1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome, which is highly associated with several metabolic disorders such as type 2 diabetes mellitus (T2DM), insulin resistance (IR) and hyperlipidemia [
1,
2]. In the absence of excessive alcohol ingestion, obesity is one of the major etiological factors of NAFLD, which is characterized by an accumulation of triglycerides inside the liver cells to a percentage exceeding 5% of liver weight [
3], with the same histopathological manifestations of an alcoholic liver injury [
4]. During the last two decades, its prevalence has reached worrying proportions as it has come to affect 25.2% of the people worldwide [
5], including 20–30% of adults and 3–10% of children in Western countries [
6]. NAFLD was reported as the main factor in many of the morbidities related to liver diseases [
7]. It causes various types of liver injuries, including simple hepatic fat accumulation, which may occur alone or associated with steatohepatitis, known as non-alcoholic steatohepatitis (NASH), with or without fibrosis [
8,
9]. It was suggested by several studies that NAFLD is the key player in increasing the incidence of hepatocellular carcinoma [
10]. The pathogenesis of NAFLD is multifactorial, but it is mainly attributed to insulin resistance and imbalance in lipids (uptake, hepatic synthesis and degradation) [
9]. Recently, gut dysbiosis has also been linked with NAFLD [
11].
Insulin resistance and oxidative stress are essential risk factors for NAFLD [
12,
13]. Oxidative stress promotes the production of reactive oxygen species (ROS) that stimulate an inflammatory process in hepatic tissues [
14]. On the other hand, insulin resistance inhibits the antilipolytic activity of insulin in the adipose tissue and increases free fatty acids (FFAs) in the serum and liver, leading to mitochondrial dysfunction as well as cardiac fat accumulation [
15].
Over the last decade, several noteworthy research initiatives have aimed at finding possible therapies to ameliorate the hepatic damage that accompanies NAFLD. The majority of pharmacological strategies include using antioxidant agents and insulin sensitizers, and also reducing the effect of dietary carbohydrate and fats by the inhibition of cholesterol micellization, pancreatic lipase and α-glucosidase [
16,
17,
18].
Recently, a considerable number of studies has investigated natural phytochemicals as anti-NAFLD agents [
19,
20,
21,
22].
P. niruri, a herb found in South East Asia, has been traditionally used to treat many pathological conditions like dyspepsia, bronchitis, influenza, asthma, dysentery, tumours, diabetes, vaginitis and tuberculosis [
23]. It was also used for its potent activity in the treatment of kidney stones and gallstones [
24] and various liver disorders, particularly hepatitis and jaundice. Recent data showed that
P. niruri had hepatoprotective properties against induced hepatitis in rats [
25], and it has a therapeutic effect on T2DM, which is associated with hypercholesterolemia [
26].
P. niruri is rich in flavonoids and phenolic compounds that are responsible for its potent antioxidant properties [
27], which could play important roles in hepatoprotective activity [
28,
29]. However, to the best of our knowledge, no reports have been published regarding the therapeutic effects of
P. niruri in the treatment of NAFLD. Thus, in this study we attempt to evaluate the potential role of
P. niruri in suppressing and/or ameliorating the development of NAFLD induced by the oral administration of a high-fat diet (HFD) in rats as a model that reflects human NAFLD, using metformin as the positive control drug.
2. Materials and Methods
2.1. High-Fat Diet Preparation
A normal diet was purchased from Gold Coin Feed mills Sdn. Bhd., Penang, Malaysia (product code 702P), which contains crude protein (21–23%), crude fibre (5%), crude fat (3%), moisture (13%), ash (8%), calcium (0.8%) and phosphorus (0.6–1.0%). The high-fat diet was prepared by mixing the normal diet with 10% margarine (wt/wt), 10% ghee fat (wt/wt), 1% cholesterol and 0.5% cholic acid.
2.2. Extract Preparation
A specimen of P. niruri including whole plant was identified by Professor Mashhor Mansor from the Botany Department, School of Biological Sciences, Universiti Sains Malaysia (USM). A voucher herbarium specimen has been deposited at the Herbarium Unit (Voucher No. 11474), School of Biological Sciences, USM. A powder of dry whole plant of P. niruri was extracted by maceration using water (WE), 50% methanol in water (50% ME) and methanol (ME) respectively.
2.3. In Vivo Model
The NAFLD animal model was developed and studied using 36 male Sprague–Dawley (SD) rats (10 weeks of age), obtained from the Animal Research and Service Centre, USM. All experimental procedures were carried out after getting the approval from Animal Ethics Committee, Universiti Sains Malaysia (protocol No.: 2013/(90) (546)).
Animals were acclimatized for one-week and randomly assigned to six groups (n = 6). Group 1 was fed a normal diet for eight weeks and served as the normal control group (NC), while the five remaining groups (2–6) were fed a high-fat diet (HFD) for eight weeks. Oral gavage treatment started in week 4. Group 1was treated with distilled water (10 mL/kg body weight). Group 2 was the HFD group, which served as a negative control and received distilled water (10 mL/kg body weight); group 3 served as a positive control and received metformin (HFD + Met 500 mg/kg body weight) at 10 mL/kg body weight; groups 4–6 received 1000 mg/kg of P. niruri WE, 50% ME, and ME, respectively, at 10 mL/kg body weight
The same protocol was repeated for evaluating the dose-response relationship, using five groups. Group 1 was fed a normal diet and served as a normal control group (NC), while the four remaining groups were fed a high-fat diet (HFD) for eight weeks. Oral gavage treatment started in week 4. Group 1 was treated with distilled water (10 mL/kg body weight). Group 2 served as a negative control and received distilled water (10 mL/kg body weight); groups 3, 4, and 5 received 1000 mg/kg, 500 mg/kg, and 250 mg/kg, respectively, of the most active extract of P. niruri to evaluate the relative potency of the three different doses (1000 mg/kg, 500 mg/kg, and 250 mg/kg) at 10 mL/kg body weight.
2.4. Determination of Blood and Serum Biochemical Parameters
After eight weeks of HFD feeding, all the rats were fasted overnight and the body weight of each rat was determined. They were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneal (i.p.)). Five millilitres of the blood were taken via cardiac puncture from each rat. Blood glucose level was determined using an automated blood glucose meter (Accu-chek Performa®, Roche Diagnostics, Mannheim, Germany). Peri-renal fats were isolated and weighed for the assessment of visceral adipose tissues. The serum was used to measure aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin, creatinine, uric acid, urea, low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), and triglycerides (TG) levels using the Olympus AU640™ multifunctional biochemistry analyser (laboratories of Gribbles, Penang, Malaysia).
2.5. Determination of Insulin and Free Fatty Acids Concentrations
Insulin levels were determined using the commercial rat insulin ELISA kit (Elabscience Biotechnology Co., Ltd., Beijing, China). Serum free fatty acids (FFAs) values were measured using a fluorometric assay kit (Cayman Chemical, Ann Arbor, MI, USA) catalogue No. 700310.
2.6. Assessment of Atherosclerosis Risk Indexes
Recent data showed that NAFLD is one of the independent predictors of atherosclerosis [
16]. Therefore, in order to identify high-risk cases of atherosclerosis and their effective therapeutic management, the predictor ratios that complement the lipid profile ratios such as atherogenic Castelli’s Risk Index I, Castelli’s Risk Index II, in addition to atherogenic coefficient (AC) were determined [
30].
Atherogenic ratios were calculated based on the following formulas:
2.7. Assessment of Lipid Peroxidation, TG and TC Contents in Liver Tissues
Total protein content of liver tissues was determined using a Bradford assay kit (Sigma-Aldrich, San Jose, CA, USA). The MDA content was measured in the hepatic tissue homogenate by a thiobarbituric acid reaction [
31]. Hepatic lipid was extracted according to Folch et al. [
32]. The content of TG was determined using triglycerides colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA), while TC content was determined using a commercial kit according to the manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA, USA).
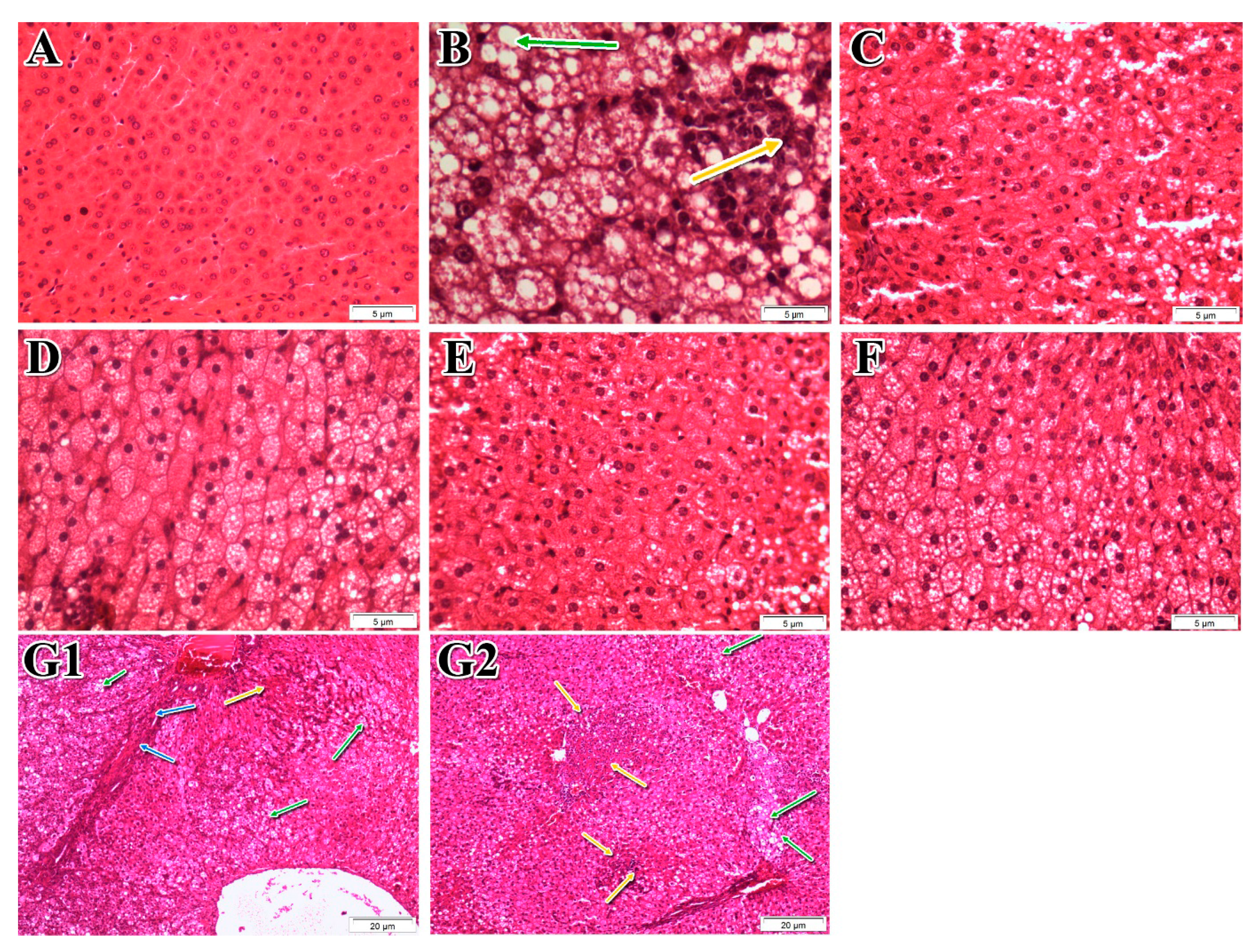
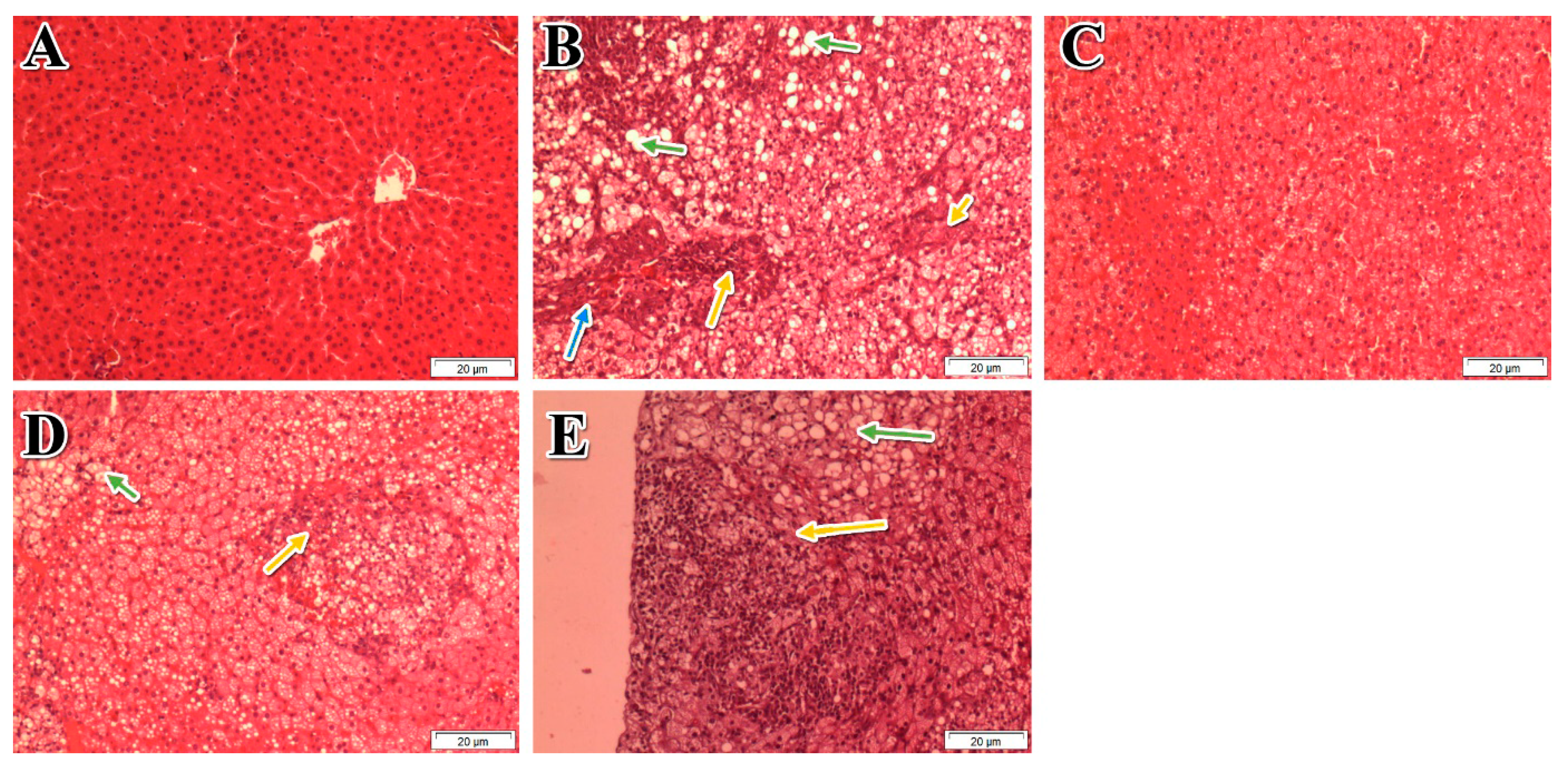
2.8. Histopathological Examinations
For histological analysis, the liver specimen was taken from the largest hepatic lobe and fixed with formaldehyde 10% (
v/
v). Histopathological examinations were performed by an experienced pathologist using a blinded approach. The liver sections were stained with hematoxylin and eosin (H&E). The histological features were examined and grouped into four broad categories: steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis. A score system was utilized to evaluate each feature. An overall score, namely the NAFLD activity score (NAS), was generated based on the individual scores of all the histological features [
33].
2.9. Antioxidant Activity Tests
The antioxidant assays of the three extracts of
P. niruri were carried out in triplicate at a concentration of 10 mg/mL. Absorbance was measured using a microplate reader (TECAN Infinite Pro
® M200, Mannedorf, Switzerland). Total phenolic content (TPC) of each extract was estimated using Folin-Ciocalteu reagent, according to the method of Kumaran and Joel Karunakaran [
34]. Gallic acid was used as the standard. TPC was expressed as µg gallic acid equivalent/mg dry extract. Total flavonoid content (TFC) of each extract was measured using the aluminium chloride method [
35]. Quercetin was used as a reference standard. TFC was expressed as µg quercetin equivalent/mg dry extract. DPPH scavenging activity was assessed according to the method developed by Al-Mansoub et al. [
36]. Ascorbic acid was used as the reference standard. The scavenging activity was expressed as IC
50. ABTS scavenging activity was performed using the method described by Al-Mansoub et al. [
36]. Ascorbic acid was used as reference standard. Results were expressed as IC
50. The ferric reducing antioxidant power of each extract (FRAP) was carried out by the method of Benzie and Strain [
37]. All results were expressed as nmol Fe
+2 equivalent/mg dry extract.
2.10. In Vitro Assay to Determine the Inhibitory Effects of the Most Active Extract on α-Glucosidase, Pancreatic Lipase and Cholesterol Micellization
The inhibitory activity of the most active extract against α-glucosidase was measured based on previous method described by Yusoff et al. [
38] with minor modification. The results were expressed as values of IC
50 which is defined as the concentration of the sample/acarbose required to inhibit 50% of α-glucosidase activity. Absorbance reading was taken using a microplate reader (TECAN Infinite Pro
® M200, Mannedorf, Switzerland). All analyses were done in triplicate. Six serial dilutions of each sample were used to obtain the IC
50 values. The inhibitory effect of the active extract towards pancreatic lipase was assessed by fluorometric assay based on the method described by Zhang et al. [
39,
40], using orlistat as the standard. The results were expressed as IC
50 which is defined as the concentration of either sample or orlistat required to inhibit 50% of pancreatic lipase activity.
The inhibitory activity of most active extract of
P. niruri against cholesterol micelle formation was carried out based on a previously reported method with minor modifications [
41], using gallic acid as the positive control and a total cholesterol test kit for cholesterol determination.
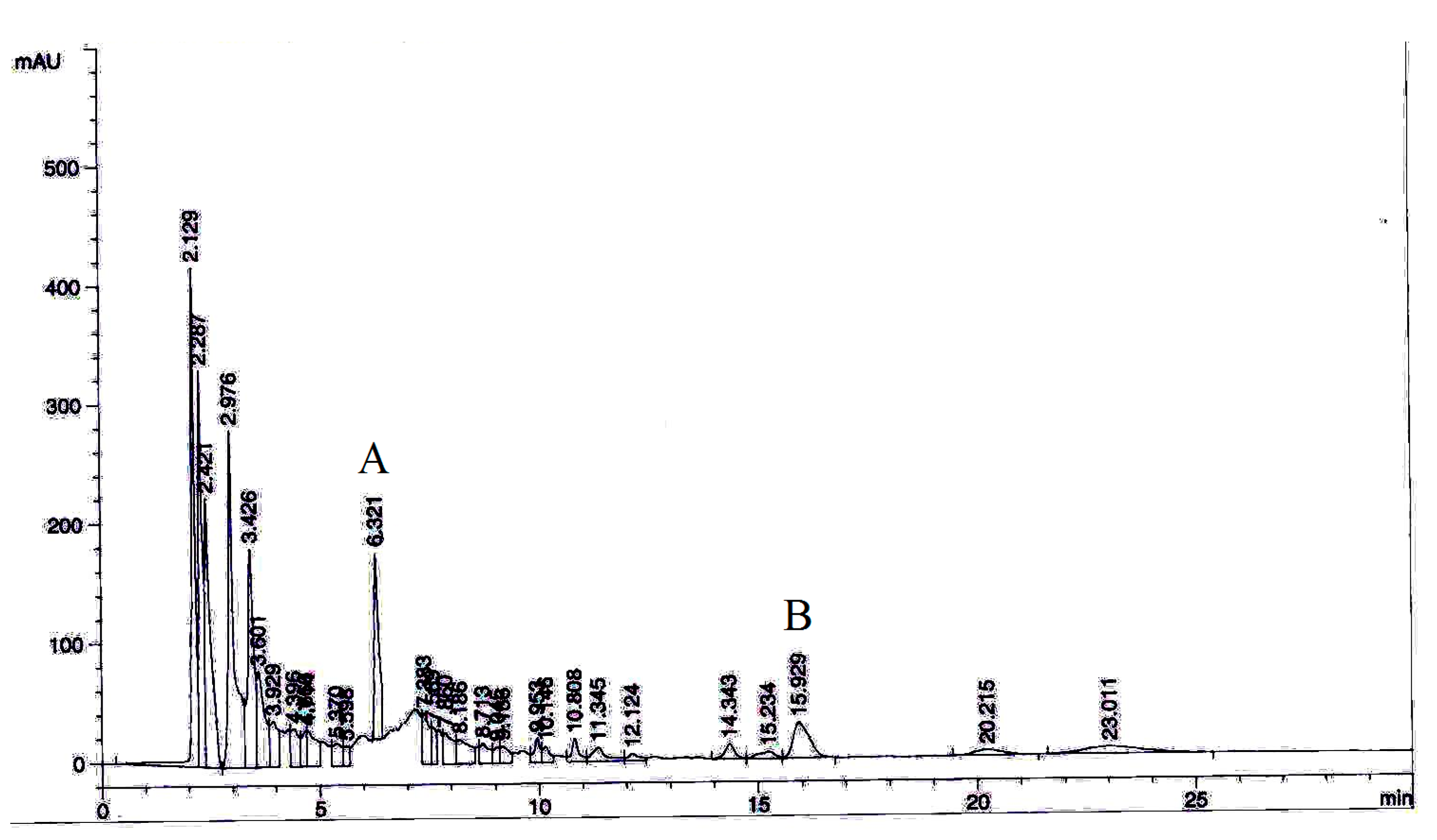
2.11. Phytochemical Analysis by High-Performance Liquid Chromatography (HPLC)
To standardize the most active extract and further elucidate its chemical composition and active compounds, an HPLC analysis was performed. Stock solutions (1000 μg/mL) of two selected marker compounds (ellagic acid and phyllanthin) were prepared in methanol. The chromatographic separation was carried out using a Zorbax Eclipse Plus C18 column and HPLC device (Agilent, Santa Clara, CA, USA). Column specifications were 250 × 4.6 mm/5 μm particle size. The samples (10 μL) were eluted at a flow rate of 1 mL/min at a λ
max of 230 nm, with a gradient mobile phase comprising 0.2% aqueous acetic acid and acetonitrile. The lowest limit of detection (LOD) value for each marker compounds was determined by analysing the standard concentrations successively with a 2-fold dilution with methanol. The concentration with the smallest detectable peak at a noise-to-signal ratio of 3 was considered as the LOD value. Moreover, the lower linearity range limit at 83 noise-to-signal ratio of 10 was deemed as the limit of quantification (LOQ) [
42]. Each working standard solution was injected (
n = 5) to determine the precision of the method. The values were expressed as the coefficient of variation (CV%). Intra-day accuracy and precision analyses were carried out by injecting the working standard concentrations (
n = 5) within one day, whereas the intra-day analyses were conducted by injecting the standard working concentrations once per day for five consecutive days (Murugaiyah and Chan, 2007). Three working standard concentrations of each selected marker compound were used to determine the percentage of recovery. The percentage of recovery was calculated for each analyte according to the equation below [
43]:
where, A = amount of marker compound detected; B = original amount of the marker compound; C = amount of the marker compound spiked.
Ellagic acid and phyllanthin were quantified in P. niruri extract. The extract (1 mg/mL) was prepared in methanol for HPLC analysis. All the samples were filtered by 0.45 μm PTFE syringe filter. The selected marker compounds were quantified in triplicates using linear regression equations of the calibration curves.
2.12. Statistical Analysis
The results were expressed as means ± S.E.M. Statistical significant difference was examined using Statistical Package of Social Sciences (SPSS) program, version 20 (IBM Corp., Armonk, NY, USA). Differences between groups were evaluated using the one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test, and was considered significant when p < 0.05.
4. Discussion
Sedentary lifestyle and overconsumption of high-calorie foods are the main reasons for central obesity and NAFLD [
48]. The present study provides strong evidence of the anti-NAFLD activity of
P. niruri in SD rats. Our optimized extract of
P. niruri not only supressed hepatic fat accumulation but also blocked inflammation in NAFLD rats, thus preventing fibrosis and inhibiting simple steatosis from progressing to NASH. Additionally, rats treated with
P. niruri exhibited significantly lower indicators of atherosclerosis than untreated rats.
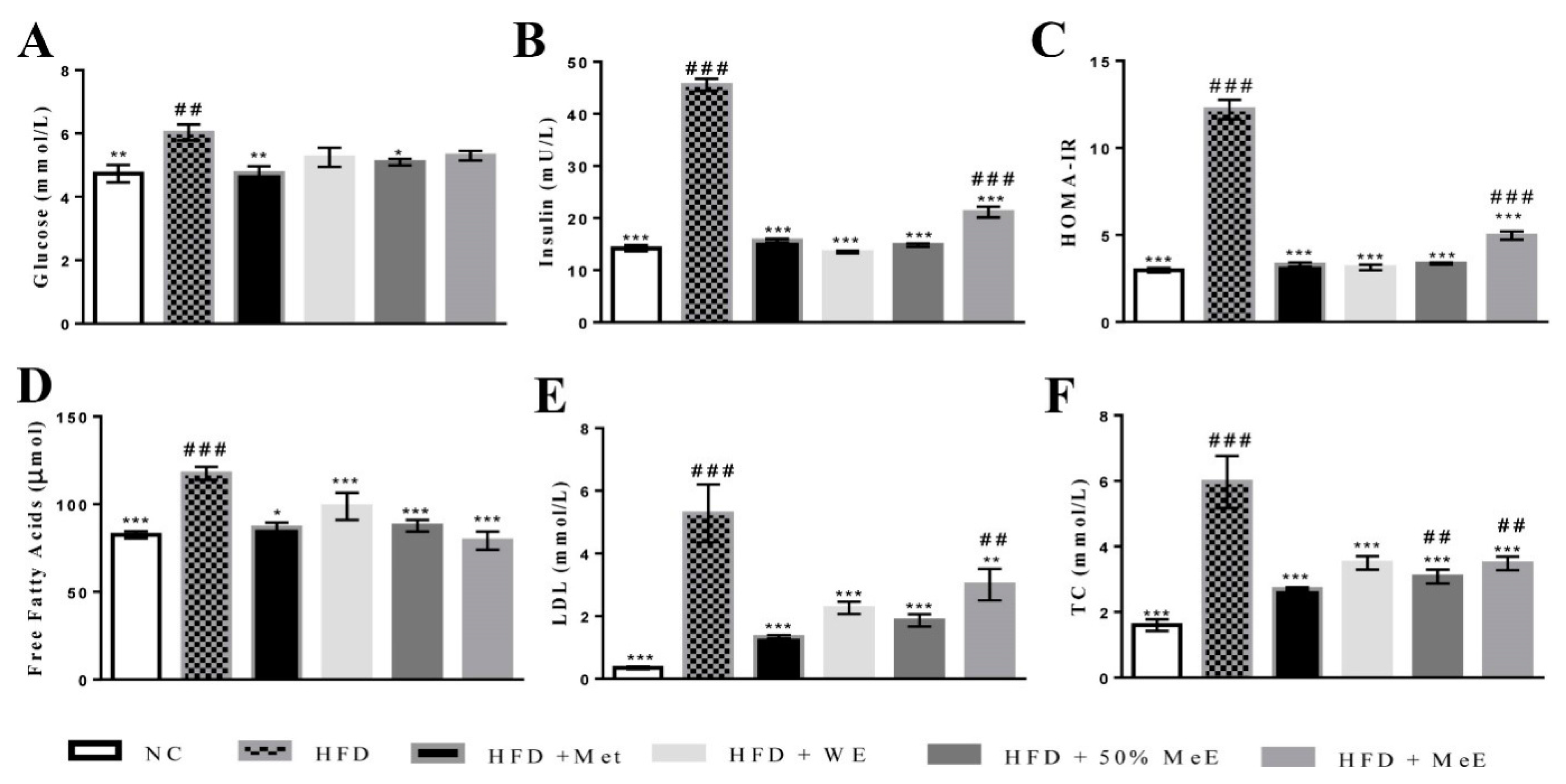
Our current in vivo model of NAFLD was successfully established and developed to NASH after feeding the rats with a HFD enriched with trans-fatty acids from margarine, and saturated fatty acids derived from animal fat [
49,
50]. The high increase in the weight of the liver and visceral fat in the HFD group is a prominent feature of NAFLD [
51,
52]. Furthermore, the hypercholesterolemia within HFD group was previously reported in 20–80% of human NAFLD cases [
53], and a greater concentration of serum FFAs is correlated with disease severity [
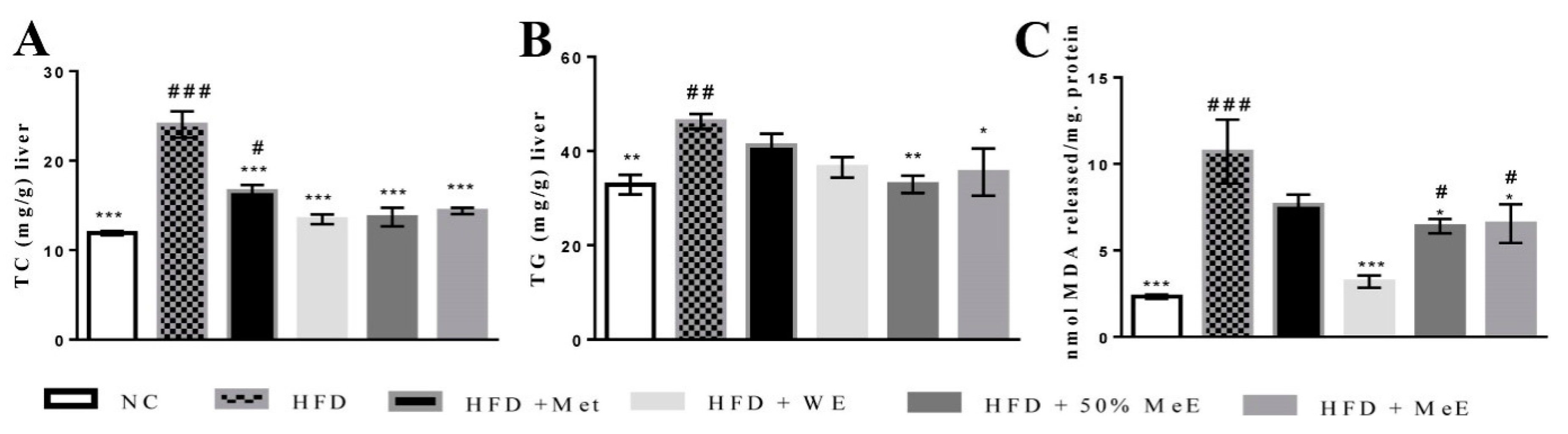
54]. Moreover, the significant increase in serum levels of ALP and ALT in addition to the elevated hepatic content of TG, TC and MDA in NAFLD rats is a strong indicator of liver damage and oxidative stress [
55]. According to the “two hit” hypothesis of NAFLD, high levels of free fatty acids lead to lipogenesis and lipid accumulation in the liver during the first hit of NAFLD progress, whereas the presence of oxidative stress and lipid peroxidation represents the second hit [
56]. More specifically, the accumulated fats in the liver induce the release of cytotoxic oxygen free radicals, which are associated with lipid peroxidation markers such as malondialdehyde (MDA) [
57] that trigger inflammation and fibrosis [
58,
59]. In the current study, HFD group exhibited higher concentrations of glucose and hyperinsulinemia, elevated HOMA-IR values, and developed significant hepatic steatosis, inflammation and fibrosis. These results are in agreement with previous studies and further prove the ability of a HFD to cause rapid fat accumulation in the liver, with distinct elevation in insulin resistance (HOMA-IR values) [
60]. The increase of insulin resistance in the HFD group would stimulate oxidative stress in the liver by releasing reactive oxygen species, causing hepatic lipid peroxidation and initiating inflammatory reactions that enhance fibrosis through the activation of hepatic stellate cell (HSC) [
3,
61].
In addition, the significant increase in serum uric acid (SUA) concentration in the HFD group led to the development of NAFLD and insulin resistance. It was reported that hyperuricemia is closely correlated with the severity of liver damage. Moreover, SUA can enhance inflammatory reactions and trigger oxidative stress in adipocytes, which in turn increases insulin resistance and induces more lipolysis in adipose tissue and greater oxidative stress in the liver cells [
62]. Consequently, the reduction in SUA could improve NAFLD [
63]. Similarly, the present findings suggest that decreasing the SUA level in WE- and ME-treated groups contributed to the suppression of NAFLD in these groups.
The considerable increase in serum atherogenic indexes indicates a higher risk of atherosclerosis in NAFLD rats. This is attributed to the sharp increase in serum levels of LDL, which is usually oxidized to form the peroxides that play a role in several atherosclerotic stages by their cytotoxic effects, leading to endothelial injury [
64].
However, 50% ME of
P. niruri was the most active among the three extracts of the plant. It prominently decreased visceral adipose tissue and liver weight, and blocked the progress of NAFLD by the reduction in hepatic steatosis and inflammation. In addition to preventing fibrosis, it improved liver function and decreased serum levels of glucose, insulin concentrations and HOMA-IR, indicating a significant decrease in insulin resistance. It was obvious from the results that
P. niruri improved cross-talk between the liver and adipose tissues. This was reflected by the lower serum concentrations of TC, LDL and FFAs in NAFLD rats [
65]. The 50% ME of
P. niruri also supressed FFA-evoked de novo lipogenesis in the liver and decreased the hepatic content of cholesterol, TG, and MDA. As a result, the atherogenic ratios were evidently decreased. The results show that all doses (1000 mg/kg, 500 mg/kg, and 250 mg/kg) of 50% ME are able to significantly reduce atherogenic ratios, ALT, AST/ALT ratio, and suppress insulin resistance. On the other hand, liver weight, visceral adipose tissue weight and liver histology were only improved by the dose of 1000 mg/kg, indicating that this is the anti-NAFLD effective dose.
The anti-NAFLD effect of
P. niruri is likely to result from the antioxidant activity shown by DPPH and ABTS tests. This is most likely attributable to phenolic compounds in all
P. niruri extracts that inhibited oxidative stress by decreasing lipid peroxidation and improving insulin signalling and β-oxidation. These results are in agreement with many previous studies that explained the potent antioxidant activities of polyphenols in suppressing lipogenesis and inhibiting fatty acid synthesis in vitro [
14]. The anti-NAFLD effect of 50% ME of
P. niruri may also be due to ellagic acid, an active antioxidant that was a major compound in the extract. It was previously found to be responsible for anti-NAFLD effect of
Phyllanthus emblica L. in vitro [
66]. Ellagic acid was further reported to decrease de novo lipogenesis, inhibit TG esterification and enhance FFAs oxidation by increasing the expression of FFAs oxidative genes in the liver, leading to significant reduction of hepatic lipid accumulation [
67]. It was suggested that this activity is due to the increase in energy expenditure by enhancing AMPK, the regulator of energy homeostasis and FFAs oxidation [
68]. It is anticipated that 50% ME of
P. niruri might act by a similar mechanism.
Another anti-oxidant agent and bioactive compound in 50% ME of
P. niruri is phyllanthin, which has been reported to be effective in ameliorating the development of NAFLD in mice [
69]. The other possible mechanism that might contribute to the anti-NAFLD effect of 50 ME of
P. niruri is inhibiting the activities of α-glucosidase, pancreatic lipase and cholesterol micellization. Since high rates of carbohydrate and fat consumption increase insulin resistance and oxidative stress [
70], improving insulin signalling can be achieved by decreasing the intake of carbohydrates and lipids, and attenuating the postprandial hyperlipidemia. This might contribute by decreasing the hepatic cholesterol and FFAs pool required for the de novo lipogenesis process in the liver [
71,
72,
73]. The same mechanism was also suggested for acarbose, orlistat and ezetimibe as a prospective treatment for lipid metabolic disorders such as obesity and other insulin resistance complications including NAFLD and diabetes [
59,
74,
75].
The oral consumption of
P. niruri is considered safe, and investigations into its toxicity in female Sprague–Dawley rats have not shown any abnormality in different body organs. The LD50 was higher than 5000 mg/kg body weight [
76]. Moreover, it is noteworthy that
P. niruri was clinically approved to be safely consumed by children [
77].
In summary, 50% ME of
P. niruri reduced visceral adiposity, improved liver enzymes abnormalities, and decreased hepatic lipid peroxidation and fat accumulation. It also decreased the risk of atherosclerosis related to NAFLD, induced by a high-fat diet in SD rats. The data suggest that the antioxidant and hypolipidemic effects of the bioactive components in 50% ME of
P. niruri (ellagic acid and phyllanthin) play essential roles in the therapeutic properties. Hence, the current study established the first-ever report on the potential therapeutic use of
P. niruri as a natural source for treating NAFLD. The general mechanism of anti-NAFLD effect of action of
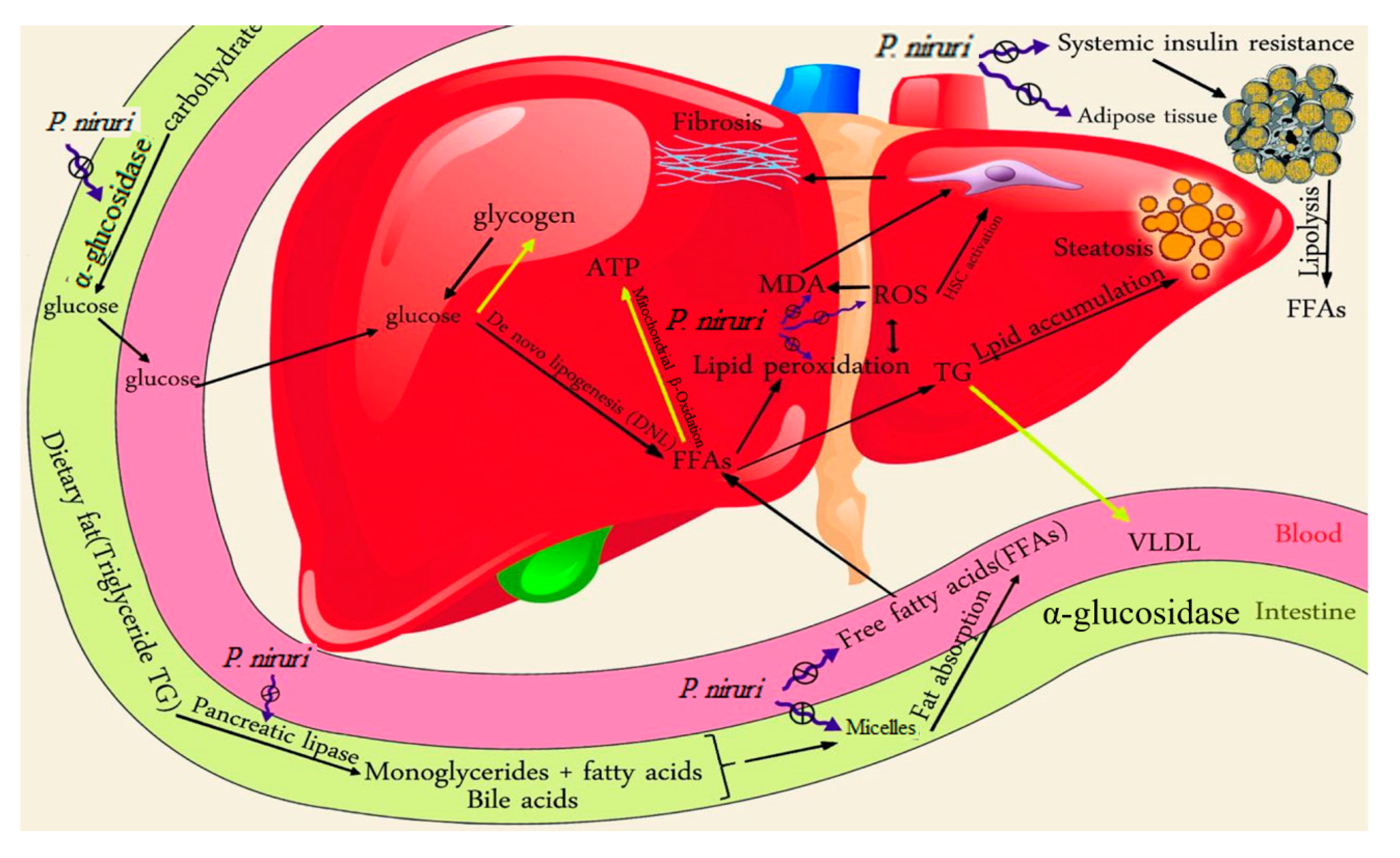
P. niruri is shown in
Scheme 1.
High dietary fat consumption combines with an increased rate of lipolysis in the insulin-resistant adipose tissues, leading to elevated free fatty acid (FFAs) concentrations in the blood. The excessive free fatty acids are delivered to the insulin-resistant liver, which has impaired mitochondrial β-oxidation. Excessive intake of carbohydrates, along with insulin resistance, result in channelling high quantities of glucose to the liver, where it is converted to either glycogen or FFAs via insulin-stimulated de novo lipogenesis (DNL), leading to excessive accumulation of triglycerides and cholesterol in the liver. P. niruri decreased insulin resistance, reduced serum fatty acids, and inhibited α-glucosidase, pancreatic lipase and cholesterol micellization, leading to a decrease in the amounts of glucose and FFAs in the liver. Consequently, fewer FFAs and less glucose will be available to the de novo lipogenesis process, and therefore less fat will accumulate in the liver. P. niruri also reduced hepatic MDA, the marker of lipid peroxidation, which stimulates hepatic stellate cells (HSC), the main cells that are responsible for triggering fibrosis in the liver. As a result, P. niruri reduced hepatic fibrosis.
The yellow arrows denote the weakened pathway in NAFLD, while the black arrows refer to the stimulated pathway in NAFLD. The blue symbol indicates the inhibitory effect of P. niruri.