Nrf2 Activation by 5-lipoxygenase Metabolites in Human Umbilical Vascular Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Immunocytochemical Analysis
2.4. Real-Time PCR
2.5. Detection of ROS
2.6. Quantitative Analysis of 5-HETE, 5-HEPE, and 5-oxo-ETE in the Cells and Medium
2.7. Statistical Analysis
3. Results
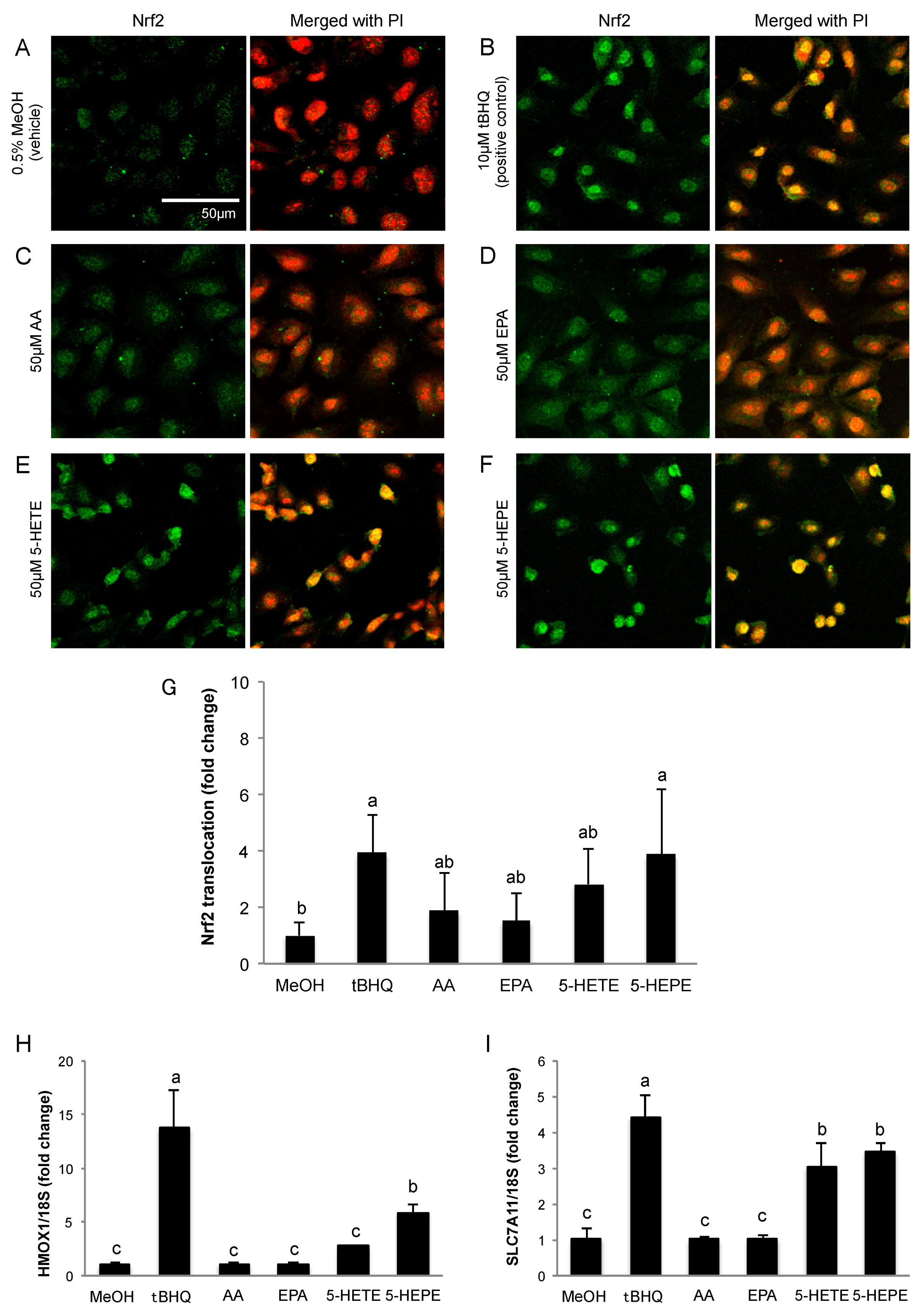
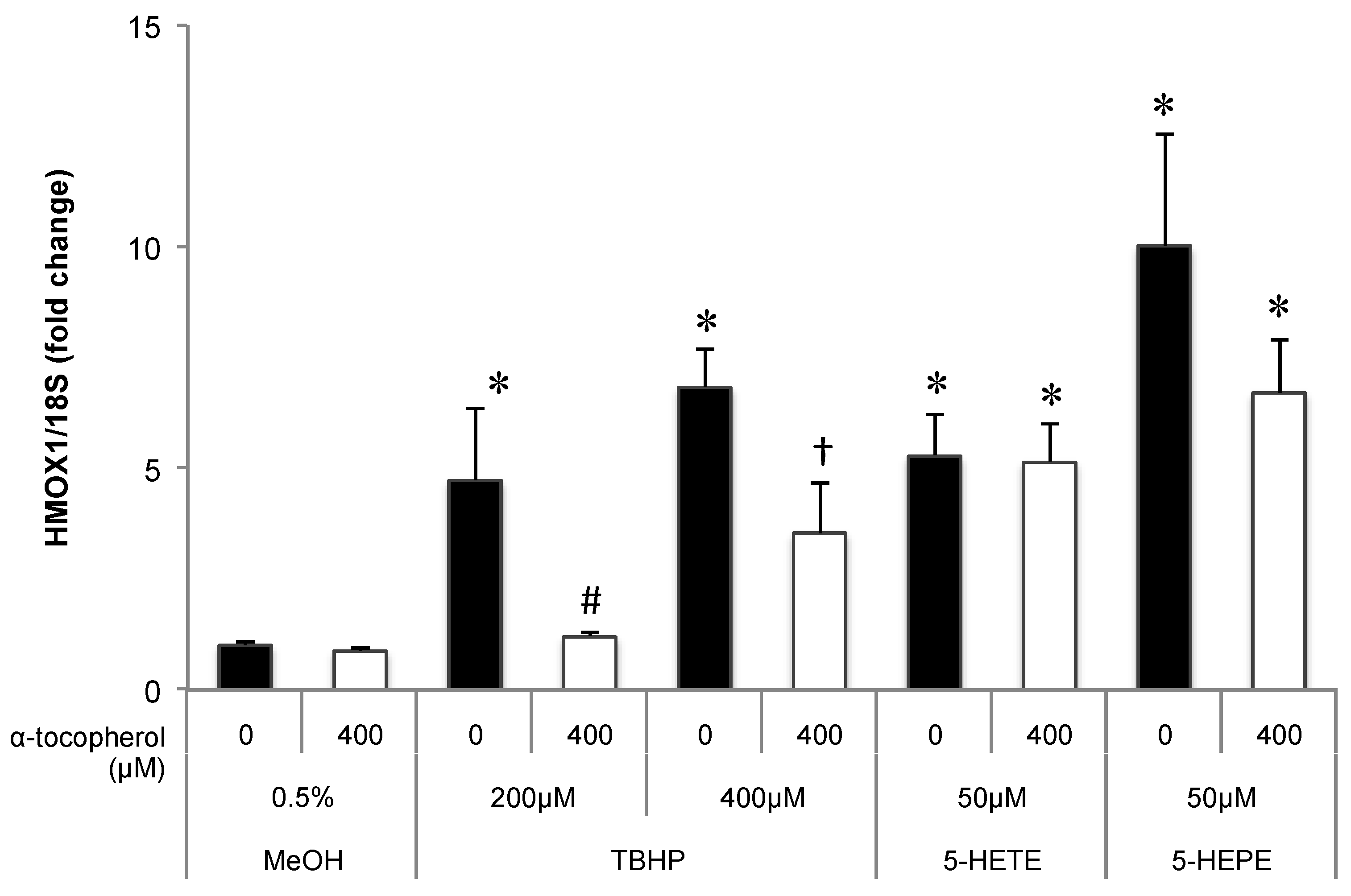
3.1. Effects of 5-HETE and 5-HEPE on the Nuclear Translocation of Nrf2 and Gene Expression of Anti-Oxidative Enzymes in HUVECs
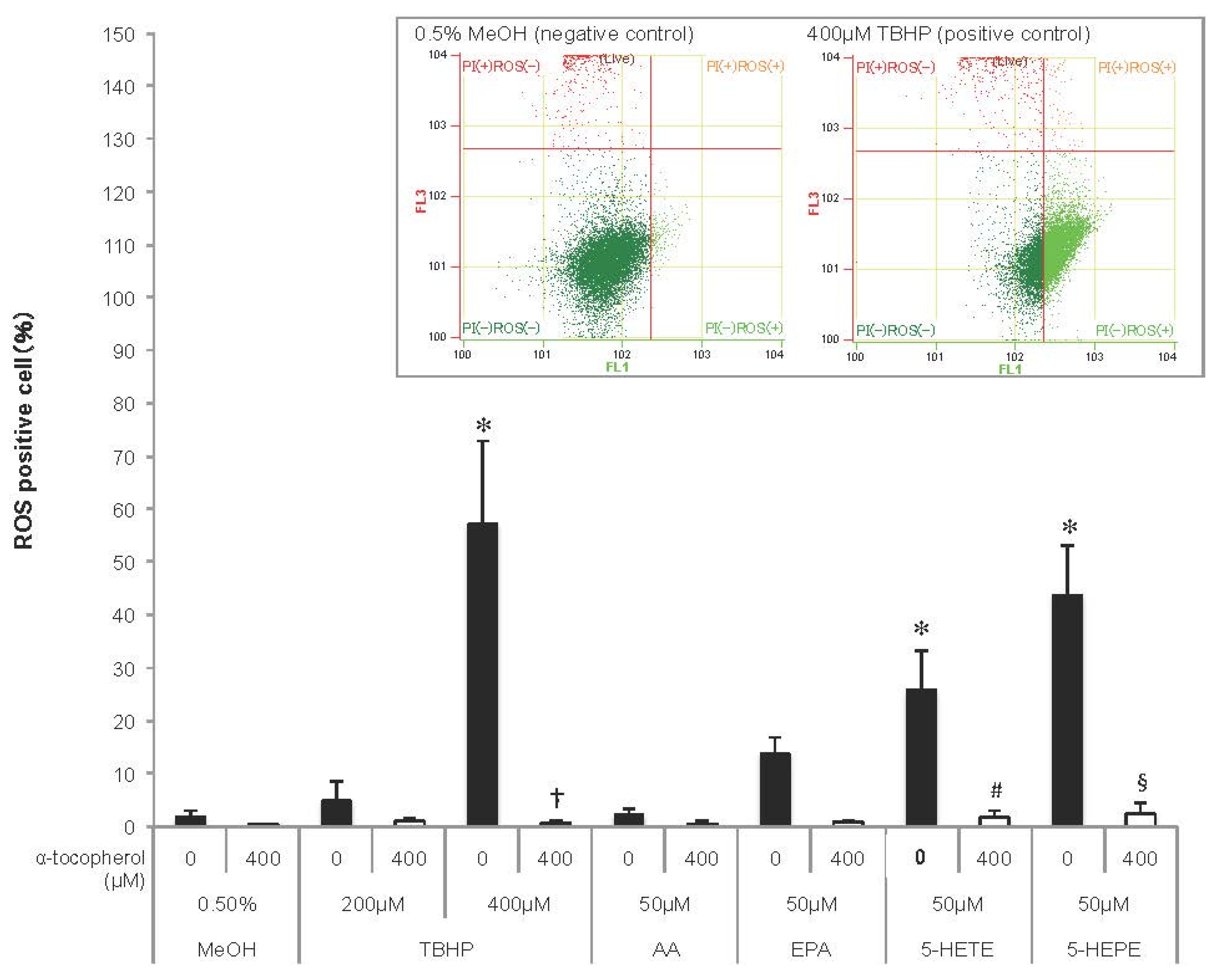
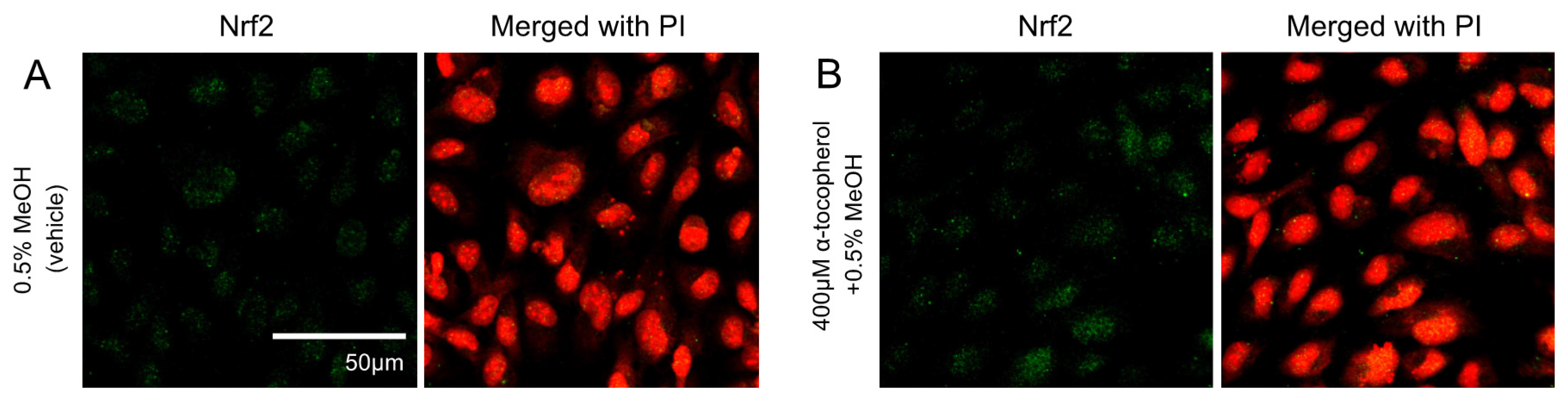
3.2. ROS Generation Induced by EPA and Hydroxy Fatty Acids in HUVECs
3.3. Metabolism of 5-HETE and 5-HEPE in HUVECs
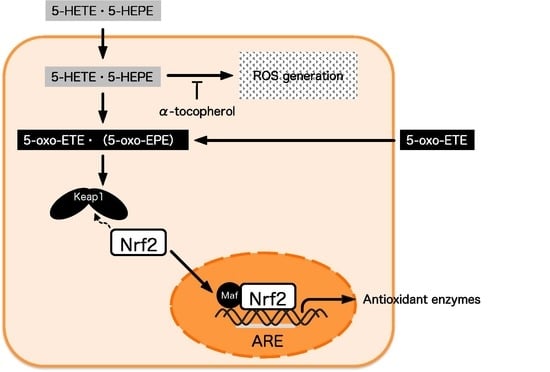
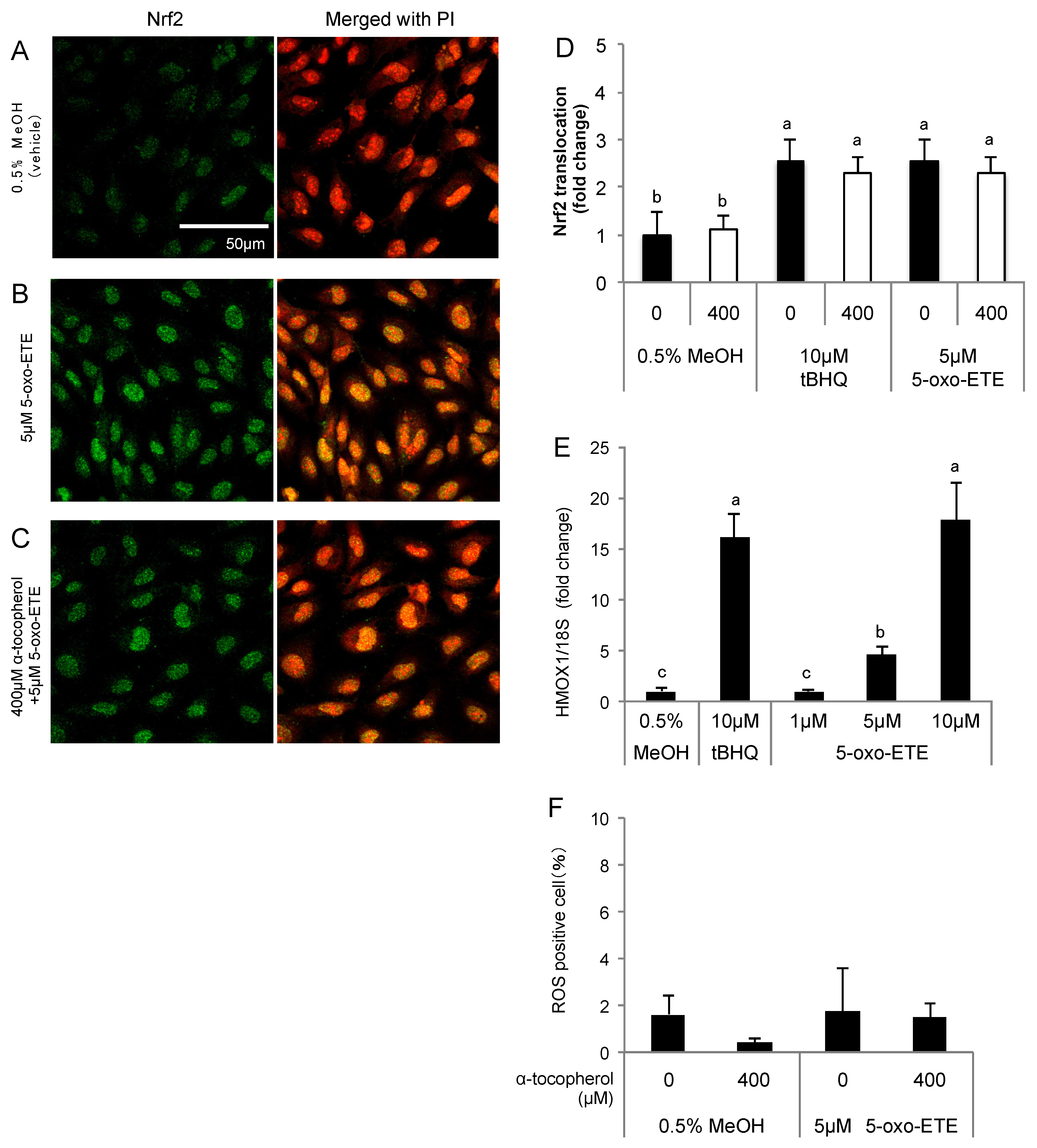
3.4. Effect of 5-oxo-ETE on Nrf2 Activation and ROS Generation in HUVECs
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and cardiovascular disease: The epidemiological evidence. Environ. Health Prev. Med. 2002, 6, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Rainger, G.E. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [PubMed]
- Matsumoto, T.; Funk, C.D.; Rådmark, O.; Höög, J.O.; Jörnvall, H.; Samuelsson, B. Molecular cloning and amino acid sequence of human 5-lipoxygenase. Proc. Natl. Acad. Sci. USA 1988, 85, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Funk, C.D. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc. Res. 2010, 86, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, P.J.; Mancini, J.A.; Riendeau, D.; Ford-Hutchinson, A.W. Identification and characterization of a novel microsomal enzyme with glutathione-dependent transferase and peroxidase activities. J. Biol. Chem. 1997, 272, 22934–22939. [Google Scholar] [CrossRef] [PubMed]
- Spanbroek, R.; Grabner, R.; Lotzer, K.; Hildner, M.; Urbach, A.; Ruhling, K.; Moos, M.P.W.; Kaiser, B.; Cohnert, T.U.; Wahlers, T.; et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Duroudier, N.P.; Tulah, A.S.; Sayers, I. Leukotriene pathway genetics and pharmacogenetics in allergy. Allergy 2009, 64, 823–839. [Google Scholar] [CrossRef]
- De Gaetano, G.; Donati, M.B.; Cerletti, C. Prevention of thrombosis and vascular inflammation: Benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol. Sci. 2003, 24, 245–252. [Google Scholar] [CrossRef]
- Bäck, M.; Powell, W.S.; Dahlén, S.-E.; Drazen, J.M.; Evans, J.F.; Serhan, C.N.; Shimizu, T.; Yokomizo, T.; Rovati, G.E. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br. J. Pharmacol. 2014, 171, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, J.T.; Thomas, M.J.; Hammett, M.J.; Carroll, C.; McCall, C.E.; Wykle, R.L. 5-L-hydroxy-6,8,11,14-eicosatetraenoate potentiates the human neutrophil degranulating action of platelet-activating factor. Biochem. Biophys. Res. Commun. 1983, 268, 1–7. [Google Scholar] [CrossRef]
- Lee, T.H.; Mencia-Huerta, J.M.; Shih, C.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophils. J. Clin. Invest. 1984, 74, 1922–1933. [Google Scholar] [CrossRef]
- Kogure, R.; Toyama, K.; Hiyamuta, S.; Kojima, I.; Takeda, S. 5-Hydroxy-eicosapentaenoic acid is an endogenous GPR119 agonist and enhances glucose-dependent insulin secretion. Biochem. Biophys. Res. Commun. 2011, 416, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Oshiro, E.; Kikuchi, S.; Hakozaki, M.; Takahashi, H.; Kimura, K.-I. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J. Lipid Res. 2014, 55, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Keenan, A.H.; Newman, J.W.; Rutledge, J.C. The Postprandial Effects of a Moderately High-Fat Meal on Lipid Profiles and Vascular Inflammation in Alzheimer’s Disease Patients: A Pilot Study. J. Gen. Pr. 2014, 2, 186. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Schmidt, S.; Kressel, G.; Dong, H.; Willenberg, I.; Hammock, B.D.; Hahn, A.; Schebb, N.H. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Wilck, N.; Baumann, G.; Stangl, V.; Stangl, K.; Meiners, S. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc. Res. 2010, 85, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ishikado, A.; Morino, K.; Nishio, Y.; Nakagawa, F.; Mukose, A.; Sono, Y.; Yoshioka, N.; Kondo, K.; Sekine, O.; Yoshizaki, T.; et al. 4-Hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation. PLoS ONE 2013, 8, e69415. [Google Scholar] [CrossRef] [PubMed]
- Ishikado, A.; Nishio, Y.; Morino, K.; Ugi, S.; Kondo, H.; Makino, T.; Kashiwagi, A.; Maegawa, H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 402, 99–104. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing, version 3.1.2. Available online: https://www.r-project.org/ (accessed on 10 September 2017).
- Erlemann, K.-R.; Cossette, C.; Gravel, S.; Stamatiou, P.B.; Lee, G.-J.; Rokach, J.; Powell, W.S. Metabolism of 5-hydroxy-6,8,11,14-eicosatetraenoic acid by human endothelial cells. Biochem. Biophys. Res. Commun. 2006, 350, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Benard, M.; Straat, K.; Omarsdottir, S.; Leghmari, K.; Bertrand, J.; Davrinche, C.; Duga-Neulat, I.; Söderberg-Nauclér, C.; Rahbar, A.; Casper, C. Human cytomegalovirus infection induces leukotriene B4 and 5-lipoxygenase expression in human placentae and umbilical vein endothelial cells. Placenta 2014, 35, 345–350. [Google Scholar] [CrossRef]
- O’Flaherty, J.T.; Rossig, A.G. 5-hydroxyicosatetraenoate stimulates neutrophils by a stereospecific, G protein-linked mechanism. J. Biol. Chem. 1993, 268, 14708–14714. [Google Scholar] [PubMed]
- Lee, S.E.; Kim, G.-D.; Yang, H.; Son, G.W.; Park, H.R.; Cho, J.-J.; Ahn, H.-J.; Park, C.-S.; Park, Y.S. Effects of Eicosapentaenoic Acid on the Cytoprotection Through Nrf2-Mediated Heme Oxygenase-1 in Human Endothelial Cells. J. Cardiovasc. Pharmacol. 2015, 66, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Sekhar, K.R.; Yin, H.; Yared, N.F.; Schneider, S.N.; Sasi, S.; Dalton, T.P.; Anderson, M.E.; Chan, J.Y.; et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 2007, 282, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Majkova, Z.; Layne, J.; Sunkara, M.; Morris, A.J.; Toborek, M.; Hennig, B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 2011, 251, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.; Qi, W.; Cheng, D.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Eicosapentaenoic acid (EPA) induced apoptosis in HepG2 cells through ROS–Ca2+–JNK mitochondrial pathways. Biochem. Biophys. Res. Commun. 2015, 456, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Ahmad, S.; Megyerdi, S.; Mussell, R.; Choksi, K.; Maddipati, K.R.; Elmarakby, A.; Rizk, N.; Al-Shabrawey, M. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: Contribution of NADPH oxidase. PLoS ONE 2013, 8, e57254. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mao, M.; Qiu, Y.; Liu, G.; Sheng, T.; Yu, X.; Wang, S.; Zhu, D. Key role of ROS in the process of 15-lipoxygenase/15-hydroxyeicosatetraenoiccid-induced pulmonary vascular remodeling in hypoxia pulmonary hypertension. PLoS ONE 2016, 11, e0149164. [Google Scholar] [CrossRef] [PubMed]
- Medhora, M.; Chen, Y.; Gruenloh, S.; Harland, D.; Bodiga, S.; Zielonka, J.; Gebremedhin, D.; Gao, Y.; Falck, J.R.; Anjaiah, S.; et al. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. AJP Lung Cell. Mol. Physiol. 2008, 294, L902–L911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.-J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Gravelle, F.; Gravel, S. Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J. Biol. Chem. 1992, 267, 19233–19241. [Google Scholar] [PubMed]
- Powell, W.S.; Gravel, S.; Gravelle, F. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J. Lipid Res. 1995, 36, 2590–2598. [Google Scholar]
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Cipollina, C.; Salvatore, S.R.; Muldoon, M.F.; Freeman, B.A.; Schopfer, F.J. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS ONE 2014, 9, e94836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schopfer, F.J.; Cipollina, C.; Freeman, B.A. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2011, 111, 5997–6021. [Google Scholar] [CrossRef] [PubMed]

| Cell (+) | Samples | 5-HETE | 5-HEPE | 5-oxo-ETE |
|---|---|---|---|---|
| Medium (nmol/dish) | MeOH | N.D. | N.D. | N.D. |
| 5-HETE | 54.691 ± 9.554 | 0.066 ± 0.039 | 3.072 ± 0.301 | |
| 5-HEPE | N.D. | 38.886 ± 4.312 | N.D. | |
| 5-oxo-ETE | 0.010 ± 0.009 | N.D. | 0.117 ± 0.010 | |
| Cell (nmol/dish) | MeOH | N.D. | N.D. | N.D. |
| 5-HETE | 0.377 ± 0.062 | 0.002 ± 0.000 | 0.283 ± 0.053 | |
| 5-HEPE | N.D | 0.418 ± 0.064 | N.D | |
| 5-oxo-ETE | 0.005 ± 0.001 | N.D. | 0.002 ± 0.000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahora, N.; Yamada, H.; Kikuchi, S.; Hakozaki, M.; Yano, A. Nrf2 Activation by 5-lipoxygenase Metabolites in Human Umbilical Vascular Endothelial Cells. Nutrients 2017, 9, 1001. https://doi.org/10.3390/nu9091001
Nagahora N, Yamada H, Kikuchi S, Hakozaki M, Yano A. Nrf2 Activation by 5-lipoxygenase Metabolites in Human Umbilical Vascular Endothelial Cells. Nutrients. 2017; 9(9):1001. https://doi.org/10.3390/nu9091001
Chicago/Turabian StyleNagahora, Nozomi, Hidetoshi Yamada, Sayaka Kikuchi, Mayuka Hakozaki, and Akira Yano. 2017. "Nrf2 Activation by 5-lipoxygenase Metabolites in Human Umbilical Vascular Endothelial Cells" Nutrients 9, no. 9: 1001. https://doi.org/10.3390/nu9091001