Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways
Abstract
:1. Introduction
2. The Plant
3. Litchi Fruit as a Functional Food
4. Antitumor Properties of Litchi Pulp-Derived Components
5. Antitumor Properties of Litchi Peel-Derived Components
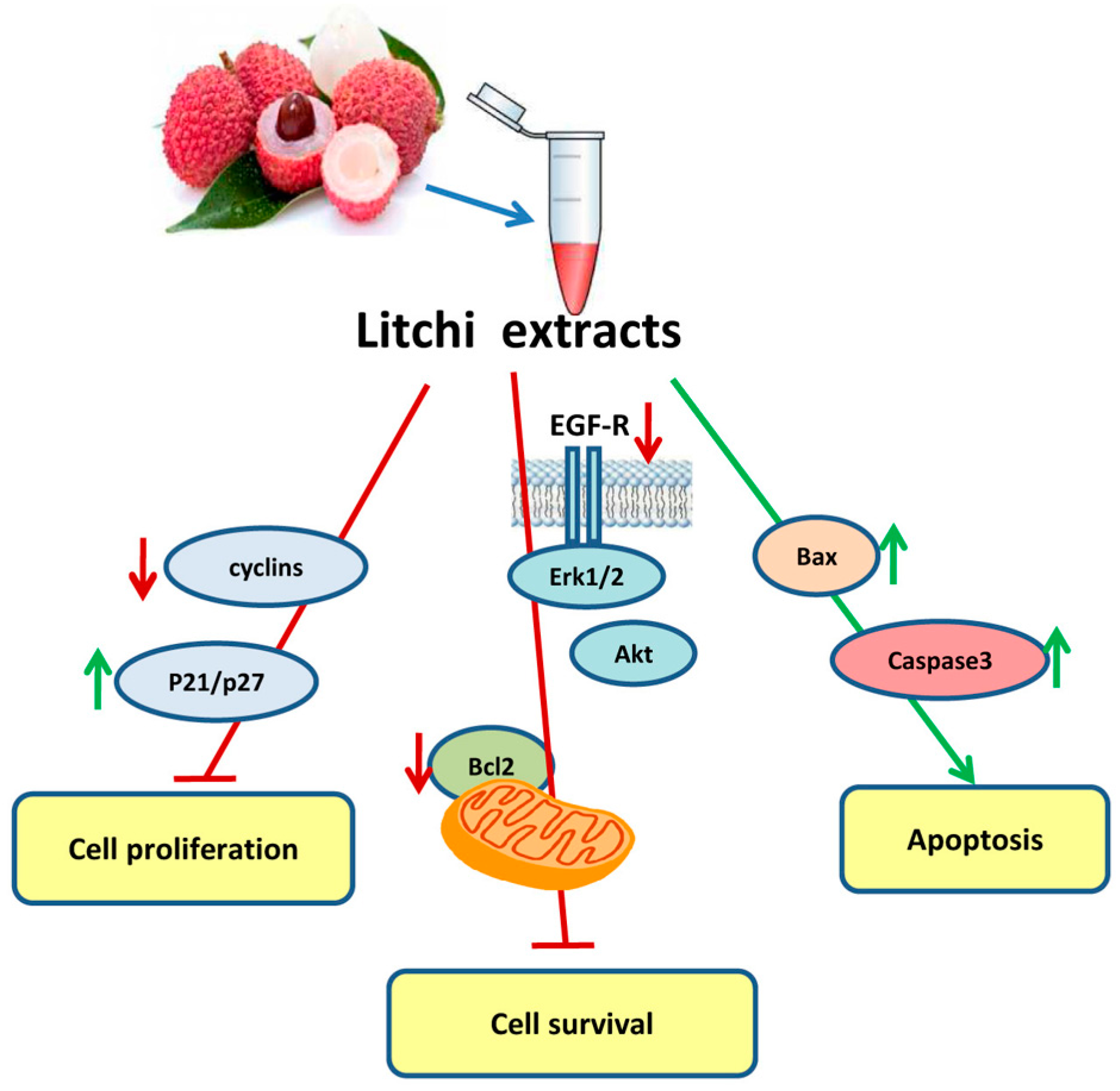
6. Antitumor Properties and Biochemical Aspects of Litchi Seed-Derived Components
7. Other Parts of the Litchi Plant That Contain Antitumor Compounds
8. Oligonol
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Menzel, C. Lychee, Its Origin, Distribution and Production around the World 1995. Available online: http://rfcarchives.org.au/index.htm (accessed on 28 July 2017).
- Menzel, C. The Lychee Crop in Asia and the Pacific; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2002. [Google Scholar]
- Menzel, C.M.; Waite, G.K. Litchi and Longan: Botany, Production and Uses; CABI Pub.: Wallingford, UK; Cambridge, MA, USA, 2005; ISBN 978-1-84593-022-6. [Google Scholar]
- Kilari, E.; Putta, S. Biological and phytopharmacological descriptions of Litchi chinensis. Pharmacogn. Rev. 2016, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G. The use of isozymes as probes to identify and label plant varieties and cultivars. Isozymes 1985, 12, 1–32. [Google Scholar] [PubMed]
- Liu, W.; Xiao, Z.; Bao, X.; Yang, X.; Fang, J.; Xiang, X. Identifying Litchi (Litchi chinensis Sonn.) cultivars and their genetic relationships using single nucleotide polymorphism (SNP) markers. PLoS ONE 2015, 10, e0135390. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted health benefits of Mangifera indica L. (Mango): The inestimable value of orchards recently planted in sicilian rural areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Padoan, D.; Farina, V.; Ferlito, B.; Barone, F.; Palazzolo, E. Qualitative features of Litchi fruits (Litchi Chinensis Sonn.) cultivated in North east Sicily. Acta Italus Hortus 2012, 11, 142–144. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service. 2012. USDA National Nutrient Database for Standard Reference, Release 25. Nutrient Data Laboratory Home Page. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 28 July 2017).
- Ramananda Singh, M.; Gupta, P.; Gupta, K. The Litchi (Litchi Chinensis) peels extract as a potential green inhibitor in prevention of corrosion of mild steel in 0.5M H2SO4 solution. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [PubMed]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.-P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, Litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture (USDA). National Nutrient Database for Standard Reference, SR-28, Full Report (All Nutrients): 09164, Litchis, Raw National Agricultural Library. USDA. Available online: https://ndb.nal.usda.gov/ndb/foods/show/2271 (accessed on 25 January 2016).
- Li, W.; Liang, H.; Zhang, M.-W.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; Zhang, Y.; Tang, X.-J. Phenolic profiles and antioxidant activity of Litchi (Litchi Chinensis Sonn.) fruit pericarp from different commercially available cultivars. Molecules 2012, 17, 14954–14967. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Isvoranu, G.; Comanescu, M.V.; Manea, A.; Debelec Butuner, B.; Korkmaz, K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015, 5, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C.D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox homeostasis and cellular antioxidant systems: Crucial players in cancer growth and therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A. Litchi chinensis: Medicinal uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2015, 174, 492–513. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Jing, Y.; Song, L.; Zhu, J.; Cui, X.; Yu, R. Structural elucidation and in vitro antioxidant activities of a new heteropolysaccharide from Litchi chinensis. Drug Discov. Ther. 2015, 9, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Huang, Y.-Y.; Dong, Q.-F.; Song, L.-Y.; Yuan, F.; Yu, R.-M. Structure characterization and antioxidant activity of a novel polysaccharide isolated from pulp tissues of Litchi chinensis. J. Agric. Food Chem. 2011, 59, 11548–11552. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Liu, Y.; Xiao, J.; Liu, L.; Wei, Z.; Yi, Y.; Zhang, M.; Liu, D. Dietary Litchi pulp polysaccharides could enhance immunomodulatory and antioxidant effects in mice. Int. J. Biol. Macromol. 2016, 92, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Yi, Y.; Tang, X.; Zhang, M.; Su, D.; Deng, Y.; Wei, Z. Comparison of physicochemical properties and immunomodulatory activity of polysaccharides from fresh and dried Litchi pulp. Molecules 2014, 19, 3909–3925. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Dong, L.; Guo, J.; Deng, Y.; Yi, Y.; Zhang, M. Antioxidant and antiproliferative activities of polysaccharide fractions from Litchi pulp. Food Funct. 2015, 6, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic profiles and antioxidant activity of Litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013, 136, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Ti, H.; Zhang, R.; Zhang, M.; Wei, Z.; Deng, Y.; Guo, J. Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chem. 2014, 158, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, R.; Zhang, C.; Huang, F.; Xiao, J.; Deng, Y.; Wei, Z.; Zhang, Y.; Chi, J.; Zhang, M. Phenolic-rich lychee (Litchi chinensis Sonn.) pulp extracts offer hepatoprotection against restraint stress-induced liver injury in mice by modulating mitochondrial dysfunction. Food Funct. 2016, 7, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Takahashi, A.; Watanabe, T.; Iida, K.; Matsuzaki, T.; Yoshikawa, H.Y.; Fujiki, H. Biophysical approach to mechanisms of cancer prevention and treatment with green tea catechins. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Kwak, T.W.; Park, S.B.; Kim, H.-J.; Jeong, Y.-I.; Kang, D.H. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. OncoTargets Ther. 2017, 10, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-B.; Huang, G.-L.; Li, H.-H.; Kong, X.; He, Z.-W. Epigallocatechin-3-gallate modulates microrna expression profiles in human nasopharyngeal carcinoma CNE2 cells. Chin. Med. J. 2017, 130, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Hong, J.; Zhou, J.; Pan, Z.; Bose, M.; Liao, J.; Yang, G.; Liu, Y.Y.; Hou, Z.; Lin, Y.; et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005, 65, 10623–10631. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Tatebe, H.; Nakagawa, T.; Hara, Y.; Weinstein, I.B.; Moriwaki, H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008, 262, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Demiroglu-Zergeroglu, A.; Candemir, G.; Turhanlar, E.; Sagir, F.; Ayvali, N. EGFR-dependent signalling reduced and p38 dependent apoptosis required by Gallic acid in Malignant Mesothelioma cells. Biomed. Pharmacother. 2016, 84, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y. Litchi flavonoids: Isolation, identification and biological activity. Molecules 2007, 12, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, S.; Wang, J.; Lin, P.; Liu, G.; Lu, Y.; Zhang, J.; Wang, W.; Wei, Y. Anticancer activity of Litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol. Appl. Pharmacol. 2006, 215, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, B.; Wang, J.; Liu, Y.; Yu, L.; Jiang, Y. Immunomodulatory and anticancer activities of flavonoids extracted from Litchi (Litchi chinensis Sonn.) pericarp. Int. Immunopharmacol. 2007, 7, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kumar, A.; Thomas, J.D.; Laserson, K.F.; Bhushan, G.; Carter, M.D.; Chhabra, M.; Mittal, V.; Khare, S.; Sejvar, J.J.; et al. Association of acute toxic encephalopathy with Litchi consumption in an outbreak in Muzaffarpur, India, 2014: A case-control study. Lancet Glob. Health 2017, 5, e458–e466. [Google Scholar] [CrossRef]
- Das, M.; ASthana, S.; Singh, S.; Dixit, S.; Tripathi, A.; John, T. Litchi fruit contains methylene cyclopropyl-glycine. Curr. Sci. 2015, 109, 2195–2197. [Google Scholar]
- Li, C.; Liao, X.; Li, X.; Guo, J.; Qu, X.; Li, L. Effect and mechanism of Litchi semen effective constituents on insulin resistance in rats with type 2 diabetes mellitus. J. Chin. Med. Mater. 2015, 38, 1466–1471. [Google Scholar]
- Choi, S.-A.; Lee, J.E.; Kyung, M.J.; Youn, J.H.; Oh, J.B.; Whang, W.K. Anti-diabetic functional food with wasted Litchi seed and standard of quality control. Appl. Biol. Chem. 2017, 60, 197–204. [Google Scholar] [CrossRef]
- Kalgaonkar, S.; Nishioka, H.; Gross, H.B.; Fujii, H.; Keen, C.L.; Hackman, R.M. Bioactivity of a flavanol-rich lychee fruit extract in adipocytes and its effects on oxidant defense and indices of metabolic syndrome in animal models. Phytother. Res. 2010, 24, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Bhoopat, L.; Srichairatanakool, S.; Kanjanapothi, D.; Taesotikul, T.; Thananchai, H.; Bhoopat, T. Hepatoprotective effects of lychee (Litchi chinensis Sonn.): A combination of antioxidant and anti-apoptotic activities. J. Ethnopharmacol. 2011, 136, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, H.; Wang, Y.; Wei, X. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J. Agric. Food Chem. 2010, 58, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-Y.; Zhang, D.; Feng, Z.; Chen, Y.; Zhang, H.; Lin, P. Studies on the antitumor effects of lychee seeds in mice. J. Chin. Med. Mater. 2004, 27, 517–518. [Google Scholar]
- Xiong, A.-H.; Shen, W.-J.; Xiao, L.-Y.; Lv, J.-H. Effect of Semen Litchi containing serum on proliferation and apoptosis of HepG2 cells. J. Chin. Med. Mater. 2008, 31, 1533–1536. [Google Scholar]
- Hsu, C.-P.; Lin, C.-C.; Huang, C.-C.; Lin, Y.-H.; Chou, J.-C.; Tsia, Y.-T.; Su, J.-R.; Chung, Y.-C. Induction of apoptosis and cell cycle arrest in human colorectal carcinoma by Litchi seed extract. J. Biomed. Biotechnol. 2012, 2012, 341479. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Qiu, Y.; He, G.; Guan, N. Effects of Litchi chinensis seed saponins on inhibiting hyperplasia of mammary glands and influence on signaling pathway of estrogen in rats. J. Chin. Med. Mater. 2015, 38, 798–802. [Google Scholar]
- Wang, X.; Wu, J.; Yu, C.; Tang, Y.; Liu, J.; Chen, H.; Jin, B.; Mei, Q.; Cao, S.; Qin, D. Lychee seed saponins improve cognitive function and prevent neuronal injury via inhibiting neuronal apoptosis in a rat model of alzheimer’s disease. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, H.; Hao, J.; Jiang, Y.; Wei, X. Flavonoid glycosides from the seeds of Litchi chinensis. J. Agric. Food Chem. 2011, 59, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Xiao, L.-Y.; Pan, J.; Lv, J.-H. Effects of Semen Litchi on the expressions of S180 and EAC tumor cells and Bax and Bcl-2 proteins in rats. China Pharm. 2008, 19, 1138–1140. [Google Scholar]
- Chung, Y.-C.; Chen, C.-H.; Tsai, Y.-T.; Lin, C.-C.; Chou, J.-C.; Kao, T.-Y.; Huang, C.-C.; Cheng, C.-H.; Hsu, C.-P. Litchi seed extract inhibits epidermal growth factor receptor signaling and growth of Two Non-small cell lung carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Luo, H.; Yuan, H.; Xia, Y.; Shu, P.; Huang, X.; Lu, Y.; Liu, X.; Keller, E.T.; Sun, D.; et al. Litchi seed extracts diminish prostate cancer progression via induction of apoptosis and attenuation of EMT through Akt/GSK-3β signaling. Sci. Rep. 2017, 7, 41656. [Google Scholar] [CrossRef] [PubMed]
- Carlisi, D.; D’Anneo, A.; Martinez, R.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. The oxygen radicals involved in the toxicity induced by parthenolide in MDA-MB-231 cells. Oncol. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Carlisi, D.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. Parthenolide induces superoxide anion production by stimulating EGF receptor in MDA-MB-231 breast cancer cells. Int. J. Oncol. 2013, 43, 1895–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauricella, M.; Carlisi, D.; Giuliano, M.; Calvaruso, G.; Cernigliaro, C.; Vento, R.; D’Anneo, A. The analysis of estrogen receptor-α positive breast cancer stem-like cells unveils a high expression of the serpin proteinase inhibitor PI-9: Possible regulatory mechanisms. Int. J. Oncol. 2016, 49, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.; Sabella, S.; Pellerito, O.; Vento, R.; Calvaruso, G.; Giuliano, M. The secreted protein acidic and rich in cysteine is a critical mediator of cell death program induced by WIN/TRAIL combined treatment in osteosarcoma cells. Int. J. Oncol. 2016, 48, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, O.; Notaro, A.; Sabella, S.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. WIN induces apoptotic cell death in human colon cancer cells through a block of autophagic flux dependent on PPARγ down-regulation. Apoptosis 2014, 19, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.; Sabella, S.; Pellerito, O.; Di Fiore, R.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. Involvement of PAR-4 in cannabinoid-dependent sensitization of osteosarcoma cells to TRAIL-induced apoptosis. Int. J. Biol. Sci. 2014, 10, 466–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; You, L.; Yang, X.; Yang, J.; Chen, F.; Jiang, Y.; Yang, B. Identification of phenolics in Litchi and evaluation of anticancer cell proliferation activity and intracellular antioxidant activity. Free Radic. Biol. Med. 2015, 84, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chang, J.-C.; Cheng, S.-Y.; Wang, C.-M.; Jhan, Y.-L.; Lo, I.-W.; Hsu, Y.-M.; Liaw, C.-C.; Hwang, C.-C.; Chou, C.-H. New bioactive chromanes from Litchi chinensis. J. Agric. Food Chem. 2015, 63, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-J.; Chang, Y.-Z.; Chen, Y.-C.; Liu, S.-C.; Hsu, C.-H.; Lin, J.-T. Antioxidant effect and active components of Litchi (Litchi chinensis Sonn.) flower. Food Chem. Toxicol. 2012, 50, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-J.; Chang, Y.-Y.; Lin, H.-W.; Chen, Y.-C.; Hsu, S.-H.; Lin, J.-T. Inhibitory effect of Litchi (Litchi chinensis Sonn.) flower on lipopolysaccharide-induced expression of proinflammatory mediators in RAW264.7 cells through NF-κB, ERK, and JAK2/STAT3 inactivation. J. Agric. Food Chem. 2014, 62, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Sun, B.; Fujii, H.; Neergheen, V.S.; Bahorun, T.; Kang, K.-S.; Sung, M.-K. Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. BioFactors 2006, 27, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Noh, J.S.; Fujii, H.; Roh, S.-S.; Song, Y.-O.; Choi, J.S.; Chung, H.Y.; Yokozawa, T. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates gluco-lipotoxicity-mediated renal disorder in type 2 diabetic db/db mice. Drug Discov. Ther. 2015, 9, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Bhakta, H.K.; Fujii, H.; Min, B.-S.; Park, C.H.; Yokozawa, T.; Jung, H.A. Inhibitory evaluation of oligonol on α-glucosidase, protein tyrosine phosphatase 1B, cholinesterase, and β-secretase 1 related to diabetes and Alzheimer’s disease. Arch. Pharm. Res. 2016, 39, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Nishioka, H.; Wakame, K.; Magnuson, B.A.; Roberts, A. Acute, subchronic and genotoxicity studies conducted with Oligonol, an oligomerized polyphenol formulated from lychee and green tea extracts. Food Chem. Toxicol. 2008, 46, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Pal, H.C.; Prasad, R. Dietary proanthocyanidins prevent ultraviolet radiation-induced non-melanoma skin cancer through enhanced repair of damaged DNA-dependent activation of immune sensitivity. Semin. Cancer Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Chung, I.-M.; Kim, M.-Y.; Park, K.-D.; Park, W.-H.; Park, W.-W.; Moon, H.-I. Inhibition of lung metastasis in mice by oligonol. Phytother. Res. 2009, 23, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Hwang, D.-M.; Lee, J.-C.; Chang, E.-J.; Shin, Y.K.; Fujii, H.; Sun, B.; Surh, Y.-J. Inhibitory effects of oligonol on phorbol ester-induced tumor promotion and COX-2 expression in mouse skin: NF-kappaB and C/EBP as potential targets. Cancer Lett. 2009, 273, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Yum, H.-W.; Zhong, X.; Park, J.; Na, H.-K.; Kim, N.; Lee, H.S.; Surh, Y.-J. Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid. Redox Signal. 2013, 19, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.-H.; Lee, S.-J.; Ahn, N.-S.; Park, J.-S.; Hwang, J.-W.; Kim, S.-H.; Aruoma, O.I.; Lee, Y.-S.; Kang, K.-S. Induction of apoptosis in MCF-7 and MDA-MB-231 breast cancer cells by Oligonol is mediated by Bcl-2 family regulation and MEK/ERK signaling. Eur. J. Cancer Prev. 2007, 16, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.; Je, N.K.; Chung, H.Y.; Yokozawa, T.; Yoon, S.; Moon, J.-O. Oligonol ameliorates CCl4 -induced liver injury in rats via the NF-Kappa B and MAPK signaling pathways. Oxid. Med. Cell. Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Yuan, S.; Liu, G.; Zhang, Y.L.J.; Wang, W. Potential anticancer activity of Litchi fruit pericarp extract against hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2006, 239, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chung, Y.-C.; Hsu, C.-P. Anti-cancer potential of Litchi seed extract. World J. Exp. Med. 2013, 3, 56–61. [Google Scholar]
- Xu, X.; Xie, H.; Hao, J.; Jiang, Y.; Wei, X. Eudesmane sesquiterpene glucosides from lychee seed and their cytotoxic activity. Food Chem. 2010, 123, 1123–1126. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C. Research progress on the antineoplastic pharmacological effects and mechanisms of Litchi seeds. Chin. Med. 2015, 06, 20–26. [Google Scholar] [CrossRef]
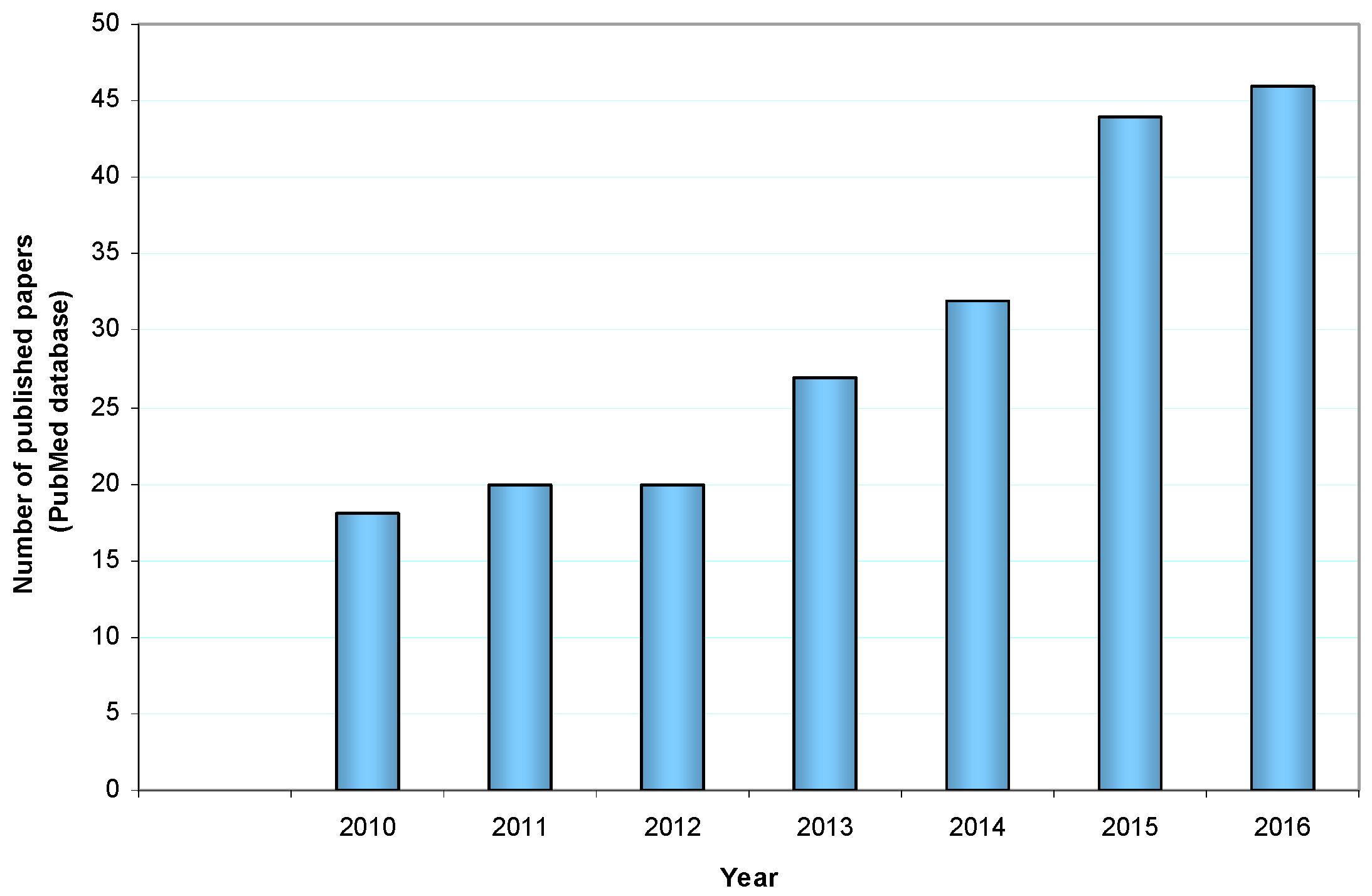

| CALORIES | 66 kcal/100 g |
|---|---|
| MACROCOMPONENTS (g/100 g) | |
| Carbohydrates | 16.53 |
| Lipid | 0.44 |
| Protein | 0.83 |
| Dietary fiber | 1.30 |
| Water | 81.76 |
| MICROCOMPONENTS | |
| Total carotenoid content (μg beta-carotene equivalent/100 g) | 571.4 ± 117.2 |
| Vitamin C content (mg ascorbic acid equivalent/100 g) | 10.1 ± 2.2 |
| Total polyphenol content (mg gallic acid equivalent/100 g) | 178.0 ± 34.7 |
| Total flavonoid content (mg quercetin equivalent/100 g) | 53.3 ± 5.9 |
| Cancer Model | Extracts or Specific Components | Antitumor Effect | Biochemical Pathways | References | ||
|---|---|---|---|---|---|---|
| Pulp | In vitro(cancer cell lines) | Lung adenocarcinoma, cervical cancer, hepatocellular carcinoma | Polysaccharides | Antiproliferative | Cell viability reduction | [22] |
| Immunomodulatory | Induction of mouse splenocyte proliferation | |||||
| Gastric cancer, hepatocellular and lung carcinoma | Galactose and mannose | Antiproliferative | Cell viability reduction | [23] | ||
| Antioxidant | Increase in cellular antioxidant activity | |||||
| In vivo(mice) | Chemical-induced liver injury | Pulp extract | Hepatoprotective | Decreased serum ALT and AST levels | [27] | |
| Antioxidant | Changes in antioxidant enzyme levels | |||||
| Peel | In vitro(cancer cell lines) | Hepatocellular carcinoma | Water-soluble crude ethanolic extract | Antiproliferative | Cell viability reduction, clonogenic growth decrease | [75] |
| Apoptosis induction | Pre G0/G1 pro-apoptotic peak in cell cycle profile | |||||
| Breast cancer cells | Water-soluble crude ethanolic extract | Antiproliferative | Cell viability reduction, Clonogenic growth decrease | [37] | ||
| Apoptosis induction | Up and down-regulation of gene clusters involved in cell death | |||||
| Breast cancer cells | Specific flavonoid components | Antiproliferative | Cell viability reduction | [36,38] | ||
| In vivo | Murine hepatoma bearing-mice | Inhibition of tumor growth | Reduction in cell proliferation | [75] | ||
| Nude mice bearing human breast infiltrating duct carcinoma | Water-soluble crude ethanolic extract | Tumor mass reduction | Reduction in cell proliferation | [37] | ||
| Apoptosis induction | Caspase-3 activation | |||||
| Seed | In vitro(cancer cell lines) | Lung adenocarcinoma, cervical, breast, ovarian cancers and hepatocellular carcinoma | Flavonoid glycosides | Anti proliferative | Cell viability reduction | [51,76] |
| Hepatocellular, lung and cervical carcinoma | Sesquiterpene glucosides | Anti proliferative | Cell viability reduction | [77] | ||
| Non-small cell lung cancer | Crude Litchi extract | Anti proliferative | Inhibition of EGF-receptor-pathway | [53] | ||
| Apoptosis induction | Bcl-2 family pro-apoptotic ratio and caspase activation | |||||
| Colorectal carcinoma | Flavonoids and tannins | Anti proliferative | G2/M phase cell cycle arrest with reduction in cyclin levels | [48] | ||
| Apoptosis induction | Increase in Bax level and caspase activation | |||||
| Prostate cancer | N-butyl alcohol extract | Anti proliferative | Clonogenic growth decrease, G1/S phase Cell cycle arrest with increase in p21 and p27 CDK inhibitors | [54] | ||
| Apoptosis induction | Activation of mitochondrial caspase cascade | |||||
| Decrease in cell migration and invasion | Increase in E-cadherin and â-catenin, decrease of vimentin and snail, inhibition of Akt pathway | |||||
| Hepatocellular carcinoma | Semen Litchi containing serum | Anti proliferative | Cell viability reduction | [47] | ||
| Apoptosis induction | Appearance of nuclear morphological features and pre G0/G1 pro-apoptotic peak in cell cycle profile | |||||
| In vivo | mouse xenografts of Ehrlich ascites cells, sarcoma S180 cells, or liver tumor cells | Water extract | Decrease in tumor size | [78] | ||
| hyperplasia of mammary glands rat model | Saponins | Reduction of mammary gland hyperplasia | [49] | |||
| Nude mice xenograft of PC3 cells | n-butyl alcohol extract | Decrease in tumor size | [54] | |||
| Oligonol | In vitro | Breast cancer cells | Low MW polyphenols from lychee fruit extract | Apoptosis induction | Modulation of pro-apoptotic Bcl-2 family proteins and MEK/ERK signaling pathway | [73] |
| In vivo | DSS-promoted adenoma in the mouse colon | Inhibition of colonic adenoma formation | Reduction of cyclins | [72] | ||
| Variation of oxidative stress markers | ||||||
| Melanoma mice models | Inhibition of lung metastasis | Inhibition of lung hexosammine content, and serum sialic acid and gamma glutamyltranspeptidase content | [70] | |||
| Mouse skin and carcinomas and papillomas bearing mice | Suppression of chemically-induced tumorigenesis | NF-êB and C/EBP DNA binding decrease, reduction of ERK 1/2 and P38 kinases, reduction in PCNA and COX2 | [71] | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emanuele, S.; Lauricella, M.; Calvaruso, G.; D’Anneo, A.; Giuliano, M. Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways. Nutrients 2017, 9, 992. https://doi.org/10.3390/nu9090992
Emanuele S, Lauricella M, Calvaruso G, D’Anneo A, Giuliano M. Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways. Nutrients. 2017; 9(9):992. https://doi.org/10.3390/nu9090992
Chicago/Turabian StyleEmanuele, Sonia, Marianna Lauricella, Giuseppe Calvaruso, Antonella D’Anneo, and Michela Giuliano. 2017. "Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways" Nutrients 9, no. 9: 992. https://doi.org/10.3390/nu9090992







