1. Introduction
Lead (Pb) is a toxic contaminating heavy metal, with no constructive biological role, that remains a public health concern. The key sources of Pb in the human environment are diet, cosmetics, Pb-based paint, soil and dust from Pb-contaminated paint, gasoline, mining, and industrial activity [
1,
2,
3]. Pb can be inhaled, as it can enter the atmosphere through industrial burning, smelting, and the emissions of vehicles with leaded gasoline. Pb can also enter drinking water through water supply pipes. Consumption of contaminated food and water are also potent sources of Pb exposure and toxicity. Pb is immensely toxic even at low concentrations, and mainly accumulates in bones, the brain [
4,
5], the liver, kidneys [
6] and muscles causing several serious disorders, such as oxidative stress [
6,
7], carcinogenesis [
8], disruption of calcium homeostasis, degenerative changes, nervous disorders [
9,
10], and sickness and tissue diseases, predominantly in children [
11]. The half-life of Pb in blood plasma is 27 days, in blood is 35 days, in the brain it is about two years, and in bone it may persist for decades [
12,
13]. Oxidative stress is an imbalance between reactive oxygen species (ROS) and cellular antioxidant systems. Many studies propose oxidative stress as one of the significant mechanisms of the toxic effects of Pb [
5,
14,
15].
The standard treatment for heavy metal poisoning is chelation therapy with the most commonly used chelating agents being CaNa
2EDTA and Meso-2,3-dimercaptosuccinic acid (DMSA). However, adverse effects, including renal injury, malaise, nausea, vomiting, skin reactions [
16,
17] and progressive deficiencies of copper, zinc and other essential trace nutrients, which are an indispensable part of the body’s antioxidant defenses [
18]. Therefore, nontoxic natural alternatives of chelating agents have been studied in recent years.
It has been reported that some lactic acid bacteria (LAB) strains, including
L. rhamnosus,
L. plantarum, and
Leuconostoc mesenteroides, are capable of binding and removing heavy metals, such as silver, cadmium and lead in vitro [
19,
20,
21]. Furthermore, various recent studies have revealed that LAB can effectively protect against metal-induced oxidative stress through antioxidation in vivo [
22,
23,
24]. Based on this, LAB may serve as a candidate to relieve the symptoms of heavy metal toxicity and their fermented products can be used as dietary fortification against heavy metal poisoning.
The aim of the present study was to select a novel probiotic LAB strain with high Pb-binding capacity and investigate different possible doses to treat acute Pb-induced tissue damage through biochemical enzyme analyses and histopathological studies. This study provides useful information on the utilization of LAB to aid recovery from Pb poisoning.
2. Materials and Methods
2.1. Chemicals and Reagents
Kits used to measure the levels of malondialdehyde (MDA; Njjcbio A003-1 kit), total superoxide dismutase (T-SOD; Njjcbio A001-1 kit), glutathione (GSH; Njjcbio A006-2kit), GSH peroxidase (GSH-PX; Njjcbio A005 kit), aspartate aminotransferase (AST; Njjcbio C010-2 kit), alanine aminotransferase (ALT; Njjcbio C009-2 kit) and a protein quantification kit, rapid (Njjcbio A045-2,) were procured from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Lead dinitrate, lead acetate and other analytical reagents were purchased from the Tianli Chemical Reagent Company (Tianjin, China).
2.2. Bacterial Strain and Culture
L. rhamnosus KLDS1.0205, KLDS1.0911 and KLDS1.0912, L. bulgaricus KLDS1.0207 and KLDS1.9201, L. plantarum KLDS1.0386 and KLDS1.0344, L. acidophilus KLDS1.1003, L. helveticus KLDS1.0903 and L. casei KLDS1.0351 were isolated from traditional dairy products in Sinkiang Province, China and identified by API 50CH strips and 16S rRNA gene similarity analysis. These LAB strains were stored at the Key Laboratory of Dairy Science (KLDS), Ministry of Education, China. All strains were anaerobically incubated in De Man, Rogosa and Sharpe (MRS) broth (Hopebio Company, Qingdao, China) at 37 °C for 18 h and were sub-cultured twice prior to the experiment.
2.3. Estimation of Pb Binding and Pb Tolerance
The Pb binding ability of 10 strains was analyzed as previously described with little modification [
25]. All strains were incubated for 18 h and the cultured biomass was centrifuged at 8000 rpm for 20 m. The centrifuged mass was washed twice with ultrapure water to obtain cell pellets. The bacterial concentration was adjusted to 1 g/L (wet weight) using ultrapure water containing 50 mg/L lead dinitrate and samples were then incubated at 37 °C for 24 h (pH 6.0). The samples were centrifuged at 8000 rpm for 20 m and the residual Pb concentrations of the supernatants were measured by flame atomic absorption spectrophotometry (Spectra AA 220; Varian, Palo Alto, CA, USA). The metal removal efficiency based on mass balance was calculated using the following equation:
where, C
i and C
e are the initial Pb concentration and residual Pb concentration after removal, respectively.
The Pb tolerance of each strain was determined by the minimum inhibitory concentration (MIC) approach [
26]. MRS agar medium containing 50 to 1000 mg/L lead dinitrate solution was prepared, and 10 μL of cultured LAB strain was spotted on the MRS agar medium at an inoculum level of 1 × 10
9 CFU/mL. LAB growth was recorded after cultivation at 37 °C for 48 h. The minimum concentration of Pb that completely inhibited LAB growth was considered as the MIC in this study.
2.4. Equilibrium Isotherm and Kinetic Study
The equilibrium isotherm was performed as previously reported [
27], and the harvested cell pellets were suspended in ultrapure water containing 5 to 75 mg/L lead dinitrate to give a final bacterial concentration of 1 g/L (dry weight). The Pb binding assay was then conducted with an initial pH of 6.0 and the equilibrium content of Pb bound by the bacterium was expressed as follows:
where, C
i and C
e are the initial Pb concentration and residual Pb concentration after removal, respectively; and m/V = 1 g/L.
The Langmuir, Freundlich and Langmuir–Freundlich models were used to determine the sorption equilibrium between the biosorbent and metal ions. Isotherm constants for the three models were obtained by non-linear regression methods [
28,
29].
The kinetic study was conducted as previously described [
27], the harvested cell pellets were suspended in ultrapure water containing 50 mg/L lead dinitrate to give a final bacterial concentration of 1 g/L (dry weight). The Pb binding assay was then conducted with an initial pH of 6.0 and the concentration of Pb in the supernatant was detected at different time intervals up to 240 m.
The pseudo first and second-order rate equations were used in the kinetic model of Pb biosorption with the integrated form of the pseudo first-order model as follows:
where q
t is adsorption capacity; and time t and k
1 are first-order rate constants. k
1 can be determined from the slope of the plot of
vs. t.
The pseudo second-order integrate equation is expressed as:
where k
2 can be determined from the interception of the linearized plot of t/q
t vs. t.
2.5. Scanning Electron Microscopy (SEM) Analysis
The samples for SEM observation were prepared as described earlier [
30]. The untreated harvested cell pellets and those treated with Pb (50 mg/L) were fixed by 2.5% glutaraldehyde (
v/
v) at 4 °C for 1.5 h, and washed thrice with phosphate buffer solution. Supernatants were discarded and the cell pellets treated with different concentrations (50%, 70%, 90% and 100%) of alcohol as well as a mixture of alcohol and t-butanol (1:1) to wash cells successively. Finally, the cell pellets were eluted with plain t-butanol. The specimens were then put in a freeze-drier for 4 h and sputter-coated with gold. The scanning and photography were performed using SEM with an energy dispersive spectrometer (EDS).
2.6. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
The untreated harvested cell pellets and those treated with Pb (50 mg/L) were lyophilized and mixed with KBr powder as KBr discs. Discs containing 2% (w/w) of finely ground powder of each sample were prepared. The FT-IR technique was employed to characterize the changes in the functional groups on the untreated cell pellets and those treated with Pb.
2.7. In Vivo Protective Potential of L. bulgaricus KLDS1.0207 Against Acute Pb Toxicity
2.7.1. Animals and Experimental Design
A total of 72 female BALB/c mice (6–7 weeks old, weighing 16 to 20 g) were purchased from the Vital River Laboratory Animal Technology Company (Beijing, China) and housed in a room under controlled environmental conditions at 25 °C and with a 12-h light/dark cycle. All the mice were put into plastic cages for one week before the begin of the experiments. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Northeast Agricultural University under the approved protocol number Specific pathogen free rodent management (SRM)-06.
Mice were fed with standard commercial pellets and water ad libitum and were randomly divided into two major groups. The prevention and therapy groups were divided into three subgroups and six subgroups, respectively. Each subgroup had a total of eight BALB/c mice. The details of the administration are shown in
Table 1. On the basis of Azar’s studies [
31], the oral dose of lead acetate [(CH
3COO)
2Pb·3H
2O] was 100 mg/kg body weight. The dose of DMSA used in this study was 50 mg/kg/day [
17,
32].
All of the therapy groups had their feces collected every week to determine Pb concentration. The animals were then sacrificed under ether anesthesia, and blood samples were collected with Pb-free needles, and all tissue samples were obtained and kept at −80 °C for biochemical assays and estimation of the Pb concentration. Some kidney samples were put into 10% neutral formalin to analyze their pathology.
2.7.2. Determination of Pb in Blood, Feces and Tissue
Samples were digested in concentrated HNO3 by using a microwave digestion system. Pb concentration in the livers, kidneys, blood, and feces was determined by a graphite furnace atomic absorption spectrophotometer.
2.7.3. Biochemical Assays
The levels of MDA and GSH and the activities of T-SOD and GSH-Px in the mouse kidneys and livers, and the activities of ALT and AST in the mouse serum were measured by using the assay kit in accordance with the recommendations of the manufacturer.
2.7.4. Histopathological Studies
The kidneys were fixed in 10% neutral formalin for 48 h. Samples were then embedded in paraffin, sliced into 5 μm thickness and stained with hematoxylin-eosin (H&E) for examination by light microscopy.
2.8. Statistical Analysis
All values are expressed as the mean ± standard deviation (SD). A minimum of three independent experiments were carried out for each assay. The statistical significance of data comparisons was determined using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test. Values of p < 0.05 were considered to be statistically significant.
4. Discussion
Pb is a prevalent environmental pollutant with no beneficial biological role. It adversely induces oxidative stress damage to the host. Many researches have reported that LAB can not only sequester Pb, but also relieve the oxidative damage [
1,
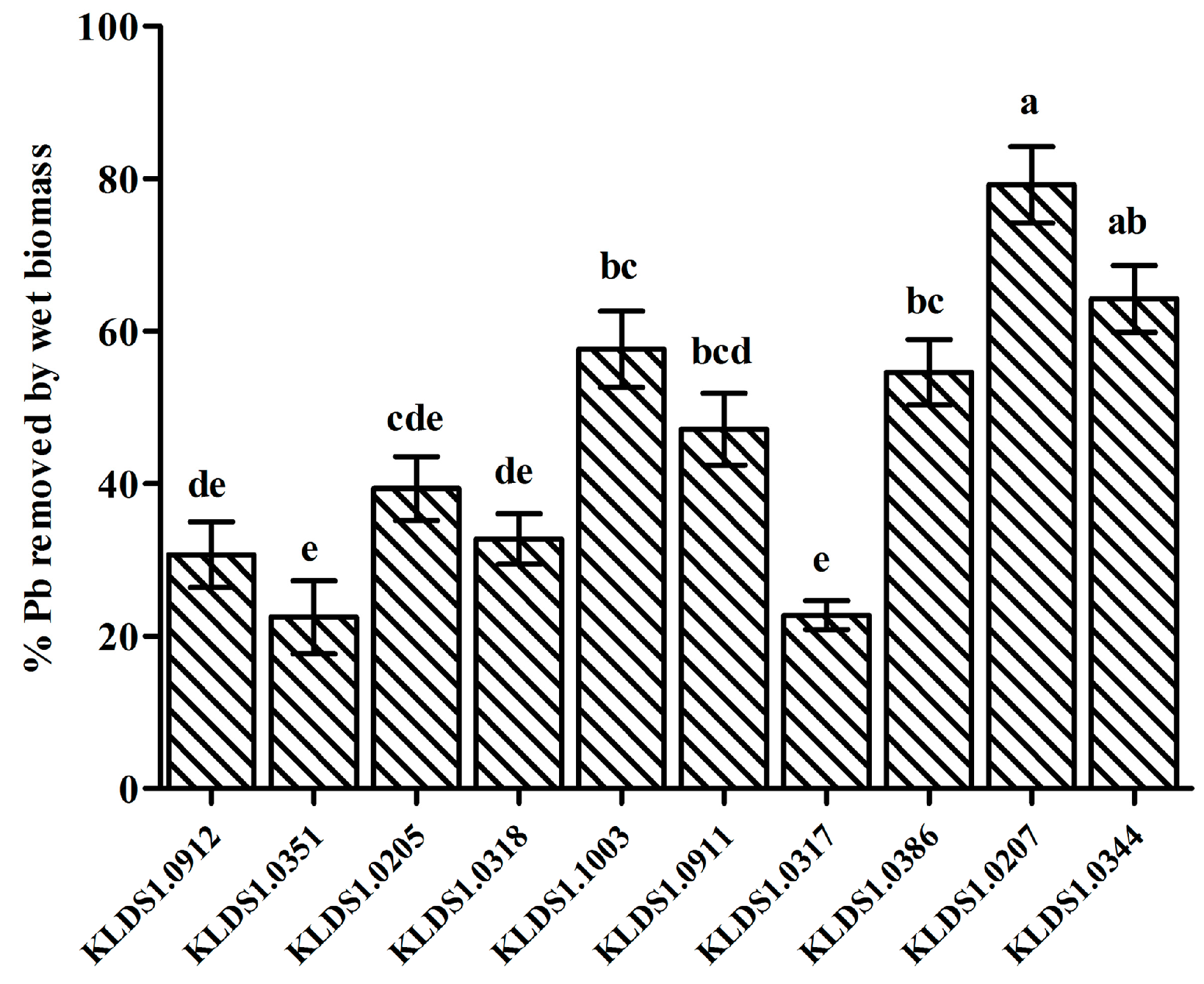
2]. Screening LAB strains against Pb toxicity should take a number of properties into consideration. First, LAB strains must show high Pb-binding ability, enabling them to bind Pb before the intestinal absorption of Pb by the host. Second, LAB strains should exhibit high resistance to Pb to avoid it being poisoned. Furthermore, to perform Pb removal in the gastrointestinal tract, it is necessary for the screened strains to remain viable in high concentrations of bile and stomach acids. In the present study,
L. bulgaricus KLDS1.0207 showed the best binding aptitude among 10 strains (
Figure 1) and had a remarkable tolerance to Pb (
Table 2). In addition,
L. bulgaricus KLDS1.0207 showed good resistance to simulated gastrointestinal tract conditions (data not shown). Based on these properties,
L. bulgaricus KLDS1.0207 was selected as a potential strain for the further study of its Pb biosorption mechanism and in vivo assays.
Various mechanisms of metal biosorption, including adsorption, ion exchange, complexation, chelation and microprecipitation have been proposed because of differences in the bacterial structures among the species [
33]. The cell walls of the gram-positive bacteria consist of peptidoglycans, teichoic acids, proteins and polysaccharides [
34,
35]. These contents also contain negatively charged functional groups, which serve as the primary sites of metal ion sorption on a bacterium surface [
36]. The phosphate and carboxyl group present in peptidoglycans and teichoic acids are the primary sites of metal ion binding on the surface of the bacterial cell [
37]. SEM micrographs and EDS analysis (
Figure 4) confirmed that the added toxic Pb metal was localized on the cell surface evenly. A previous study has reported a similar phenomenon in
L. mesenteroides after Pb binding [
21], this phenomenon may be related to the passive physicochemical adsorption mechanism.
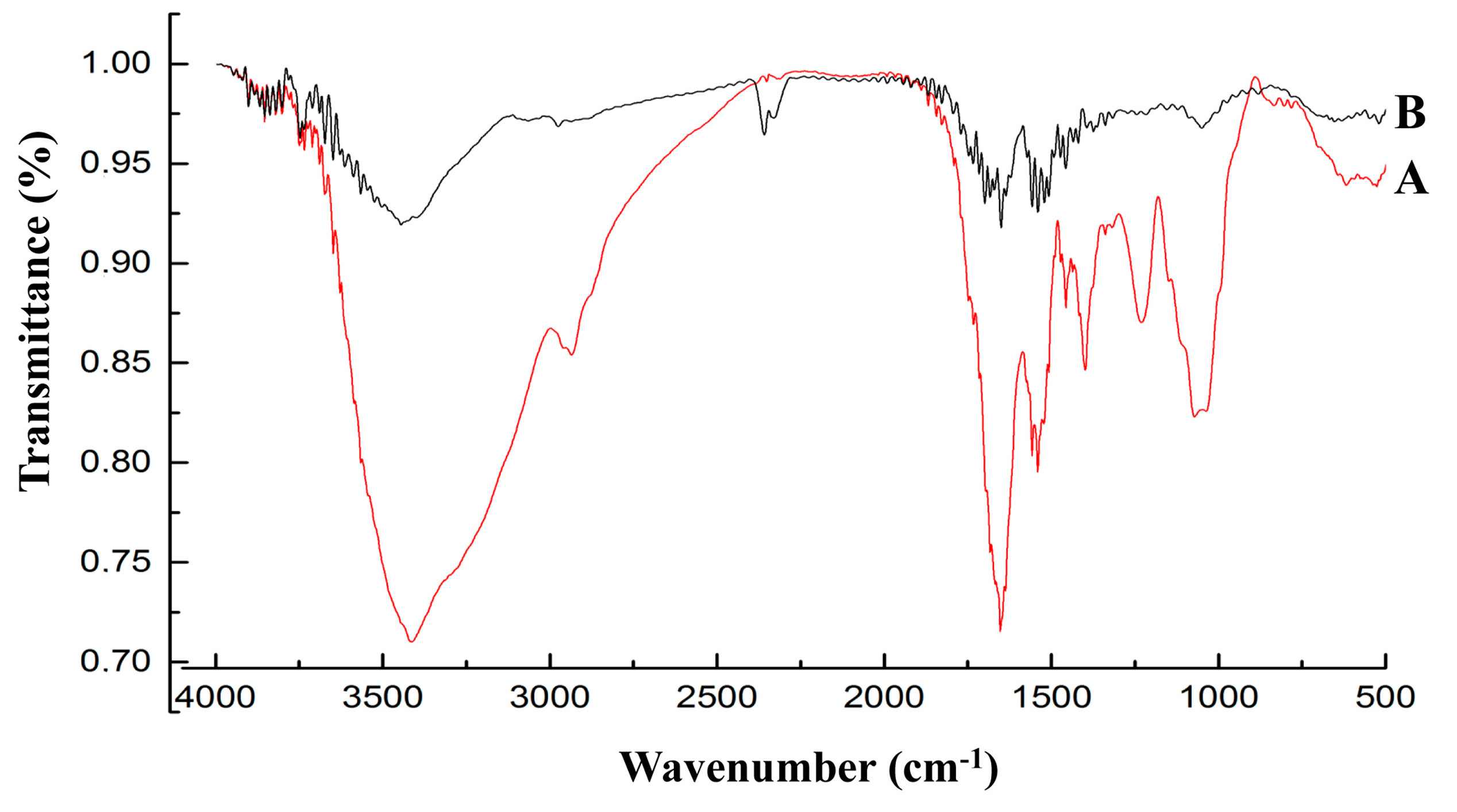
By analyzing the FT-IR spectrum (
Figure 5), it was able to be speculated that the functional groups (carboxyl, phosphoryl, hydroxyl, amino and amide) of biological macromolecules (fatty acids, polysaccharides, S-layer proteins and teichoic acid) bind Pb through complexation, ion exchange and physical adsorption (electrostatic attraction). These predictions were consistent with the previous assertion that the carboxyl, hydroxyl and amide groups were involved in Pb uptake [
38,
39,
40]. It may be necessary in future studies to elucidate the effects of the specific groups in this mechanism.
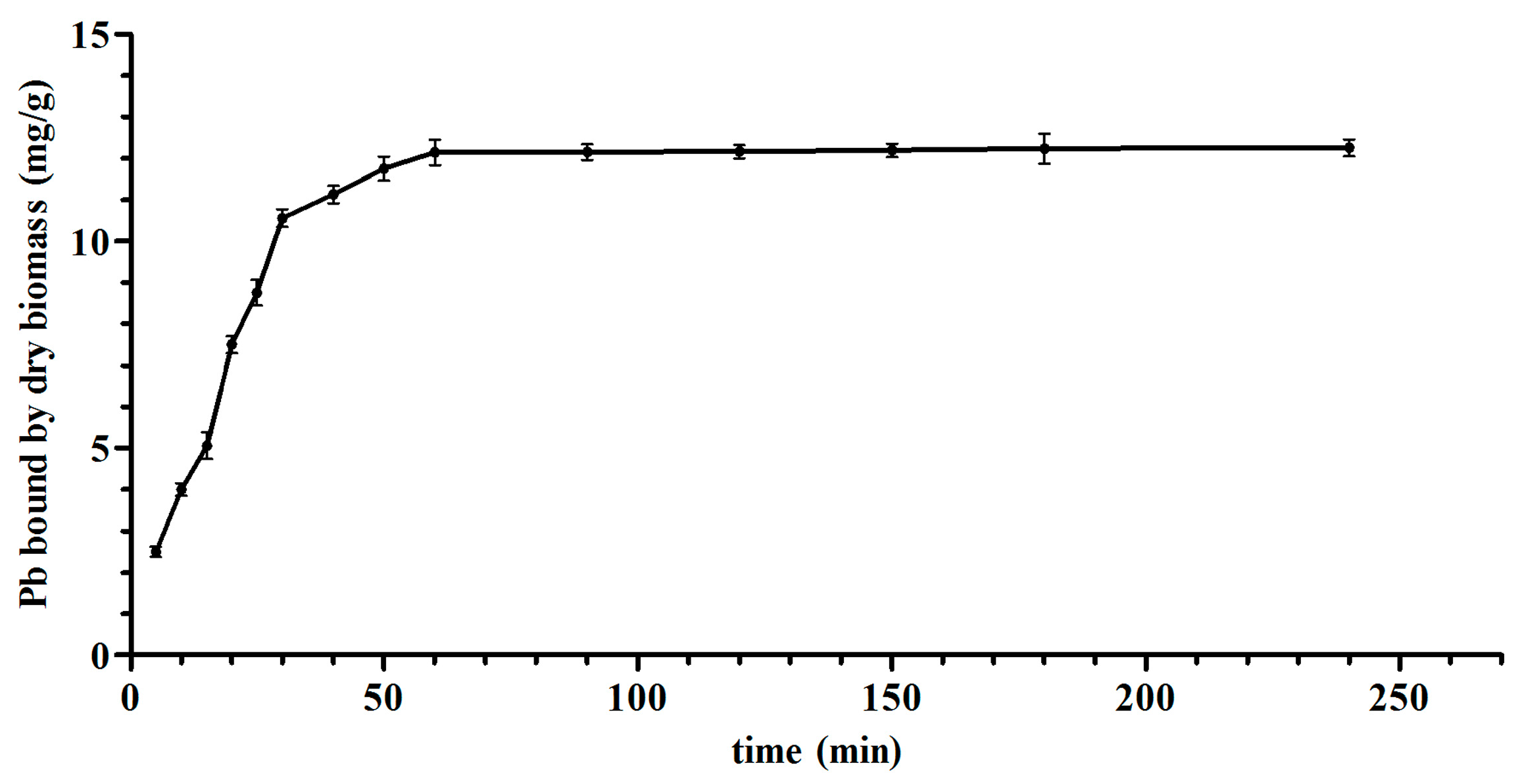
The thermodynamic and kinetic models of
L. bulgaricus KLDS1.0207 binding Pb fit with the Langmuir–Freundlich model and pseudo second-order kinetic model, respectively (
Figure 2 and
Figure S1), this is in line with the cadmium binding characterization of an acidophilic bacterium [
27], indicating that adsorption (physical and chemical) is a complex process.
As
L. bulgaricus KLDS1.0207 had an excellent Pb-binding capacity, the protective effects of it against acute Pb toxicity in mice was evaluated using prevention and therapy groups. In the present study, mice were orally given a lead acetate solution of 100 mg/kg bodyweight to stimulate acute Pb toxicity, followed by treatment with different concentrations of
L. bulgaricus KLDS1.0207 for two weeks. Our study showed that the high dose therapy group had a higher ability to increase fecal excretion of Pb than low dose groups (
Table 4). Similar results were observed for the other
Lactobacillus strains, which could modulate intestinal heavy metal absorption in mice by increasing fecal heavy metal excretion [
22,
23,
41].
The high concentration of Pb in different tissues was associated with increased oxidative reaction, which might be responsible, at least in part, for Pb-induced toxic effects [
42]. Several studies have reported a possible link between oxidative stress and the disruption of metal ion homeostasis [
43,
44,
45]. Our results showed that
L. bulgaricus KLDS1.0207 lowered Pb-induced oxidative stress and facilitated a protective role in reducing the lipid peroxide by decreasing MDA concentration and improving other antioxidants, such as T-SOD, GSH and GSH-Px in the liver and kidneys (
Figure 6).
Changes in the ALT and AST levels are often used for liver pathological examination, and AST/ALT is an important biochemical indicator [
46,
47]. ALT mainly exists in the liver cell plasma. When liver cell damage is lower, changes in liver-cell-membrane permeability elevate ALT levels in the blood. On the other hand, increased AST blood levels are a result of severely damaged liver cells. It thus follows that higher AST/ALT ratios imply more severe liver cell damage. The treatment of
L. bulgaricus KLDS1.0207 could improve the ALT and AST levels (
Table 6).
Compared with the dose groups, the drug group was able to increase Pb excretion. Nonetheless, the drug DMSA has been shown in a previous study to have some adverse effects, including depletion of zinc and copper in the body [
17]. In all, the results from the present study have shown that the most effective dose in the therapy groups was 1 × 10
10 CFU/mL of
L. bulgaricus KLDS1.0207 in 0.4 mL skim milk. This was similar to the findings of earlier studies that 2 × 10
10 CFU/mL of
L. plantarum CCFM8246 (0.2 mL) and 1 × 10
9 Colony-Forming Units (CFU) of
L.
plantarum CCFM8610 (0.5 mL) were effective against copper and cadmium toxicity in mice, respectively [
22,
41]. These effective LAB strains can be freeze-dried or spray-dried to obtain the strain powders for the practical administration. Interestingly, LAB strains such as
L. plantarum and
L. rhamnosus can improve the absorption and bioavailability of several trace elements in animals [
48,
49]. Thus, it is very significant to exploit LAB as a heavy metal removal agent added to food or feed.