Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Animal Models of IBD—The Chemical-Induced Models
2.1. DSS
2.2. TNBS
2.3. Acetic Acid
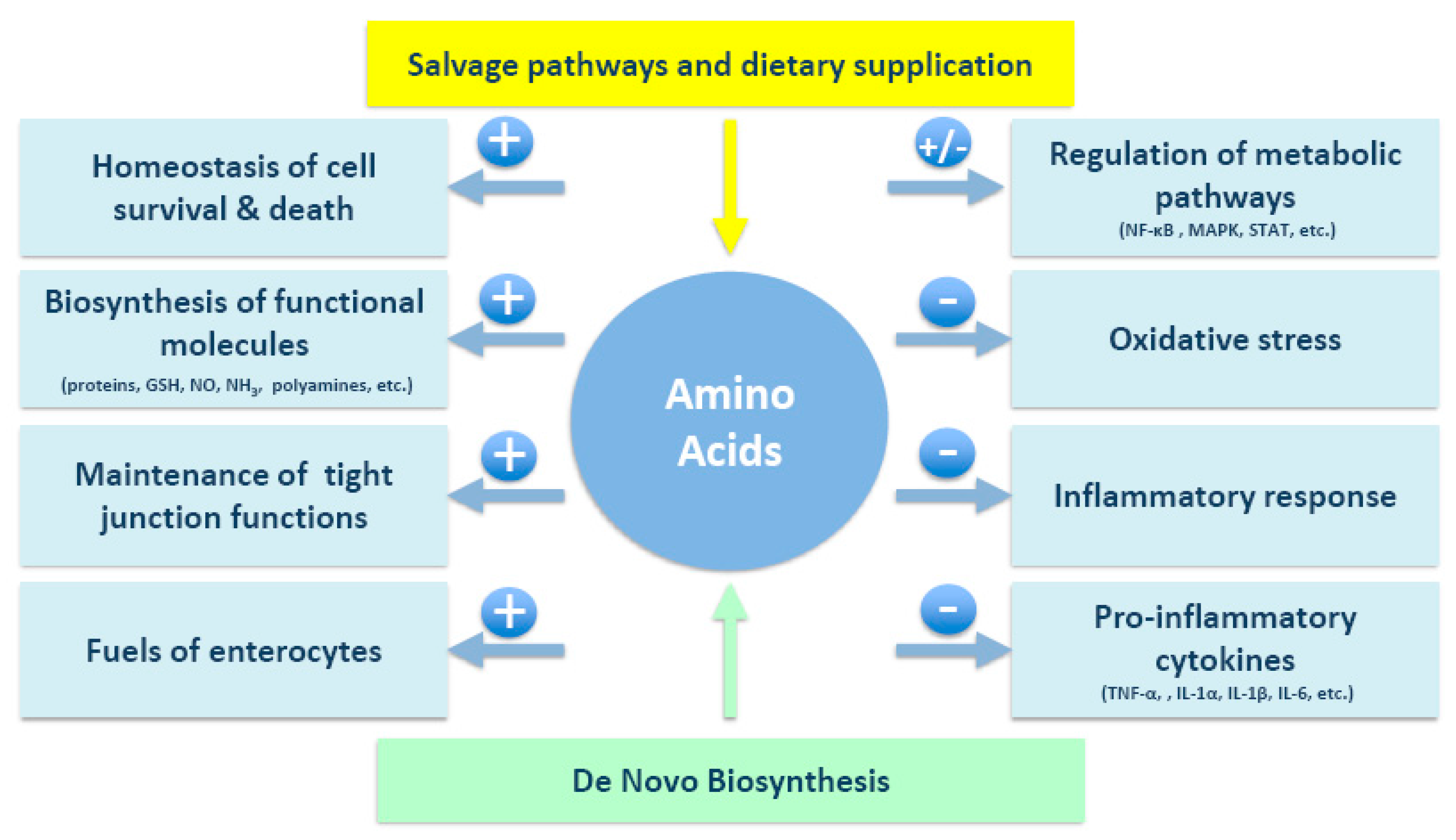
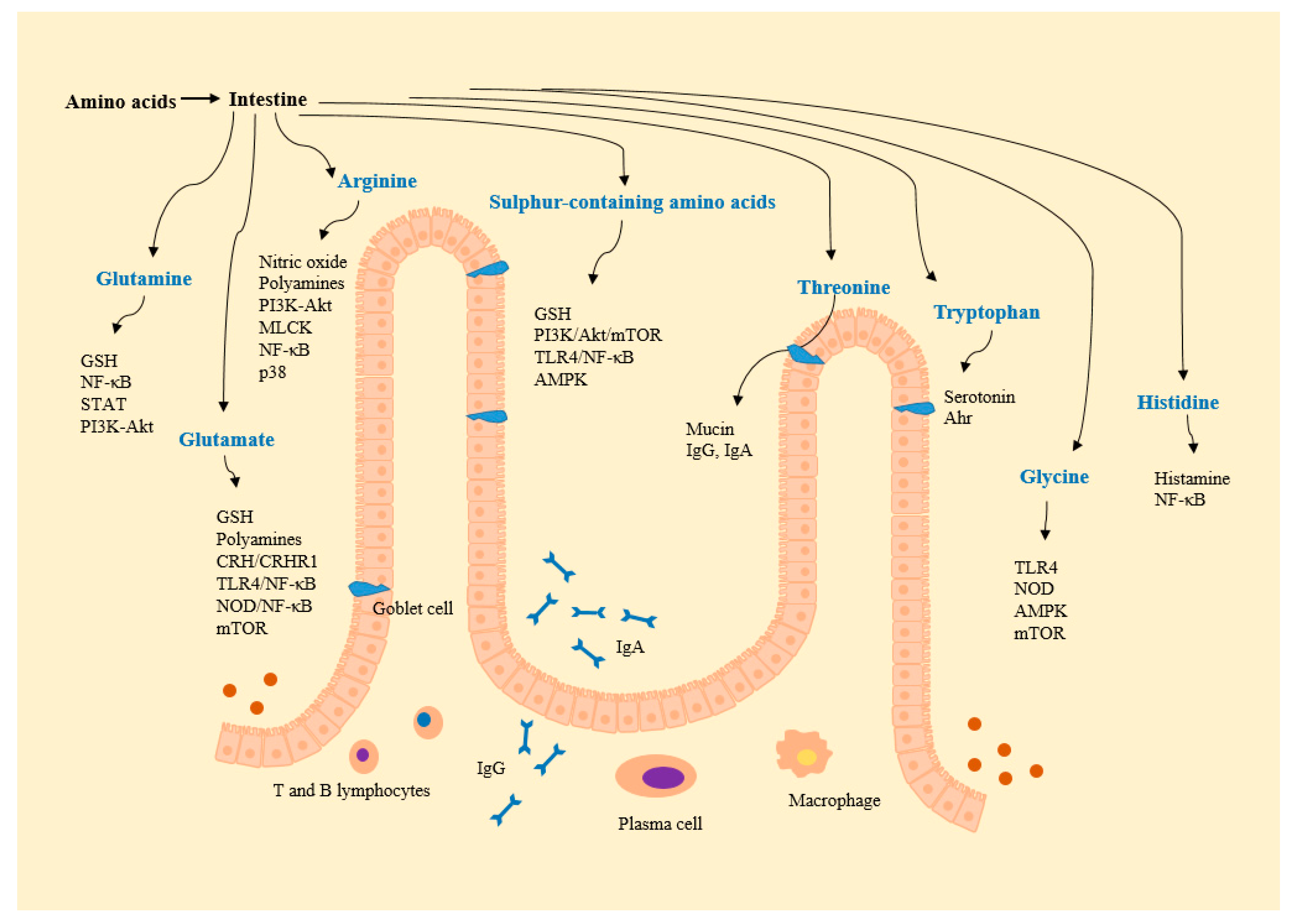
3. Potential Roles of Amino Acids in IBD
3.1. Glutamine
3.2. Glutamate
3.3. Arginine
3.4. Sulfur-Containing Amino Acids (Methionine and Cysteine)
3.5. Threonine
3.6. Tryptophan
3.7. Glycine
3.8. Histidine
3.9. Other Amino Acids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, X.Z.; Winston, J.H.; Sarna, S.K. Differential immune and genetic responses in rat models of Crohn’s colitis and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G41–G51. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Snider, A.J.; Bialkowska, A.B.; Ghaleb, A.M.; Yang, V.W.; Obeid, L.M.; Hannun, Y.A. Murine model for colitis-associated cancer of the colon. Methods Mol. Biol. 2016, 1438, 245–254. [Google Scholar] [PubMed]
- Corridoni, D.; Arseneau, K.O.; Cominelli, F. Inflammatory bowel disease. Immunol. Lett. 2014, 161, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [PubMed]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary protein and amino acid supplementation in inflammatory bowel disease course: What impact on the colonic mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Z.; Hang, S.; Zhu, W.; Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015, 21, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Cho, K.A.; Kang, J.L.; Kim, K.H.; Woo, S.Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [PubMed]
- Hou, Y.C.; Wu, J.M.; Wang, M.Y.; Wu, M.H.; Chen, K.Y.; Yeh, S.L.; Lin, M.T. Glutamine supplementation attenuates expressions of adhesion molecules and chemokine receptors on T cells in a murine model of acute colitis. Mediat. Inflamm. 2014, 2014, 837107. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, Y.C.; Liu, J.J.; Hou, Y.C.; Yeh, C.L.; Yeh, S.L. Effects of dietary glutamine on the homeostasis of CD4+ T cells in mice with dextran sulfate sodium-induced acute colitis. PLoS ONE 2014, 9, e84410. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum amino acids profile and the beneficial effects of l-arginine or l-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Hou, Y.C.; Pai, M.H.; Chao, C.J.; Yeh, S.L. Pretreatment with alanyl-glutamine suppresses T-helper-cell-associated cytokine expression and reduces inflammatory responses in mice with acute DSS-induced colitis. J. Nutr. Biochem. 2012, 23, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Gong, X.; Singh, K.; Asim, M.; Scull, B.P.; Allaman, M.M.; Williams, C.S.; Rosen, M.J.; Washington, M.K.; Barry, D.P.; et al. l-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS ONE 2012, 7, e33546. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Sufit, A.J.; Wischmeyer, P.E. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J. Parenter. Enteral Nutr. 2011, 35, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. l-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim. Biophys. Acta 2009, 1790, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, B.; Crespo, I.; Kretzmann, N.A.; Mauriz, J.L.; Marroni, N.; Tuñón, M.J.; González-Gallego, J. Glutamine prevents fibrosis development in rats with colitis induced by 2,4,6-trinitrobenzene sulfonic acid. J. Nutr. 2010, 140, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Ancha, H.R.; Kurella, R.R.; McKimmey, C.C.; Lightfoot, S.; Harty, R.F. Effects of N-acetylcysteine plus mesalamine on prostaglandin synthesis and nitric oxide generation in TNBS-induced colitis in rats. Dig. Dis. Sci. 2009, 54, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Pouillart, P.R.; Dépeint, F.; Abdelnour, A.; Deremaux, L.; Vincent, O.; Mazière, J.C.; Madec, J.Y.; Chatelain, D.; Younes, H.; Wils, D.; et al. Nutriose, a prebiotic low-digestible carbohydrate, stimulates gut mucosal immunity and prevents TNBS-induced colitis in piglets. Inflamm. Bowel. Dis. 2010, 16, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Buffière, C.; Godin, J.P.; Mirand, P.P.; Obled, C.; Papet, I.; Dardevet, D.; Williamson, G.; Breuillé, D.; Faure, M. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J. Nutr. 2009, 139, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Taruishi, M.; Ashida, T. Experimental ileitis in dogs and colitis in rats with trinitrobenzene sulfonic acid—Colonoscopic and histopathologic studies. Gastroenterol. Jpn. 1993, 28, 518–527. [Google Scholar] [PubMed]
- Fabia, R.; Willén, R.; Ar’Rajab, A.; Andersson, R.; Ahrén, B.; Bengmark, S. Acetic acid-induced colitis in the rat: A reproducible experimental model for acute ulcerative colitis. Eur. Surg. Res. 1992, 24, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Uraz, S.; Tahan, G.; Aytekin, H.; Tahan, V. N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scand. J. Clin. Lab. Investig. 2013, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Takuma, S.; Morimoto, M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp. Anim. 2000, 49, 9–15. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Fu, J.J.; Meng, J.; Huang, G.D.; Liu, Y.H. Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Dig. Dis. Sci. 2009, 54, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; McClain, C.J.; de Villiers, W.J. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, Å.; Tormo-Badia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslätt, M.-L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrné, S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 2015, 15, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.-E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Vicario, M.; Amat, C.; Rivero, M.; Moretó, M.; Pelegrí, C. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J. Nutr. 2007, 137, 1931–1937. [Google Scholar] [PubMed]
- Amrouche-Mekkioui, I.; Djerdjouri, B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur. J. Pharmacol. 2012, 691, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, M.; Douglas, T.; Saleh, M. Role of programmed necrosis and cell death in intestinal inflammation. Curr. Opin. Gastroenterol. 2014, 30, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Yuan, Z.W.; Yu, X.T.; Huang, Y.F.; Yang, G.H.; Chen, J.N.; Lai, X.P.; Su, Z.R.; Zeng, H.F.; Xie, Y.; et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol. Res. 2017, 121, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wu, B.; Guo, Y.S.; Zhou, Y.H.; Fu, Z.G.; Xu, B.Q.; Li, J.H.; Jing, L.; Jiang, J.L.; Tang, J.; Chen, Z.N. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am. J. Cancer Res. 2015, 5, 3174–3185. [Google Scholar] [PubMed]
- Welz, P.S.; Wullaert, A.; Vlantis, K.; Kondylis, V.; Fernández-Majada, V.; Ermolaeva, M.; Kirsch, P.; Sterner-Kock, A.; van Loo, G.; Pasparakis, M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011, 477, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Motavallian-Naeini, A.; Andalib, S.; Rabbani, M.; Mahzouni, P.; Afsharipour, M.; Minaiyan, M. Validation and optimization of experimental colitis induction in rats using 2,4,6-trinitrobenzene sulfonic acid. Res. Pharm. Sci. 2012, 7, 159–169. [Google Scholar] [PubMed]
- Zheng, L.; Gao, Z.Q.; Wang, S.X. A chronic ulcerative colitis model in rats. World J. Gastroenterol. 2000, 6, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Katsikeros, R.; Abimosleh, S.M. Current and Novel Treatments for Ulcerative Colitis. In Ulcerative Colitis from Genetics to Complications; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Wang, Y.; Cai, X.; Zou, M.; Xu, T.; Wang, M.; Wang, J.; Xu, D. Therapeutic efficacy of a mutant of keratinocyte growth factor-2 on trinitrobenzene sulfonic acid-induced rat model of Crohn’s disease. Am. J. Transl. Res. 2016, 8, 530–543. [Google Scholar] [PubMed]
- Giriş, M.; Erbil, Y.; Doğru-Abbasoğlu, S.; Yanik, B.T.; Aliş, H.; Olgaç, V.; Toker, G.A. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int. J. Colorectal. Dis. 2007, 22, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; San-Miguel, B.; Prause, C.; Marroni, N.; Cuevas, M.J.; González-Gallego, J.; Tuñón, M.J. Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS ONE 2012, 7, e50407. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Ancha, H.; Tedesco, D.; Lightfoot, S.; Stewart, C.A.; Harty, R.F. Antioxidant therapy with N-acetylcysteine plus mesalamine accelerates mucosal healing in a rodent model of colitis. Dig. Dis. Sci. 2006, 51, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal models of ulcerative colitis and their application in drug research. Drug Des. Devel. Ther. 2013, 7, 1341–1357. [Google Scholar] [PubMed]
- Nakao, K.; Ro, A.; Kibayashi, K. Evaluation of the morphological changes of gastric mucosa induced by a low concentration of acetic acid using a rat model. J. Forensic Leg. Med. 2014, 22, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fillmann, H.; Kretzmann, N.A.; San-Miguel, B.; Llesuy, S.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Glutamine inhibits over-expression of pro-inflammatory genes and down-regulates the nuclear factor kappaB pathway in an experimental model of colitis in the rat. Toxicology 2007, 236, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.M.; Guruvayoorappan, C. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis. Int. Immunopharmacol. 2013, 17, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ola, M.S.; Parmar, M.Y.; Ahmed, M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Fan, T.; Li, W.; Huang, H.; Zhang, Y.; Xing, W. Protective effect of sanguinarine against acetic acid-induced ulcerative colitis in mice. Toxicol. Appl. Pharmacol. 2013, 267, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.E.; Abo-Salem, O.M.; El-Sayed, S.M.; Taha, E.I.; El-Halawany, N. l-arginine augments the antioxidant effect of garlic against acetic acid-induced ulcerative colitis in rats. Pak. J. Pharm. Sci. 2009, 22, 373–380. [Google Scholar] [PubMed]
- Wang, Q.; Hou, Y.; Yi, D.; Wang, L.; Ding, B.; Chen, X.; Long, M.; Liu, Y.; Wu, G. Protective effects of N-acetylcysteine on acetic acid-induced colitis in a porcine model. BMC Gastroenterol. 2013, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abd Al Haleem, E.N.; Khaleel, S.A.; Sallam, A.S. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol. Rep. 2017, 69, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Kuzyk, O.; Rauch, I.; Heider, S.; Schwab, C.; Hainzl, E.; Decker, T.; Müller, M.; Strobl, B.; Schleper, C.; et al. Intestinal microbiota signatures associated with inflammation history in mice experiencing recurring colitis. Front. Microbiol. 2015, 6, 1408. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Li, N.; Zhu, W.; Wu, K.; Zhang, L.; Zhai, J.; Wang, Y.; Zhu, J.; Wang, X.; Shi, Y.; et al. An analysis of amino acid metabolic profile and its clinical significance in ulcerative colitis. Zhonghua Nei Ke Za Zhi 2015, 54, 210–213. [Google Scholar] [PubMed]
- Swaid, F.; Sukhotnik, I.; Matter, I.; Berkowitz, D.; Hadjittofi, C.; Pollak, Y.; Lavy, A. Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following acetic acid induced intestinal injury in rats. Nutr. Metab. 2013, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Değer, C.; Erbil, Y.; Giriş, M.; Yanik, B.T.; Tunca, F.; Olgaç, V.; Abbasoğlu, S.D.; Oztezcan, S.; Toker, G. The effect of glutamine on pancreatic damage in TNBS-induced colitis. Dig. Dis. Sci. 2006, 51, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Kretzmann, N.A.; Fillmann, H.; Mauriz, J.L.; Marroni, C.A.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm. Bowel Dis. 2008, 14, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Zhang, J.F.; Fei, S.J.; Zhu, S.P.; Zhu, J.Z.; Qiao, X.; Liu, Z.B. Glutamate microinjection into the hypothalamic paraventricular nucleus attenuates ulcerative colitis in rats. Acta Pharmacol. Sin. 2014, 35, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, H.S.; Thabit, R.H. l-arginine and aminoguanidine reduce colonic damage of acetic acid-induced colitis in rats: Potential modulation of nuclear factor-κB/p65. Clin. Exp. Pharmacol. Physiol. 2014, 41, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, A.; Bulbuloglu, E.; Kurutas, E.B.; Ciralik, H.; Kantarceken, B.; Buyukbese, M.A. Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J. Exp. Med. 2005, 206, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Seril, D.N.; Liao, J.; Ho, K.L.; Yang, C.S.; Yang, G.Y. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis 2002, 23, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Shizuma, T.; Mori, H.; Fukuyama, N. Protective effect of tryptophan against dextran sulfate sodium- induced experimental colitis. Turk. J. Gastroenterol. 2013, 24, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kovacs-Nolan, J.A.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 2010, 21, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah; Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tsune, I.; Ikejima, K.; Hirose, M.; Yoshikawa, M.; Enomoto, N.; Takei, Y.; Sato, N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology 2003, 125, 775–785. [Google Scholar] [CrossRef]
- Andou, A.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kamada, N.; Kobayashi, T.; Hashimoto, M.; Okutsu, T.; Shimbo, K.; Takeda, T.; et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology 2009, 136, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Sido, B.; Seel, C.; Hochlehnert, A.; Breitkreutz, R.; Dröge, W. Low intestinal glutamine level and low glutaminase activity in Crohn’s disease: A rational for glutamine supplementation? Dig. Dis. Sci. 2006, 51, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Fasina, Y.O.; Bowers, J.B.; Hess, J.B.; McKee, S.R. Effect of dietary glutamine supplementation on Salmonella colonization in the ceca of young broiler chicks. Poult. Sci. 2010, 89, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, T.; Han, L.; Zhao, L.; Niu, Y.; Chen, H. l-glutamine supplementation alleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.; Makharia, G.; Ahuja, V.; Anand Rajan, K.D.; Kalaivani, M.; Gupta, S.D.; Joshi, Y.K. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: A randomized controlled trial. Dig. Dis. Sci. 2012, 57, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Ockenga, J.; Borchert, K.; Stüber, E.; Lochs, H.; Manns, M.P.; Bischoff, S.C. Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur. J. Clin. Nutr. 2005, 59, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Hiele, M.; Peeters, M.; Ghoos, Y.; Rutgeerts, P. Effect of long-term oral glutamine supplements on small intestinal permeability in patients with Crohn’s disease. JPEN J. Parenter. Enter. Nutr. 1999, 23, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K.; Elawad, M.; Gordon, M. Glutamine for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 2, CD007348. [Google Scholar] [PubMed]
- Newsholme, P.; Lima, M.M.; Procopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D., II. Glutamine and glutamate. Biomed. Pharmacother. 2002, 56, 446–457. [Google Scholar] [CrossRef]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814S–821S. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. l-Glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiao, H.; Ren, W.; Yin, J.; Tan, B.; Liu, G.; Li, L.; Nyachoti, C.M.; Xiong, X.; Wu, G. Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS ONE 2014, 9, e100591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y. Regulative Effect of Glutamate on Intestinal Injury and Muscle Protein Synthesis and Degradation of Piglets after Lipopolysaccharide Challenge. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2015. [Google Scholar]
- Ren, X.R. Regulatory Effect of Glutamic Acid or Glycine on Intestinal Mucosal Immune Barrier Injury in Piglets after Lipopolysaccharide Challenge. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2015. [Google Scholar]
- Ren, W.; Chen, S.; Yin, J.; Duan, J.; Li, T.; Liu, G.; Feng, Z.; Tan, B.; Yin, Y.; Wu, G. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J. Nutr. 2014, 144, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meininger, C.J.; Knabe, D.A.; Bazer, F.W.; Rhoads, J.M. Arginine nutrition in development, health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. l-Arginine availability and metabolism is altered in ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Han, J.; Huang, J.J.; Wang, X.Q.; Wang, F.L.; Wang, J.J. Dietary l-Arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide. Asian-Aust. J. Anim. Sci. 2009, 22, 1667–1675. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Hou, Y.; Zhu, H.; Zhao, S.; Ding, B.; Yin, Y.; Yi, G.; Shi, J.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Lecleire, S.; Hassan, A.; Marion-Letellier, R.; Antonietti, M.; Savoye, G.; Bôle-Feysot, C.; Lerebours, E.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Combined glutamine and arginine decrease proinflammatory cytokine production by biopsies from Crohn’s patients in association with changes in nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways. J. Nutr. 2008, 138, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, D.; Dai, Z.; Piao, X.; Wu, Z.; Wang, B.; Zhu, Y.; Zeng, Z. l-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 2014, 46, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Weaver, A.C.; Kim, S.W. Effect of feed grade l-methionine on growth performance and gut health in nursery pigs compared with conventional dl-methionine. J. Anim. Sci. 2014, 92, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, A.; Wang, X.; Gabello, M.; Valenzano, M.C.; Soler, A.P.; Ko, A.; Morin, P.J.; Mullin, J.M. Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern. Am. J. Physiol. Cell Physiol. 2010, 299, C1028–C1035. [Google Scholar] [CrossRef] [PubMed]
- Komninou, D.; Leutzinger, Y.; Reddy, B.S.; Richie, J.P., Jr. Methionine restriction inhibits colon carcinogenesis. Nutr. Cancer 2006, 54, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.X.; Chen, J.; Chen, L.L.; Gu, W.Z. The impact of dietary methionine-restriction on tight junction expression and function in a rat colonitis model. Zhonghua Nei Ke Za Zhi 2013, 52, 503–509. [Google Scholar] [PubMed]
- Tang, Y.; Tan, B.; Xiong, X.; Li, F.; Ren, W.; Kong, X.; Qiu, W.; Hardwidge, P.R.; Yin, Y. Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids 2015, 47, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Li, T.W.; Yang, H.; Peng, H.; Xia, M.; Mato, J.M.; Lu, S.C. Effects of S-adenosylmethionine and methylthioadenosine on inflammation-induced colon cancer in mice. Carcinogenesis 2012, 33, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wu, Z.; Dai, Z.; Sun, K.; Zhang, Q.; Wu, G. Excessive l-cysteine induces vacuole-like cell death by activating endoplasmic reticulum stress and mitogen-activated protein kinase signaling in intestinal porcine epithelial cells. Amino Acids 2016, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Tong, G.; Xiao, K.; Jiao, L.F.; Ke, Y.L.; Hu, C.H. l-cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, L.; Yi, D.; Wu, G. N-acetylcysteine and intestinal health: A focus on its mechanism of action. Front. Biosci. 2015, 20, 872–891. [Google Scholar] [CrossRef]
- Xu, C.C.; Yang, S.F.; Zhu, L.H.; Cai, X.; Sheng, Y.S.; Zhu, S.W.; Xu, J.X. Regulation of N-acetyl cysteine on gut redox status and major microbiota in weaned piglets. J. Anim. Sci. 2014, 92, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, Y.; Ma, C.Y.; Xu, X.M.; Zhang, Y.Q.; Wang, C.G.; Jin, J.; Shen, X.; Gao, J.L.; Li, N.; et al. Dimethyl fumarate induces necroptosis in colon cancer cells through GSH depletion/ROS increase/MAPKs activation pathway. Br. J. Pharmacol. 2015, 172, 3929–3943. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Hou, Y.Q.; Xiao, H.; Wang, L.; Zhang, Y.; Chen, H.B.; Wu, T.; Ding, B.Y.; Hu, C.A.; Wu, G.Y. N-Acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways. Amino Acids 2017. [CrossRef] [PubMed]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. 2011, 3, 1192–1200. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cheng, Y.F.; Li, X.H.; Yang, W.L.; Wen, C.; Zhuang, S.; Zhou, Y.M. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 2017, 96, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Baird, C.H.; Niederlechner, S.; Beck, R.; Kallweit, A.R.; Wischmeyer, P.E. l-Threonine induces heat shock protein expression and decreases apoptosis in heat-stressed intestinal epithelial cells. Nutrition 2013, 29, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P.; Myerscough, N.; Longman, R.; Sylvester, P.; Arul, S.; Pignatelli, M. Mucins and mucosal protection in the gastrointestinal tract: New prospects for mucins in the pathology of gastrointestinal disease. Gut 2000, 47, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Mettraux, C.; Moennoz, D.; Godin, J.P.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 2006, 136, 1558–1564. [Google Scholar] [PubMed]
- Wang, H.; Ji, Y.; Wu, G.; Sun, K.; Sun, Y.; Li, W.; Wang, B.; He, B.; Zhang, Q.; Dai, Z.; Wu, Z. l-Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J. Nutr. 2015, 145, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Messori, S.; Trevisi, P.; Simongiovanni, A.; Priori, D.; Bosi, P. Effect of susceptibility to enterotoxigenic Escherichia coli F4 and of dietary tryptophan on gut microbiota diversity observed in healthy young pigs. Vet. Microbiol. 2013, 162, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Gewirtz, A.T. Tryptophan: A gut microbiota-derived metabolites regulating inflammation. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Ghia, J.E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El-Sharkawy, R.T.; Côté, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Al-Massad, N.; Bethge, J.; Waetzig, G.H.; Rosenstiel, P.C.; Junker, R.; Thieme, F.; Schreiber, S. Su1079 Tryptophan deficiency in Crohn disease. Gastroenterology 2014, 146, S366–S367. [Google Scholar] [CrossRef]
- Schröcksnadel, K.; Wirleitner, B.; Winkler, C.; Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 2006, 364, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Petrat, F.; Boengler, K.; Schulz, R.; de Groot, H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: Current knowledge. Br. J. Pharmacol. 2012, 165, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Effenberger-Neidnicht, K.; Jägers, J.; Verhaegh, R.; de Groot, H. Glycine selectively reduces intestinal injury during endotoxemia. J. Surg. Res. 2014, 192, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.F.; Martins, J.L.; de Freitas Filho, L.G.; Oliva, M.L.; Patrício, F.R.; Macedo, M.; Wang, L. Glycine reduces tissue lipid peroxidation in hypoxia-reoxygenation-induced necrotizing enterocolitis in rats. Acta Cir. Bras. 2006, 21, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. l-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T. Regulative Effect of Glycine on Intestinal Injury and Muscle Protein Sythesis and Degradation of Piglets after Lipopolysaccharide Challenge. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2015. [Google Scholar]
- Liu, Y.; Wang, X.; Wu, H.; Chen, S.; Zhu, H.; Zhang, J.; Hou, Y.; Hu, C.A.; Zhang, G. Glycine enhances muscle protein mass associated with maintaining Akt-mTOR-FOXO1 signaling and suppressing TLR4 and NOD2 signaling in piglets challenged with LPS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R365–R373. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of l-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Jutel, M.; Akdis, M.; Akdis, C.A. Histamine, histamine receptors and their role in immune pathology. Clin. Exp. Allergy 2009, 39, 1786–1800. [Google Scholar] [CrossRef] [PubMed]
- Son, D.O.; Satsu, H.; Shimizu, M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005, 579, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Hirano, R.; Haneda, Y.; Fukano, R.; Hara, M.; Furukawa, S. Amino acids exhibit anti-inflammatory effects in human monocytic leukemia cell line, THP-1 cells. Inflamm. Res. 2011, 60, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Ono, N.; Imaizumi, A.; Mori, M.; Suzuki, H.; Uo, M.; Hashimoto, M.; Naganuma, M.; Matsuoka, K.; Mizuno, S.; et al. Decreased plasma histidine level predicts risk of relapse in patients with ulcerative colitis in remission. PLoS ONE 2015, 10, e0140716. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Shi, H.; Wang, X.; Zhu, H.; Pi, D.; Leng, W.; Li, S. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-κB and p38 signaling in weaned pigs after LPS challenge. Eur. J. Nutr. 2017, 56, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Wang, X.; Wang, H.; Li, S.; Shi, H.; Zhu, H.; Zhang, J.; Pi, D.; Hu, C.A.; Lin, X.; Odle, J. Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun. 2016, 22, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Zhang, L.; Hou, Y.; Ding, B.; Yi, D.; Wang, L.; Zhu, H.; Liu, Y.; Yin, Y.; Wu, G. Effects of l-proline on the growth performance, and blood parameters in weaned lipopolysaccharide (LPS)-challenged pigs. Asian-Australas. J. Anim. Sci. 2014, 27, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Coëffier, M.; Marion-Letellier, R.; Déchelotte, P. Potential for amino acids supplementation during inflammatory bowel diseases. Inflamm. Bowel Dis. 2010, 16, 518–524. [Google Scholar] [CrossRef] [PubMed]
| Colitis Models | Procedure | Animals | References |
|---|---|---|---|
| DSS | 1.5–5% (wt/vol) DSS (molecular weight 36–50 kDa) in the drinking water for 5–7 days | rodent | [9,10,11,12,13,14,15] |
| 1.25 g DSS per kg body weight twice a day for 5 days | piglets | [16] | |
| Chronic colitis: cyclic treatment with 2% DSS repeated 3 times for 30 days | C57BL/6 mice | [9] | |
| TNBS | 2 mg/100 mL of TNBS in 45% ethanol | C57BL/6 mice | [9] |
| 30 mg TNBS in 0.25 mL 50% ethanol | Wistar rats | [17] | |
| 15 mg TNBS in 0.6 mL 50% ethanol | Sprague-Dawley rats | [18] | |
| 15 mg/kg TNBS in 5 mL 50% ethanol | piglets | [19] | |
| 30-mL of 120 mg/kg TNBS in 50% ethanol | minipigs | [20] | |
| 10 mL of 100% ethanol and 1 g of TNBS in 10 mL of distilled water | adult mongrel dogs | [21] | |
| acetic acid | 4% acetic acid for 15 s | rats | [22] |
| 1 mL of 4% acetic acid in 0.9% NaCl | rats | [23,24] |
| Amino Acids | Effects | Animal Models | References |
|---|---|---|---|
| Glutamine | ↓body weight loss, colon edema, endothelial adhesion molecules, infiltrating Th cells, PSGL-1, LFA-1, CCR9 | C57BL/6 mice; DSS | [10] |
| ↓epithelium injury and loss, mucosal hypoplasia | Sprague-Dawley rats; acetic acid | [60] | |
| ↓disruption of colonic architecture, submucosal and serosal fibrosis, collagen Iα2, collagen III, TGFβ, phosphorylated Smad3, PDGF, CTGF | Wistar rats; TNBS | [17] | |
| ↑HO-1, GSH ↓MDA, caspase-3, NF-κB | Wistar-albino rats; TNBS | [44] | |
| ↑GSH ↓MDA, caspase-3 | Wistar-albino rats; TNBS | [61] | |
| ↓histopathological scores, cytosolic concentration of TBARS, hydroperoxide-initiated chemiluminescence, NF-κB, iNOS, COX-2 | Wistars rats; acetic acid | [49] | |
| ↓histopathological scores, cytosolic concentration of TBARS, hydroperoxide-initiated chemiluminescence, MPO, iNOS, COX-2, NF-κB, TNF-α, IFN-γ, phosphorylated forms of STAT1, STAT5, Akt | Wistar rats; TNBS | [62] | |
| ↑Th22 and Treg cell expression ↓Th1/Th17-associated cytokine expression | C57BL/6 mice; DSS | [11] | |
| ↓NF-κB, PI3K-Akt | ICR mice; DSS | [12] | |
| ↑HSP25, HSP70 ↓diarrhea | Sprague-Dawley rats; DSS | [15] | |
| ↓oxidative stress, ER stress, apoptosis | Wistar rats; TNBS | [45] | |
| Glutamate | ↑PCNA-positive cells, SOD, Bcl-2 ↓MDA, Bax, caspase-3, TNF-α, IL-1β | Sprague-Dawley rats; TNBS | [63] |
| Arginine | ↑iNOS ↓mucosal permeability, number of MPO-positive neutrophils, and expression of pro-inflammatory cytokine and chemokine | iNOS−/− C57BL/6 mice; DSS | [14] |
| ↑T-SOD ↓Akt, MLCK | ICR mice; DSS | [12] | |
| ↓body weight loss, colon weights, macroscopic and microscopic damage of colonic tissues | Wistar rats; acetic acid | [64] | |
| Sulphur-containing amino acids | ↓colon lesions, cytoskeleton damage, serum amyloid A, TNF-α | BALB/C mice; DSS | [27] |
| ↑caspase-8 ↓chemokine, neutrophil influx, TNF-α, IL-6, IL-12p40, IL-1β, cFLIP and Bcl-xL | Yorkshire piglets; DSS | [16] | |
| ↑goblet cell number, protein/DNA ratio, claudin-1 ↓IEL number, caspase-3, MPO, MDA, TNF-α | piglets; acetic acid | [54] | |
| ↑GSH, SOD, CAT ↓MPO, MDA | Wistar-albino rats; acetic acid | [65] | |
| ↑PON1,GSH ↓macroscopic colonic damage, histopathologic changes, MPO, ROS, TNF-α, IL-1β | BALB/c mice; DSS | [26] | |
| ↑GSH, SOD ↓MPO, MDA, TNF-α, IL-1β, IL-6 | Wistar albino rats; acetic acid | [24] | |
| ↓COX2, PGE2 | Sprague-Dawley rats; TNBS | [18] | |
| ↓chronic ulcerative colitis-associated colorectal adenocarcinoma development | C57BL/6J mice; DSS | [66] | |
| Tryptophan | ↓body weight loss, frequency of bloody stools, nitrotyrosine content of the colonic tissues | C57black6 mice; DSS | [67] |
| ↑caspase-8, Bax ↓gut permeability, TNF-α, IL-6, IFN-γ, IL-12p40, IL-1β, IL-17, IL-8, ICAM-1 | Piglets; DSS | [68] | |
| ↑Ahr, IL-22, STAT3 ↓colitis symptoms, IL-6, TNF-α, IL-1β, Ccl2, Cxcl1, Cxcl2 | C57BL/6 WT and KO mice; DSS | [69] | |
| Glycine | ↓diarrhea, body weight loss, ulceration, MPO, IL-1β, TNF-α, CINC, MIP-2 | Wistar rats; TNBS/DSS | [70] |
| Histidine | ↓histologic damage, colon weight, TNF-α, IL-6, NF-κB | IL-10−/− mice | [71] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. https://doi.org/10.3390/nu9090920
Liu Y, Wang X, Hu C-AA. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients. 2017; 9(9):920. https://doi.org/10.3390/nu9090920
Chicago/Turabian StyleLiu, Yulan, Xiuying Wang, and Chien-An Andy Hu. 2017. "Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease" Nutrients 9, no. 9: 920. https://doi.org/10.3390/nu9090920





