Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Definition of Diseases
2.4. Evaluation of Body Composition
2.5. Blood Biochemistry Examination
2.6. Dietary Assessment
2.7. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Associations among Serum Iron:Ferritin Ratio, Body Composition, and Fatty Liver Severity
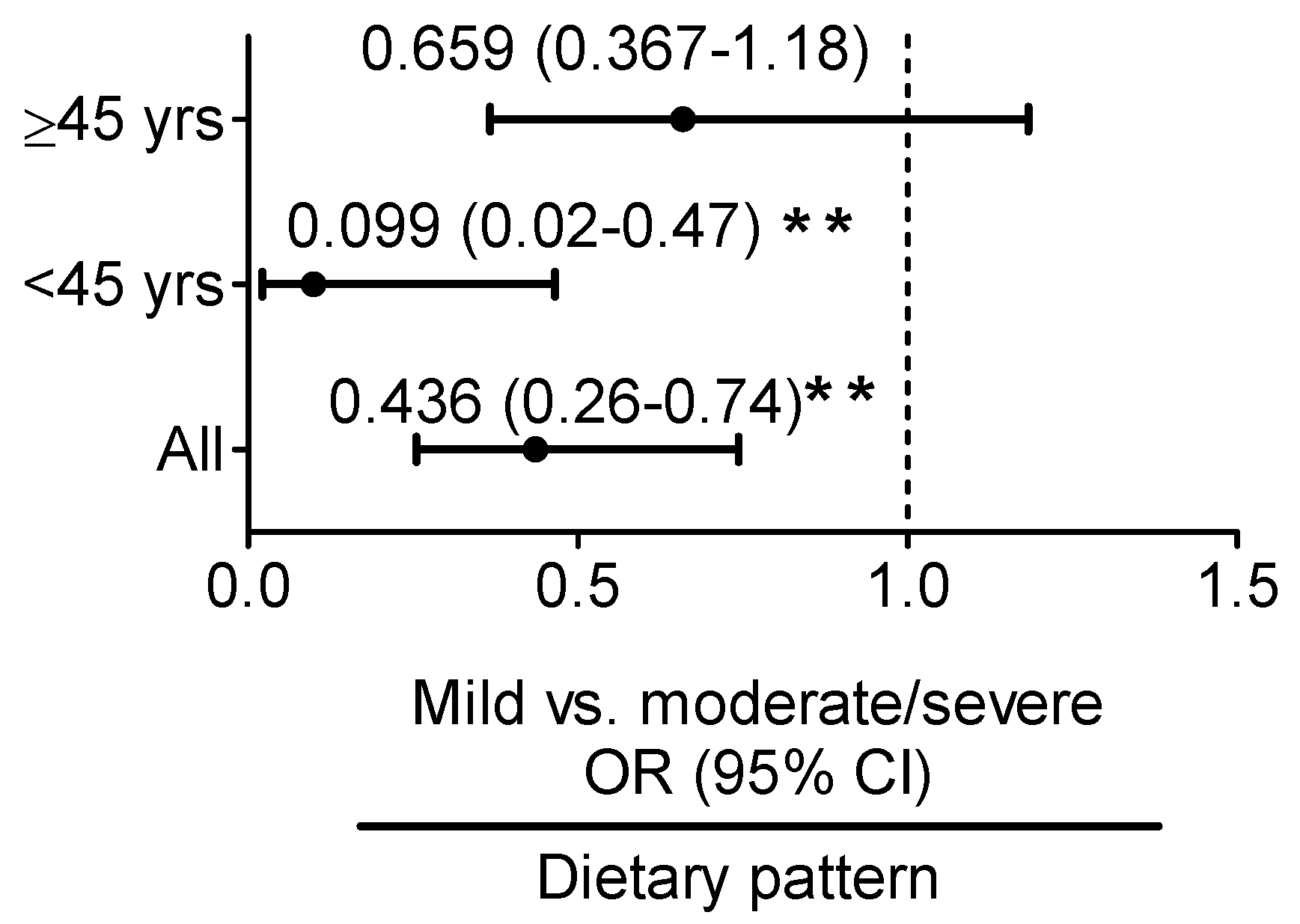
3.3. Iron:Ferritin-Specific Dietary Pattern Is Associated with Reduced Risk of Fatty Liver Progression in Young Adult Women
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Non-Alcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatology 2016. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medic-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef] [PubMed]
- George, D.K.; Goldwurm, S.; MacDonald, G.A.; Cowley, L.L.; Walker, N.I.; Ward, P.J.; Jazwinska, E.C.; Powell, L.W. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology 1998, 114, 311–318. [Google Scholar] [CrossRef]
- Deugnier, Y.M.; Loreal, O.; Turlin, B.; Guyader, D.; Jouanolle, H.; Moirand, R.; Jacquelinet, C.; Brissot, P. Liver pathology in genetic hemochromatosis: A review of 135 homozygous cases and their bioclinical correlations. Gastroenterology 1992, 102, 2050–2059. [Google Scholar] [CrossRef]
- Ahmed, U.; Latham, P.S.; Oates, P.S. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J. Gastroenterol. 2012, 18, 4651–4658. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S.; et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am. J. Pathol. 2013, 182, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.E.; Klintworth, H.; Kowdley, K.V. Iron metabolism in Nonalcoholic Fatty Liver Disease. Curr. Gastroenterol. Rep. 2012, 14, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.H.; Wu, M.S.; Owaga, E.; Cheng, S.Y.; Pan, W.H.; Chang, J.S. Relationship between hemoglobin levels and risk for suspected non-alcoholic fatty liver in Taiwanese adults. Chin. J. Physiol. 2014, 57, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qi, Q.; Yu, Z.; Sun, L.; Li, H.; Lin, X. Opposite associations of trunk and leg fat depots with plasma ferritin levels in middle-aged and older Chinese men and women. PLoS ONE 2010, 5, e13316. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Nakajima, A.; Yoneda, M.; Yamada, Y.; Mukasa, K.; Fujita, K.; Fujisawa, N.; Wada, K.; Terauchi, Y. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care 2005, 28, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.-W.; Kang, E.S.; Cha, B.-S.; Han, K.-H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Lanti, C.; Gatti, S.; Rametta, R.; Recalcati, S.; Maggioni, M.; Fracanzani, A.L.; Riso, P.; Cairo, G.; Fargion, S. High fat diet subverts hepatocellular iron uptake determining dysmetabolic iron overload. PLoS ONE 2015, 10, e0116855. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Ebata, Y.; Sakabe, T.; Hama, S.; Kogure, K.; Shiota, G. High-fat, high-fructose diet induces hepatic iron overload via a hepcidin-independent mechanism prior to the onset of liver steatosis and insulin resistance in mice. Metabolism 2013, 62, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Aigner, E.; Theurl, I.; Theurl, M.; Lederer, D.; Haufe, H.; Dietze, O.; Strasser, M.; Datz, C.; Weiss, G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2008, 87, 1374–1383. [Google Scholar] [PubMed]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Rusu, E.; Enache, G.; Jinga, M.; Dragut, R.; Nan, R.; Popescu, H.; Parpala, C.; Homentcovschi, C.; Nitescu, M.; Stoian, M.; et al. Medical nutrition therapy in non-alcoholic fatty liver disease—A review of literature. J. Med. Life 2015, 8, 258–262. [Google Scholar] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Peta, V.; Alfieri, F.; De Lorenzo, A.; Bellentani, S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J. Gastroenterol. 2014, 20, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Park, S.H. Radiologic evaluation of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 7392–7402. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.E.; Ma, S.; Wai, D.; Chew, S.K.; Tai, E.S. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004, 27, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Yeh, W.T.; Chang, Y.H.; Tsai, K.S.; Pan, W.H. Prevalence of dyslipidemia and mean blood lipid values in Taiwan: Results from the Nutrition and Health Survey in Taiwan (NAHSIT, 1993–1996). Chin. J. Physiol. 2002, 45, 187–197. [Google Scholar] [PubMed]
- World Health Organization (WHO). Iron deficiency anaemia: Assessment, prevention and control: A guide for programme managers. Geneva Switz. Who 2001, 21, 42. [Google Scholar]
- Sun, L.; Franco, O.H.; Hu, F.B.; Cai, L.; Yu, Z.; Li, H.; Ye, X.; Qi, Q.; Wang, J.; Pan, A.; et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly Chinese. J. Clin. Endocrinol. Metab. 2008, 93, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Lee, M.S.; Pan, W.H.; Liu, K.L.; Yu, M.S. Reproducibility and validity of a Chinese food frequency questionnaire used in Taiwan. Asia Pac. J. Clin. Nutr. 2006, 15, 161–169. [Google Scholar] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nothlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Schulze, M.; Hu, F.B.; Hankinson, S.E.; Holmes, M.D. A dietary pattern derived to correlate with estrogens and risk of postmenopausal breast cancer. Breast Cancer Res. Treat. 2012, 132, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Manson, J.E.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; Colditz, G.A.; Hu, F.B. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am. J. Clin. Nutr. 2005, 82, 675–684, quiz 714–675. [Google Scholar] [PubMed]
- Weikert, C.; Hoffmann, K.; Dierkes, J.; Zyriax, B.C.; Klipstein-Grobusch, K.; Schulze, M.B.; Jung, R.; Windler, E.; Boeing, H. A homocysteine metabolism-related dietary pattern and the risk of coronary heart disease in two independent German study populations. J. Nutr. 2005, 135, 1981–1988. [Google Scholar] [PubMed]
- Rumberger, J.M.; Peters, T., Jr.; Burrington, C.; Green, A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes 2004, 53, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Ward, N.C.; Burke, V.; Beilin, L.J.; Puddey, I.B. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J. Nutr. 2007, 137, 363–367. [Google Scholar] [PubMed]
- Doyle, W.; Crawley, H.; Robert, H.; Bates, C.J. Iron deficiency in older people: Interactions between food and nutrient intakes with biochemical measures of iron; further analysis of the National Diet and Nutrition Survey of people aged 65 years and over. Eur. J. Clin. Nutr. 1999, 53, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Monsen, E.R.; Balintfy, J.L. Calculating dietary iron bioavailability: Refinement and computerization. J. Am. Diet. Assoc. 1982, 80, 307–311. [Google Scholar] [PubMed]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2016; The Organisation for Economic Co-operation and Development: Paris, France, 2016. [Google Scholar]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia: Protein, amino acid metabolism and therapy. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Poustchi, H.; Eslamparast, T.; Hekmatdoost, A. Egg consumption and risk of non-alcoholic fatty liver disease. World J. Hepatol. 2017, 9, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Kontogianni, M.D.; Tileli, N.; Margariti, A.; Fragopoulou, E.; Tiniakos, D.; Zafiropoulou, R.; Papatheodoridis, G. The impact of cereal grain consumption on the development and severity of non-alcoholic fatty liver disease. Eur. J. Nutr. 2014, 53, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Gaens, K.H.J.; Niessen, P.M.G.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.M.; Driessen, A.; Wolfs, M.G.M.; Hofker, M.H.; Bloemen, J.G.; Dejong, C.H.; et al. Endogenous formation of Nε-(carboxymethyl)lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J. Hepatol. 2012, 56, 647–655. [Google Scholar] [CrossRef] [PubMed]

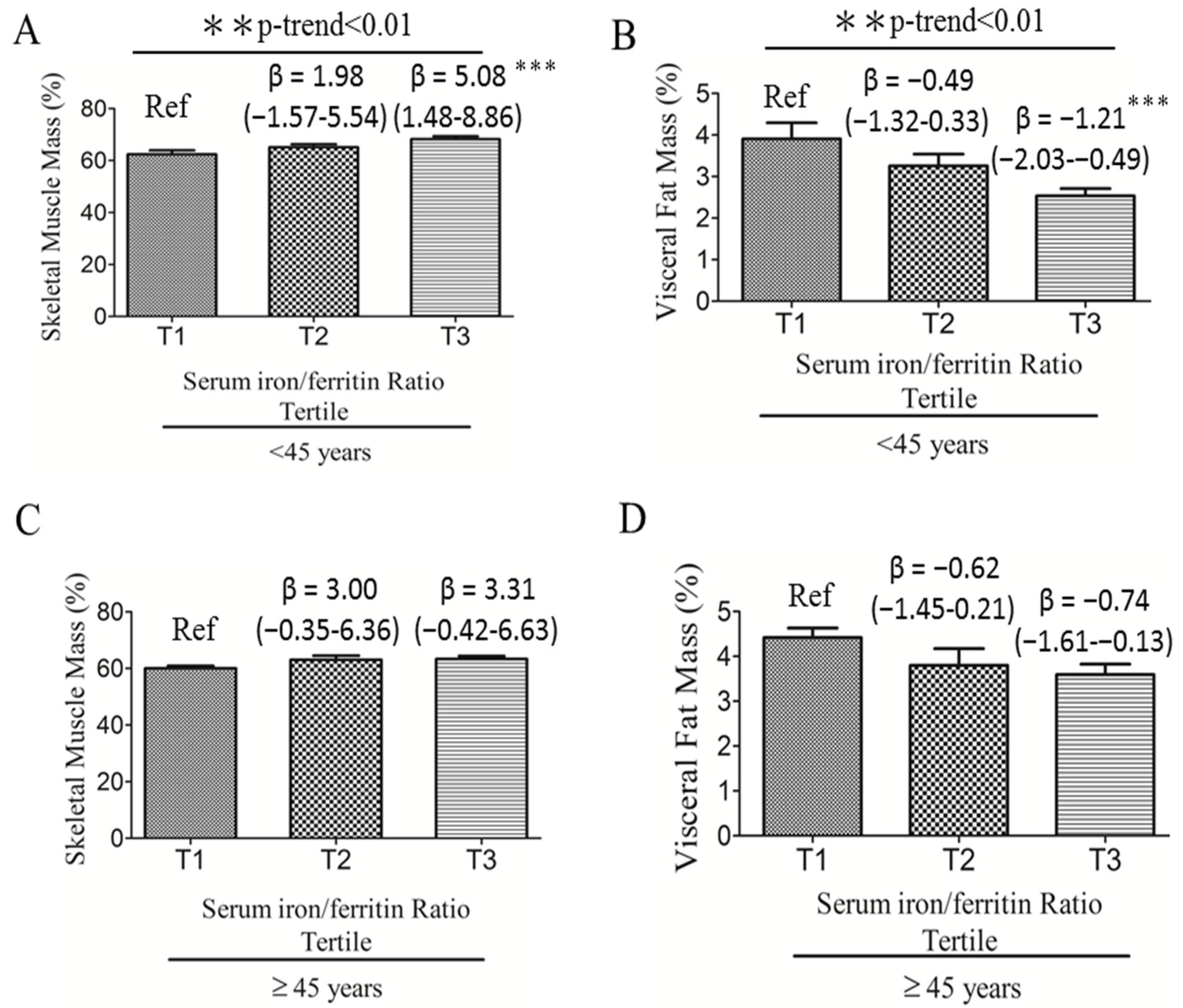
| Characteristics | <45 Y (n = 65) | ≥45 Y (n = 52) | p Value * |
|---|---|---|---|
| Age (y) | 32.85 ± 7.30 | 55.73 ± 6.30 | <0.001 |
| BMI (kg/m2) | 23.27 ± 6.49 | 23.60 ± 3.97 | 0.040 |
| Waist circumference (cm) | 79.57 ± 15.09 | 80.63 ± 9.3 | 0.017 |
| Overweight and obese (n, %) | 17 (26.2%) | 21 (40.4%) | 0.102 |
| Central obesity (n, %) | 19 (29.7%) | 30 (58.7%) | 0.002 |
| Diabetes mellitus (n, %) | 2 (3.1%) | 5 (9.6%) | 0.318 |
| Dyslipidemia (n, %) | 18 (28.1%) | 24 (49.0%) | 0.023 |
| MetS (n, %) | 8 (12.7%) | 17 (34.7%) | 0.006 |
| NAFLD stage (n, %) | 0.006 | ||
| Control | 14 (21.5%) | 5 (9.6%) | |
| Mild | 41 (63.1%) | 26 (50.0%) | |
| Moderate/severe | 10 (15.4%) | 21 (40.4%) | |
| Self-reported post-menopause | 0 (0%) | 33 (63.5%) | <0.001 |
| Anemia (n, %) | 9 (13.8%) | 6 (11.5%) | 0.778 |
| Iron deficiency (n, %) | 5 (7.7%) | 3 (5.7%) | 0.728 |
| Iron deficiency anemia (n, %) | 5 (7.7%) | 3 (5.7%) | 0.728 |
| Iron overload (n, %) | 6 (9.2%) | 5 (9.6%) | 0.883 |
| Inflammation markers | |||
| AST (U/L) | 21.94 ± 9.69 | 28.39 ± 13.47 | <0.001 |
| ALT (U/L) | 22.52 ± 18.21 | 32.14 ± 25.42 | <0.001 |
| Body compositions | |||
| Body fat mass (%) | 29.02 ± 6.37 | 32.31 ± 5.07 | 0.001 |
| Skeletal muscle mass (%) | 65.27 ± 6.30 | 62.00 ± 5.02 | 0.001 |
| Visceral fat (%) | 3.22 ± 1.43 | 3.97 ± 1.25 | <0.001 |
| Subcutaneous fat (%) | 25.92 ± 5.02 | 28.34 ± 3.85 | 0.002 |
| Serum iron biomarkers | |||
| Iron (µg/dL) | 99.66 ± 42.35 | 92.12 ± 35.19 | <0.001 |
| TS (%) | 26.67 ± 12.65 | 25.91 ± 10.88 | 0.993 |
| Ferritin (ng/mL) | 54.30 ± 72.72 | 98.81 ± 83.97 | <0.001 |
| Iron:ferritin ratio | 4.41 ± 3.39 | 2.12 ± 2.59 | <0.001 |
| Hepcidin (ng/mL) | 79.90 ± 107.59 | 139.17 ± 121.39 | 0.027 |
| Hb (g/dL) | 14.03 ± 3.01 | 13.30 ± 2.13 | 0.402 |
| HbA1c (%) | 5.50 ± 0.66 | 6.16 ± 1.44 | <0.001 |
| Free Hb (µg/mL) | 133.34 ± 55.73 | 164.95 ± 56.03 | 0.016 |
| CML (µg/mL) | 258.14 ± 149.01 | 246.81 ± 145.19 | 0.720 |
| Iron:Ferritin Ratio | Control vs. Mild Fatty Liver | Mild vs. Moderate/Severe Fatty Liver | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| All | ||||
| Univariate | 0.923 (0.795–1.072) | 0.295 | 0.734 (0.576–0.935) | 0.012 |
| model 1 | 0.929 (0.791–1.092) | 0.373 | 0.799 (0.626–1.021) | 0.073 |
| <45 years | ||||
| Univariate | 0.931 (0.783–1.107) | 0.419 | 0.508 (0.281–0.917) | 0.025 |
| model 1 | 0.932 (0.783–1.110) | 0.430 | 0.531 (0.298–0.946) | 0.032 |
| ≥45 years | ||||
| Univariate | 1.017 (0.621–1.666) | 0.947 | 0.953 (0.752–1.208) | 0.691 |
| model 1 | 0.933 (0.551–1.579) | 0.796 | 1.006 (0.908–1.114) | 0.912 |
| Food Groups | Explained Variations (%) | Factor Loadings * |
|---|---|---|
| Beef and lamb | 17.79 | 0.38 |
| Dairy products | 9.07 | 0.27 |
| Fruits | 8.03 | 0.26 |
| Whole grains | 6.74 | 0.24 |
| Eggs | 4.71 | 0.20 |
| Rice | 12.54 | −0.32 |
| Noodles | 9.20 | −0.28 |
| Bread and pastries | 8.26 | −0.26 |
| Stir-fried food | 7.87 | −0.26 |
| Deep-fried food | 6.50 | −0.23 |
| Organs | 4.99 | −0.20 |
| Steamed/boiled/raw food | 3.94 | 0.18 |
| Dark green vegetables | 3.78 | 0.18 |
| Western dishes | 2.80 | 0.15 |
| White and light green vegetables | 2.09 | 0.13 |
| Orange, red, and purple vegetables | 2.07 | 0.13 |
| Coffee | 1.63 | −0.12 |
| Stew food | 1.57 | −0.11 |
| Seafood | 1.30 | 0.10 |
| Fried desserts | 1.03 | 0.09 |
| Homemade food | 0.80 | −0.08 |
| Animal fats | 0.75 | 0.08 |
| Grilled or barbecued food | 0.74 | 0.08 |
| Root starch | 0.54 | −0.07 |
| Seaweed | 0.48 | −0.06 |
| Desserts | 0.40 | 0.06 |
| Eat away from home | 0.30 | −0.05 |
| Duck and goose | 0.28 | −0.05 |
| Processed meats | 0.17 | 0.04 |
| Soy products | 0.02 | 0.01 |
| Chicken and pork | 0.01 | 0.01 |
| Sugar beverages | 0.00 | 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabrina, N.; Bai, C.-H.; Chang, C.-C.; Chien, Y.-W.; Chen, J.-R.; Chang, J.-S. Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women. Nutrients 2017, 9, 833. https://doi.org/10.3390/nu9080833
Sabrina N, Bai C-H, Chang C-C, Chien Y-W, Chen J-R, Chang J-S. Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women. Nutrients. 2017; 9(8):833. https://doi.org/10.3390/nu9080833
Chicago/Turabian StyleSabrina, Nindy, Chyi-Huey Bai, Chun-Chao Chang, Yi-Wen Chien, Jiun-Rong Chen, and Jung-Su Chang. 2017. "Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women" Nutrients 9, no. 8: 833. https://doi.org/10.3390/nu9080833






