Emotion-Based Cognition in Mice Is Differentially Influenced by Dose and Chemical Form of Dietary Docosahexaenoic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Behavioral Tasks
2.3. Lipid Extraction and Quantification
2.4. Statistical Analysis
3. Results
3.1. Body and Tissue Weight and Food Intake
3.2. Behavioral Tests
3.3. Brain Fatty Acid Composition
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-Y.; Tsao, Y.-Y.; Leung, Y.-M.; Su, K.-P. Docosahexaenoic Acid Suppresses Neuroinflammatory Responses and Induces Heme Oxygenase-1 Expression in BV-2 Microglia: Implications of Antidepressant Effects for Omega-3 Fatty Acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Bazinet, R.P. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Investig. Drugs 2008, 9, 735–743. [Google Scholar] [PubMed]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Levant, B.; Reichel, M.; Gulbins, E.; Kornhuber, J.; Müller, C.P. Lipids in psychiatric disorders and preventive medicine. Neurosci. Biobehav. Rev. 2017, 76, 336–362. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Bazinet, R.P.; Sinclair, A.J. Fat intake and CNS functioning: Ageing and disease. Ann. Nutr. Metab. 2009, 55, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Ma, Q.-L.; Frautschy, S.A. Dietary fatty acids and the aging brain. Nutr. Rev. 2010, 68 (Suppl. S2), S102–S111. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Gupta, S.; Dherange, P.; De Meester, F.; Wilczynska, A.; Alam, S.E.; Pella, D.; Wilson, D.W. Metabolic syndrome: A brain disease. Can. J. Physiol. Pharmacol. 2012, 90, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Begg, D.; Mathai, M.; Weisinger, R.S. Omega 3 fatty acids and the brain: Review of studies in depression. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 391–397. [Google Scholar] [PubMed]
- Delion, S.; Chalon, S.; Guilloteau, D.; Lejeune, B.; Besnard, J.C.; Durand, G. Age-related changes in phospholipid fatty acid composition and monoaminergic neurotransmission in the hippocampus of rats fed a balanced or an n-3 polyunsaturated fatty acid-deficient diet. J. Lipid Res. 1997, 38, 680–689. [Google Scholar] [PubMed]
- Favrelère, S.; Stadelmann-Ingrand, S.; Huguet, F.; De Javel, D.; Piriou, A.; Tallineau, C.; Durand, G. Age-related changes in ethanolamine glycerophospholipid fatty acid levels in rat frontal cortex and hippocampus. Neurobiol. Aging 2000, 21, 653–660. [Google Scholar] [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Messamore, E.; Almeida, D.M.; Jandacek, R.J.; McNamara, R.K. Polyunsaturated fatty acids and recurrent mood disorders: Phenomenology, mechanisms, and clinical application. Prog. Lipid Res. 2017, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M.; Seguin, J.; Sieswerda, L.E. Omega-3 fatty acids as treatments for mental illness: Which disorder and which fatty acid? Lipids Health Dis. 2007, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Maclean, C.H.; Issa, A.M.; Newberry, S.J.; Mojica, W.A.; Morton, S.C.; Garland, R.H.; Hilton, L.G.; Traina, S.B.; Shekelle, P.G. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evid. Rep. Technol. Assess. (Summary) 2005, 114, 1–3. [Google Scholar]
- Dauncey, M.J. Genomic and Epigenomic Insights into Nutrition and Brain Disorders. Nutrients 2013, 5, 887–914. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Tyagi, E. Diet and cognition: Interplay between cell metabolism and neuronal plasticity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Walach, H. The omega-6/omega-3 ratio and dementia or cognitive decline: A systematic review on human studies and biological evidence. J. Nutr. Gerontol. Geriatr. 2013, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.F.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Frautschy, S.A. DHA May Prevent Age-Related Dementia. J. Nutr. 2010, 140, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [PubMed]
- Denis, I.; Potier, B.; Vancassel, S.; Heberden, C.; Lavialle, M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Res. Rev. 2013, 12, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Palmblad, J. Are omega-3 fatty acids options for prevention and treatment of cognitive decline and dementia? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. MAPT Study Group Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Graf, B.A.; Duchateau, G.S.; Patterson, A.B.; Mitchell, E.S.; van Bruggen, P.; Koek, J.H.; Melville, S.; Verkade, H.J. Age dependent incorporation of 14C-DHA into rat brain and body tissues after dosing various 14C-DHA-esters. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Nieto, S.; Sanhueza, J.; Nuñez, M.J.; Ferrer, C. Tissue accretion and milk content of docosahexaenoic acid in female rats after supplementation with different docosahexaenoic acid sources. Ann. Nutr. Metab. 2005, 49, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Arabo, A.; Potier, C.; Ollivier, G.; Lorivel, T.; Roy, V. Temporal analysis of free exploration of an elevated plus-maze in mice. J. Exp. Psychol. Anim. Learn. Cogn. 2014, 40, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Smedes, F. Determination of total lipid using non-chlorinated solvents. Analyst 1999, 124, 1711–1718. [Google Scholar] [CrossRef]
- Fedor, D.M.; Adkins, Y.; Newman, J.W.; Mackey, B.E.; Kelley, D.S. The effect of docosahexaenoic acid on t10, c12-conjugated linoleic acid-induced changes in fatty acid composition of mouse liver, adipose, and muscle. Metab. Syndr. Relat. Disord. 2013, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.A.; Zidell, R.H.; Perry, R.W. Relationships between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol. Pathol. 2004, 32, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K.; Pitsch, S. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Colicchio, P.; Conca, P.; Finelli, C.; Di Minno, M.N.D.; Tarantino, M.; Capone, D.; Pasanisi, F. Young adult obese subjects with and without insulin resistance: What is the role of chronic inflammation and how to weigh it non-invasively? J. Inflamm. Lond. Engl. 2009, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Conca, P.; Pasanisi, F.; Ariello, M.; Mastrolia, M.; Arena, A.; Tarantino, M.; Scopacasa, F.; Vecchione, R. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur. J. Gastroenterol. Hepatol. 2009, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Iwanaga, M.; Harada, E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res. 2003, 964, 136–143. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Delattre, A.M.; Almendra, R.G.; Sonagli, M.; Borges, C.; Araujo, P.; Andersen, M.L.; Tufik, S.; Lima, M.M.S. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav. Brain Res. 2011, 219, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.; Salem, N. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Trofimiuk, E.; Braszko, J.J. Concomitant docosahexaenoic acid administration ameliorates stress-induced cognitive impairment in rats. Physiol. Behav. 2013, 118, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.Á.; Terreros, G.; Dagnino-Subiabre, A. Long-term ω-3 fatty acid supplementation induces anti-stress effects and improves learning in rats. Behav. Brain Funct. BBF 2013, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J. Nutr. Biochem. 2010, 21, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Schipper, P.; Kiliaan, A.J.; Homberg, J.R. A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav. Pharmacol. 2011, 22, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Venna, V.R.; Deplanque, D.; Allet, C.; Belarbi, K.; Hamdane, M.; Bordet, R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 2009, 34, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; Nolan, Y.M.; Green, H.F.; Robertson, R.C.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. The Omega-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid (DHA) Reverses Corticosterone-Induced Changes in Cortical Neurons. Int. J. Neuropsychopharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Brinks, V.; van der Mark, M.; de Kloet, R.; Oitzl, M. Emotion and Cognition in High and Low Stress Sensitive Mouse Strains: A Combined Neuroendocrine and Behavioral Study in BALB/c and C57BL/6J Mice. Front. Behav. Neurosci. 2007, 1. [Google Scholar] [CrossRef] [PubMed]
- Messamore, E.; McNamara, R.K. Detection and treatment of omega-3 fatty acid deficiency in psychiatric practice: Rationale and implementation. Lipids Health Dis. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Smirnova, M.; Clément, M.; De, J.D.; Francès, H.; Bourre, J.M. Docosahexaenoic acid-rich phospholipid supplementation: Effect on behavior, learning ability, and retinal function in control and n-3 polyunsaturated fatty acid deficient old mice. Nutr. Neurosci. 2002, 5, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Clément, M.; de Javel, D.; Francès, H.; Bourre, J.M. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [PubMed]
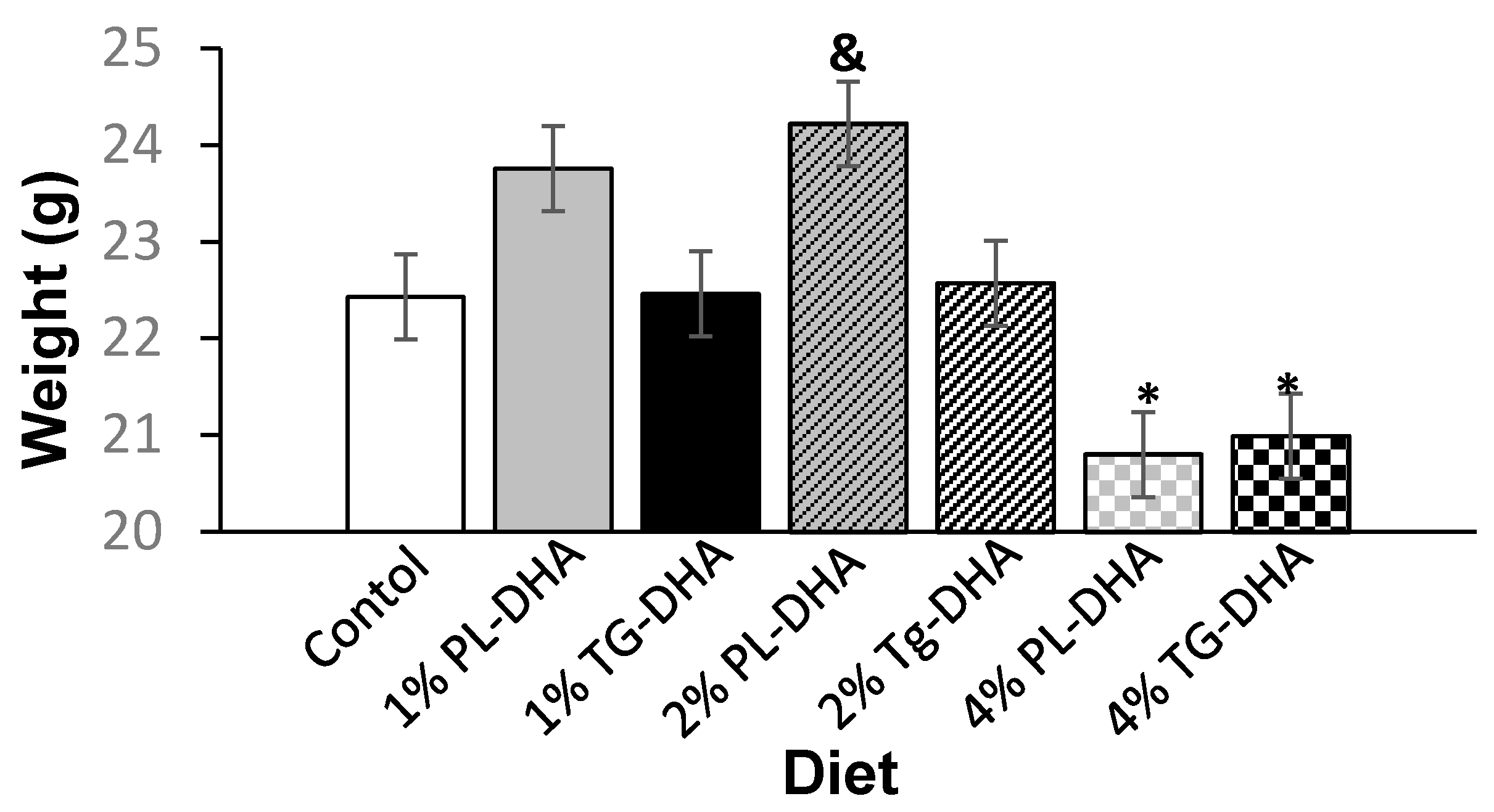
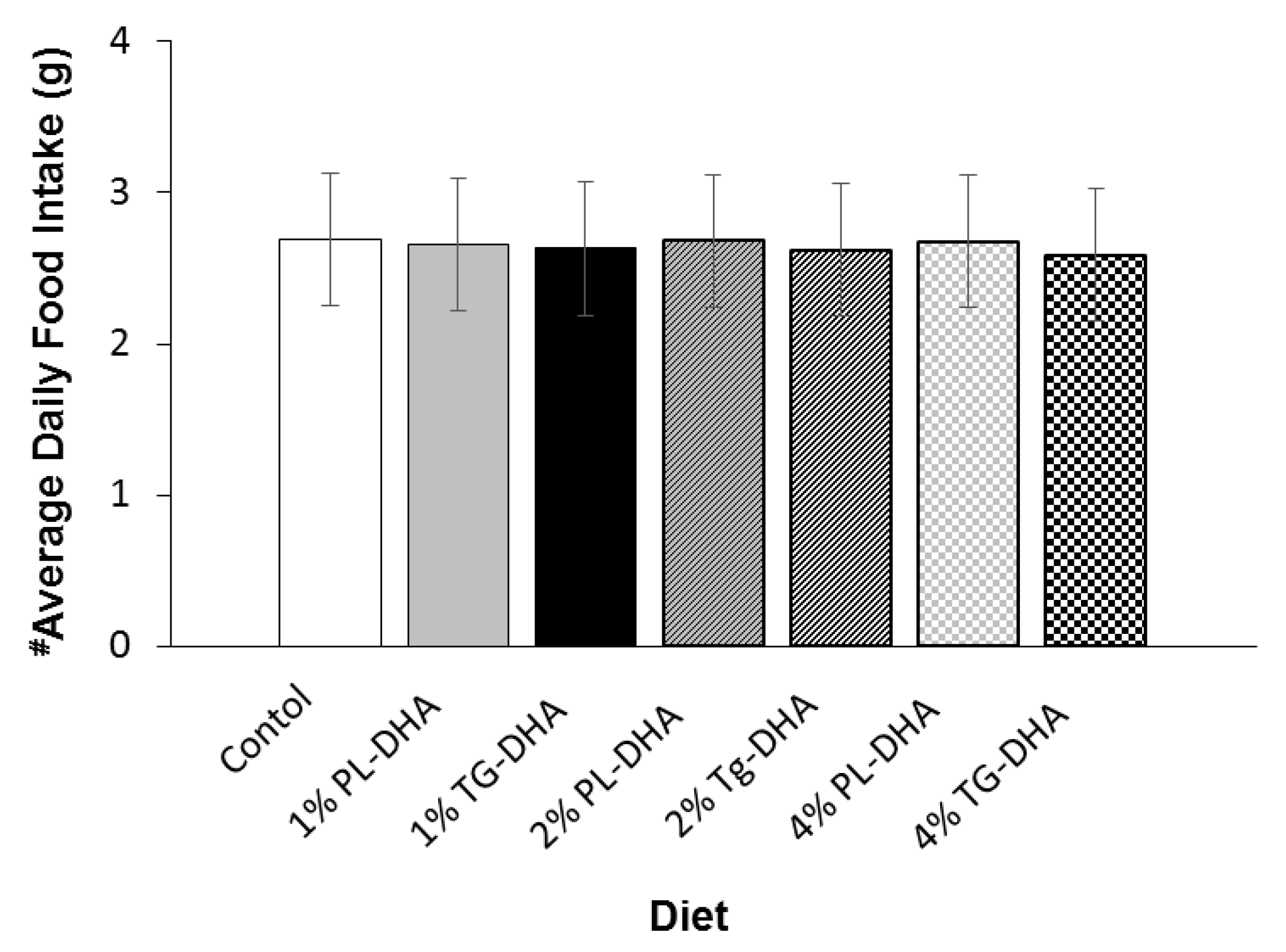
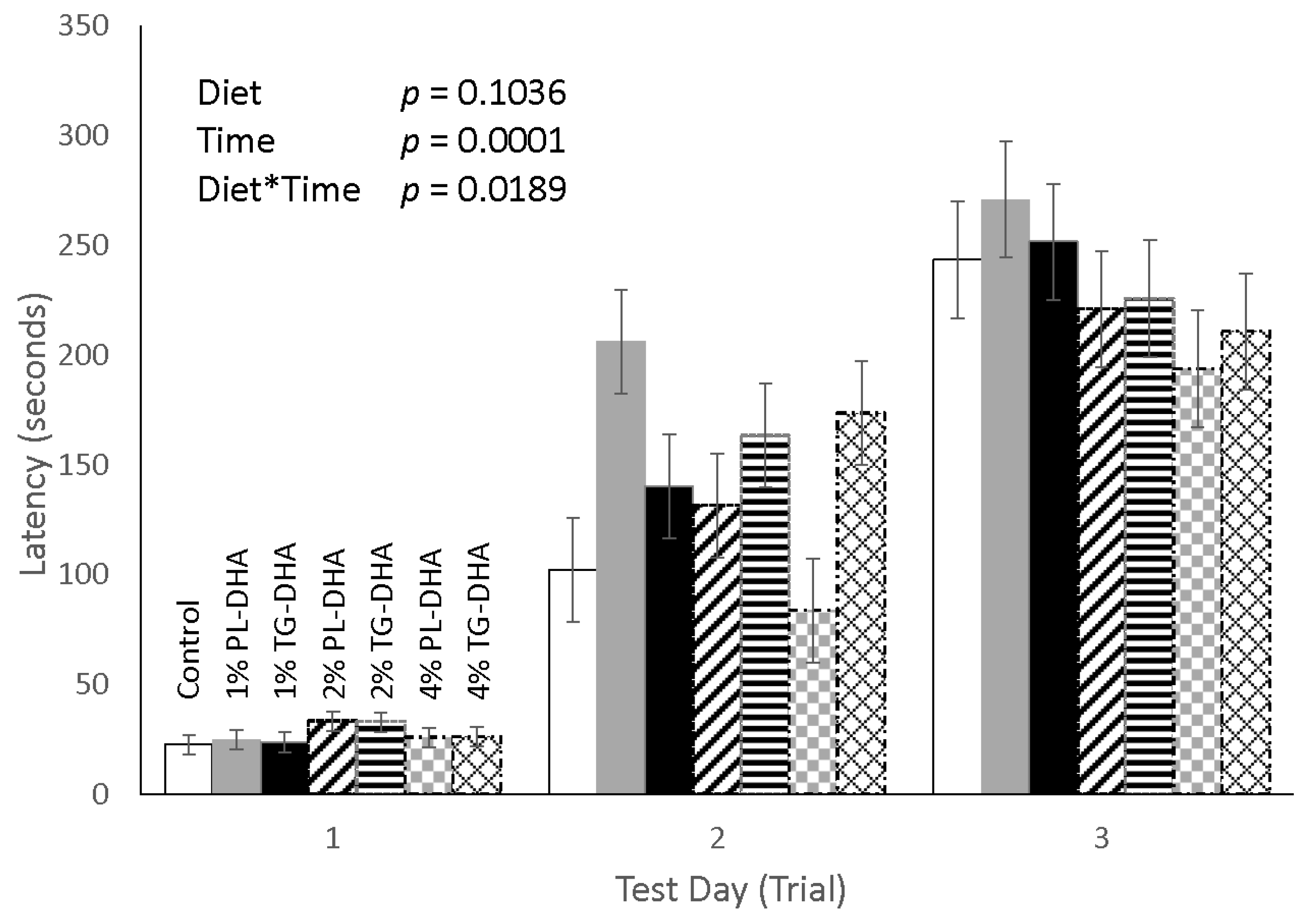
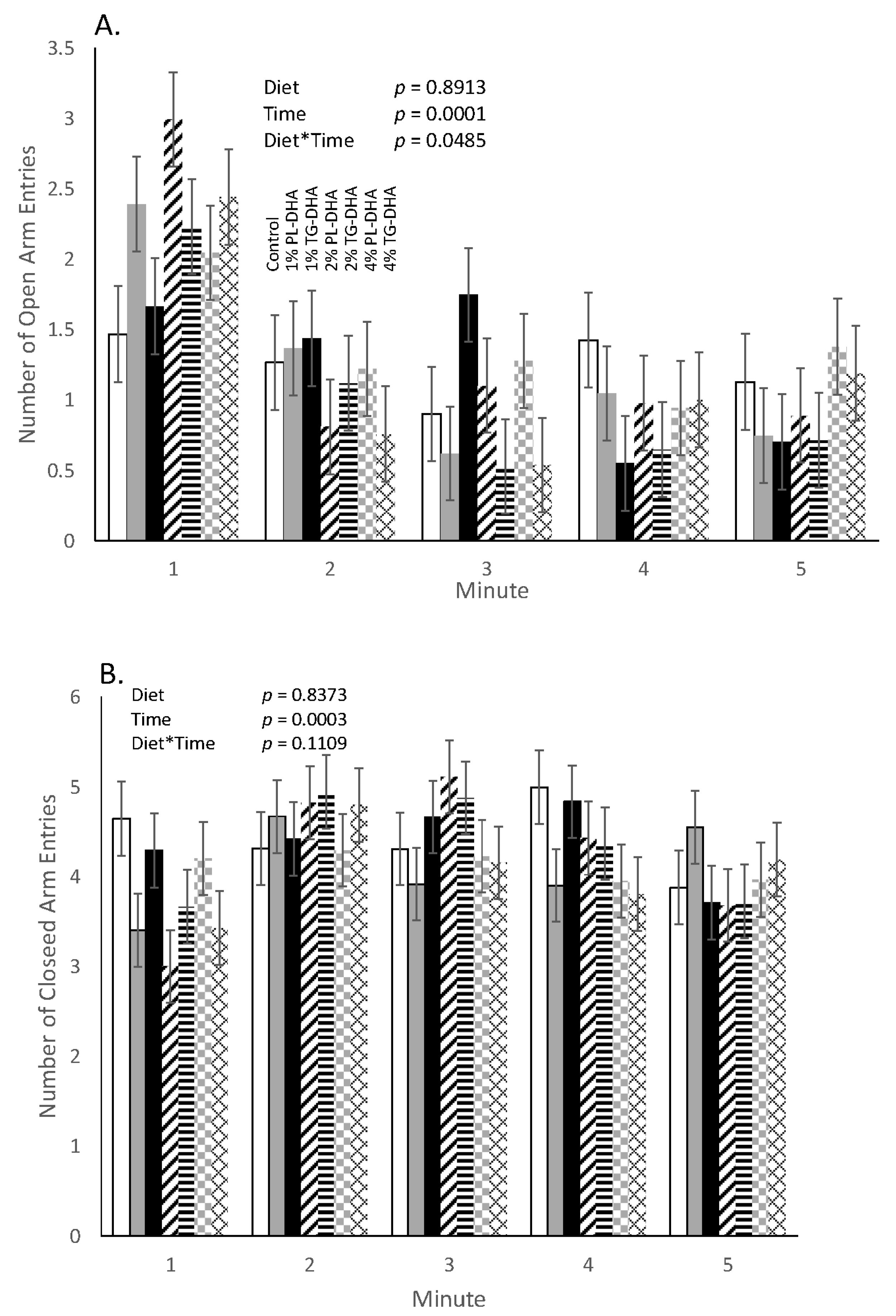
| Tissue (g) | Control | 1% PL-DHA | 1% TG-DHA | 2% PL-DHA | 2% TG-DHA | 4% PL-DHA | 4% TG-DHA |
|---|---|---|---|---|---|---|---|
| #Mesenteric Fat | 0.205 ± 0.017 ab | 0.174 ± 0.017 a | 0.203 ± 0.017 ab | 0.189 ± 0.018 a | 0.217 ± 0.017 ab | 0.225 ± 0.017 ab | 0.244 ± 0.017 b |
| Subcutaneous Fat | 0.654 ± 0.084 | 0.564 ± 0.085 | 0.751 ± 0.084 | 0.539 ± 0.086 | 0.655 ± 0.084 | 0.626 ± 0.085 | 0.689 ± 0.085 |
| Peri-Uterine Fat | 0.755 ± 0.075 | 0.659 ± 0.076 | 0.790 ± 0.075 | 0.643 ± 0.077 | 0.770 ± 0.075 | 0.767 ± 0.076 | 0.824 ± 0.076 |
| Brain | 0.463 ± 0.007 | 0.461 ± 0.007 | 0.471 ± 0.007 | 0.450 ± 0.007 | 0.467 ± 0.007 | 0.457 ± 0.007 | 0.462 ± 0.007 |
| #Liver | 0.787 ± 0.042 ab | 0.707 ± 0.043 a | 0.705 ± 0.042 a | 0.744 ± 0.043 ab | 0.751 ± 0.042 ab | 0.789 ± 0.043 ab | 0.849 ± 0.043 b |
| #Spleen | 0.053 ± 0.003 a | 0.058 ± 0.003 a | 0.056 ± 0.003 a | 0.061 ± 0.003 a | 0.061 ± 0.003 a | 0.074 ± 0.003 b | 0.090 ± 0.003 c |
| #Quadriceps | 0.151 ± 0.006 a | 0.145 ± 0.007 ab | 0.142 ± 0.007 ab | 0.137 ± 0.007 ab | 0.146 ± 0.007 ab | 0.126 ± 0.007 b | 0.143 ± 0.016 ab |
| #Gastrocnemius | 0.115 ± 0.003 a | 0.113 ± 0.003 ab | 0.120 ± 0.003 ab | 0.114 ± 0.003 ab | 0.119 ± 0.003 ab | 0.104 ± 0.003 b | 0.113 ± 0.003 ab |
| Soleus | 0.006 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 | 0.006 ± 0.001 | 0.007 ± 0.001 | 0.008 ± 0.001 | 0.007 ± 0.001 |
| Heart | 0.098 ± 0.008 | 0.096 ± 0.008 | 0.092 ± 0.007 | 0.099 ± 0.009 | 0.097 ± 0.001 | 0.910 ± 0.001 | 0.089 ± 0.008 |
| Eyes | 0.039 ± 0.001 | 0.040 ± 0.002 | 0.040 ± 0.002 | 0.041 ± 0.001 | 0.041 ± 0.002 | 0.041 ± 0.003 | 0.041 ± 0.003 |
| Fatty Acid μmol/g | Control | 1% PL-DHA | 1% TG-DHA | 2% PL-DHA | 2% TG-DHA | 4% PL-DHA | 4% TG-DHA |
|---|---|---|---|---|---|---|---|
| SFA | |||||||
| 16:0 | 26.44 ± 1.22 | 24.24 ± 1.00 | 26.70 ± 1.31 | 26.24 ± 1.13 | 26.37 ± 1.12 | 25.25 ± 0.99 | 27.37 ± 1.68 |
| 18:0 | 23.29 ± 0.84 | 20.88 ± 0.89 | 22.69 ± 1.21 | 23.02 ± 1.02 | 22.58 ± 0.95 | 21.69 ± 0.78 | 23.54 ± 1.54 |
| Total | 49.73 ± 1.98 | 45.12 ± 1.80 | 49.39 ± 2.44 | 49.25 ± 2.12 | 48.96 ± 1.97 | 46.94 ± 1.70 | 50.91 ± 3.17 |
| MUFA | |||||||
| 18:1n9 | 20.91 ± 1.27 | 17.65 ± 1.20 | 19.74 ± 1.70 | 21.45 ± 1.19 | 23.22 ± 1.39 | 19.98 ± 1.35 | 22.59 ± 2.29 |
| 18:1n7 | 4.55 ± 0.30 | 3.13 ± 0.19 | 3.69 ± 0.35 | 3.47 ± 0.39 | 4.54 ± 0.41 | 3.46 ± 0.30 | 3.91 ± 0.47 |
| 20:1n9 | 2.66 ± 0.52 | 1.86 ± 0.44 | 2.08 ± 0.41 | 2.12 ± 0.26 | 2.94 ± 0.46 | 2.22 ± 0.47 | 2.48 ± 0.43 |
| 24:1n9 | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.09 ± 0.03 | 0.13 ± 0.04 | 0.09 ± 0.01 | 0.12 ± 0.02 |
| Total | 28.21 ± 1.98 | 22.73 ± 1.79 | 25.57 ± 2.39 | 27.12 ± 1.43 | 30.84 ± 2.13 | 25.75 ± 2.00 | 29.10 ± 3.11 |
| n-6 PUFA | |||||||
| 18:2n6 | 0.79 ± 0.03 a | 0.81 ± 0.03 ab | 0.95 ± 0.07 ab | 0.83 ± 0.04 ab | 0.91 ± 0.04 ab | 0.45 ± 0.02 b | 0.65 ± 0.05 a* |
| 20:4n6 | 14.11 ± 0.84 a | 10.31 ± 0.57 b | 10.50 ± 0.61 b | 10.08 ± 0.56 b | 9.25 ± 0.63 b | 8.68 ± 0.36 b | 8.87 ± 0.67 b |
| 22:4n6 | 3.10 ± 0.16 a | 2.00 ± 0.13 b | 1.87 ± 0.15 b | 1.97 ± 0.17 b | 1.88 ± 0.11 b | 1.73 ± 0.06 b | 1.78 ± 0.13 b |
| Total | 18.00 ± 0.98 a | 13.12 ± 0.65 b | 13.32 ± 0.73 b | 12.88 ± 0.71 b | 12.03 ± 0.72 b | 10.86 ± 0.34 b | 11.30 ± 0.81 b |
| n-3 PUFA | |||||||
| 20:5n3 | 0.03 ± 0.00 a | 0.24 ± 0.01 b | 0.27 ± 0.03 b | 0.41 ± 0.02 b | 0.44 ± 0.03 b | 0.70 ± 0.02 b | 0.71 ± 0.06 b |
| 22:5n3 | 0.12 ± 0.01 a | 0.34 ± 0.02 b | 0.35 ± 0.03 b | 0.46 ± 0.02 b | 0.49 ± 0.03 b | 0.67 ± 0.05 b | 0.64 ± 0.06 b |
| 22:6n3 | 16.41 ± 0.70 | 15.83 ± 0.64 | 17.89 ± 1.20 | 18.48 ± 1.00 | 18.79 ± 0.96 | 17.05 ± 0.61 | 19.66 ± 1.57 |
| Total | 16.55 ± 0.71 a | 16.41 ± 0.65 ab | 18.51 ± 1.23 ab | 19.35 ± 1.01 ab | 19.71 ± 0.99 ab | 18.42 ± 0.60 ab | 20.98 ± 1.67 b |
| n-6:n-3 ratio | 1.09 ± 0.03 a | 0.80 ± 0.03 b | 0.73 ± 0.03 b | 0.67 ± 0.03 b | 0.62 ± 0.03 b | 0.59 ± 0.01 b | 0.55 ± 0.03 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laugero, K.D.; Adkins, Y.; Mackey, B.E.; Kelley, D.S. Emotion-Based Cognition in Mice Is Differentially Influenced by Dose and Chemical Form of Dietary Docosahexaenoic Acid. Nutrients 2017, 9, 993. https://doi.org/10.3390/nu9090993
Laugero KD, Adkins Y, Mackey BE, Kelley DS. Emotion-Based Cognition in Mice Is Differentially Influenced by Dose and Chemical Form of Dietary Docosahexaenoic Acid. Nutrients. 2017; 9(9):993. https://doi.org/10.3390/nu9090993
Chicago/Turabian StyleLaugero, Kevin D., Yuriko Adkins, Bruce E. Mackey, and Darshan S. Kelley. 2017. "Emotion-Based Cognition in Mice Is Differentially Influenced by Dose and Chemical Form of Dietary Docosahexaenoic Acid" Nutrients 9, no. 9: 993. https://doi.org/10.3390/nu9090993
APA StyleLaugero, K. D., Adkins, Y., Mackey, B. E., & Kelley, D. S. (2017). Emotion-Based Cognition in Mice Is Differentially Influenced by Dose and Chemical Form of Dietary Docosahexaenoic Acid. Nutrients, 9(9), 993. https://doi.org/10.3390/nu9090993





