/L) | 31.85 ± 7.75 b | 89.52 ± 22.90 a | 70.43 ± 11.76 a,b | 22.11 ± 5.57 b |
| BUN (mg/dL) | 22.55 ± 0.78 a | 17.49 ± 0.32 b | 17.73 ± 0.47 b | 7.65 ± 0.6 c |
| CREA (mg/dL) | 0.41 ± 0.02 b | 0.45 ± 0.01 a | 0.42 ± 0.01 b | 0.21 ± 0.01 c |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| δ 1H (ppm) | Type | Metabolites | Group 1 | |||||
| NFD | HFD | GRD | VDD | |||||
| (A) | 5.32 | Fatty acids | MUFA/PUFA | 7.78 ± 0.80 a | 29.8 ± 4.38 b | 9.63 ± 1.76 a | 6.71 ± 0.90 a | |
| (B) | 3.16 | Phospholipid | N+(CH3)3 | 0.18 ± 0.00 a | 2.11 ± 0.34 b | 0.33 ± 0.00 c | 0.23 ± 0.01 d | |
| (C) | 2.78 | Lipid moieties | -CH=CH(-CH2CH=CH-) | } | 3.56 ± 0.29 a | 18.2 ± 4.98 b | 3.96 ± 0.14 a | 3.2 ± 0.37 a |
| (D) | 2.72 | Lipid moieties | -CH=CH-CH2CH=CH- | |||||
| (E) | 2.27 | Lipid moieties | αRCH2CH2CO | 7.4 ± 1.02 a | 31.67 ± 9.92 b | 10.61 ± 2.46 a,b | 6.17 ± 0.10 a | |
| (F) | 2.00 | Lipid moieties | -CH2CH=CH- | 6.92 ± 0.43 a | 39.22 ± 11.61 b | 12.91 ± 3.26 a,b | 9.1 ± 1.02 a | |
| (G) | 1.55 | Lipid moieties | βRCH2CH2CO | 6.49 ± 0.20 a | 23.61 ± 2.53 b | 10.17 ± 2.48 a | 6.59 ± 1.06 a | |
| (H) | 1.24 | Lipid moieties | -(CH2)n- | 54.65 ± 3.47 a | 201.94 ± 32.30 b | 78.24 ± 21.31 a | 48.02 ± 9.28 a | |
| (I) | 0.83 | Lipid moieties | RCH3 | 4.6 ± 0.55 a | 33.57 ± 7.76 c | 10.63 ± 2.27 b | 7.53 ± 1.43 a,b | |
| (J) | 0.64 | Cholesterol | Chol-C18 | 0.05 ± 0.03 a | 0.51 ± 0.35 a | 0.20 ± 0.73 a | 0.17 ± 0.39 a | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hwang, S.H.; Kim, J.H.; Lim, S.S. Anti-Obesity Effect of the Above-Ground Part of Valeriana dageletiana Nakai ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice. Nutrients 2017, 9, 689. https://doi.org/10.3390/nu9070689
Wang Z, Hwang SH, Kim JH, Lim SS. Anti-Obesity Effect of the Above-Ground Part of Valeriana dageletiana Nakai ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice. Nutrients. 2017; 9(7):689. https://doi.org/10.3390/nu9070689
Chicago/Turabian StyleWang, Zhiqiang, Seung Hwan Hwang, Ju Hee Kim, and Soon Sung Lim. 2017. "Anti-Obesity Effect of the Above-Ground Part of Valeriana dageletiana Nakai ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice" Nutrients 9, no. 7: 689. https://doi.org/10.3390/nu9070689





