Biological Control Products for Aflatoxin Prevention in Italy: Commercial Field Evaluation of Atoxigenic Aspergillus flavus Active Ingredients
Abstract
:1. Introduction
2. Results
2.1. Pin-Bar Inoculation Experiment
2.2. Field Application of Atoxigenic Aspergillus flavus Strains
2.2.1. Aspergillus flavus Population
2.2.2. Mycotoxin Contamination
2.3. Mating-Type and Microsatellites
3. Discussion
4. Materials and Methods
4.1. Isolates of Aspergillus flavus
4.2. Pin-Bar Inoculation Experiment
4.3. Natural Contamination Experiment
4.3.1. Preparation of Atoxigenic A. flavus Based Product
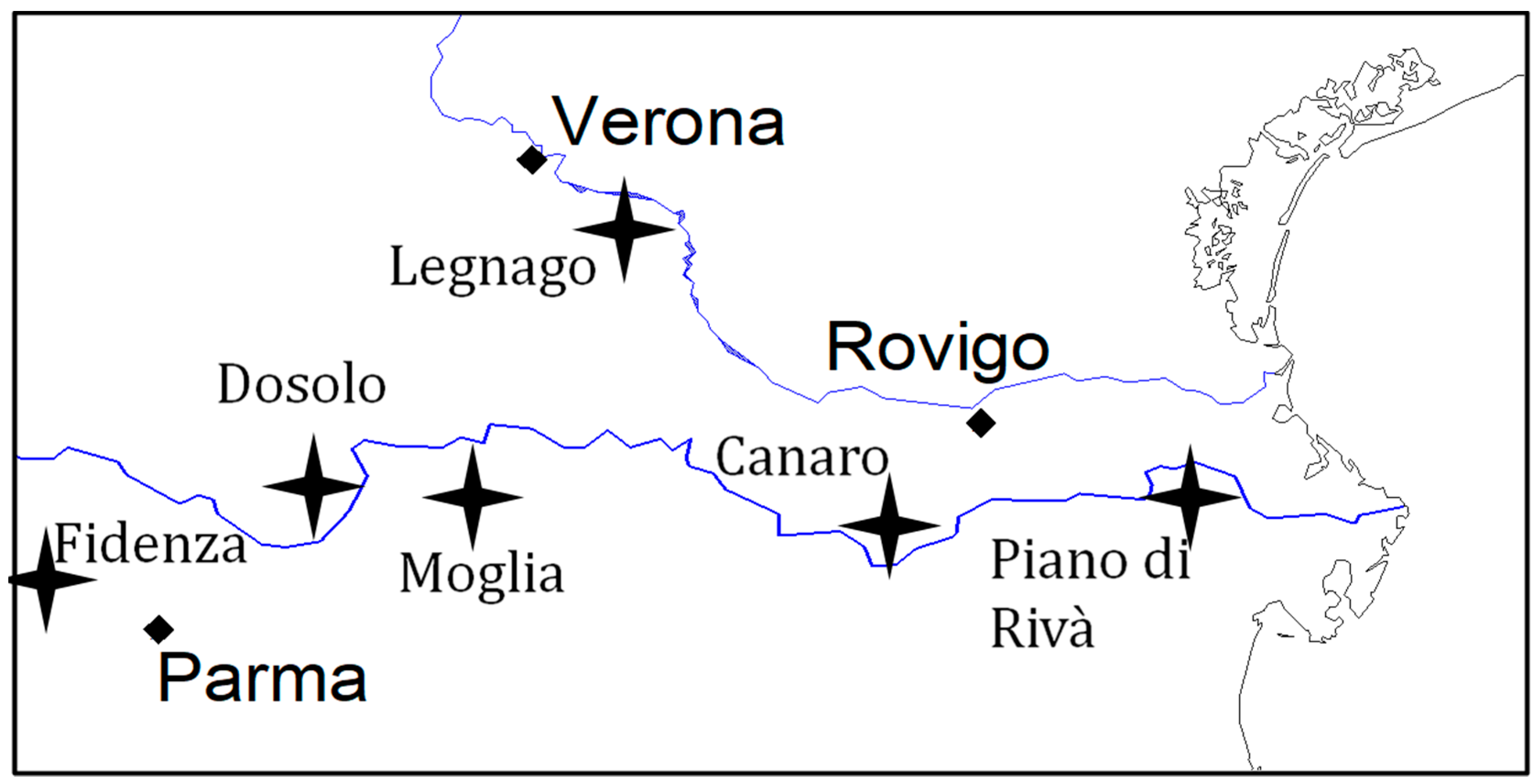
4.3.2. Maize Field Locations and Experimental Design
4.3.3. Recovery of Applied Atoxigenic Strains
4.4. Aflatoxin Quantification
4.5. Fumonisin Quantification
4.6. Verification of Fungal Identities
4.7. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.S.; Tang, L. Epidemiology of aflatoxin exposure and human liver cancer. Toxin Rev. 2004, 23, 249–271. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bayman, P.; Egel, D.S.; Elias, K.S. Agriculture, aflatoxins and Aspergillus. In The Genus Aspergillus: From Taxonomy and Genetics to Industrial Applications; Powell, K.A., Renwick, A., Peberdy, J.F., Eds.; Plenum Publishing: New York, NY, USA, 1994; pp. 1–27. ISBN 0306447010. [Google Scholar]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Istituto Nazionale di Statistica (Istat). 2016. Available online: www.istat.it (accessed on 20 November 2017).
- European Commission (EC). Commission Regulation (EC) No 165/2010 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Communities 2010, L50, 8–12. [Google Scholar]
- Piva, G.; Battilani, P.; Pietri, A. Emerging issues in southern Europe: Aflatoxins in Italy. In The Mycotoxin Factbook: Food and Feed Topics; Barug, D., Bhatnagar, D., van Egmond, H.P., van der Kamp, J.W., van Osenbruggen, W.A., Visconti, A., Eds.; Wageningen Academic Publisher: Wageningen, The Netherlands, 2006; pp. 139–153. ISBN 978-90-8686-006-7. [Google Scholar]
- Cotty, P.J.; Antilla, L.; Wakelyn, P.J. Competitive exclusion of aflatoxin producers: Farmer Driven Research and Development. In Biological Control: A Global Perspective; Vincent, C., Goettel, N., Lazarovits, G., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 241–253. ISBN 9781845932657. [Google Scholar]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Atehnkeng, J.; Ojiambo, P.S.; Cotty, P.J.; Bandyopadhyay, R. Field efficacy of a mixture of atoxigenic Aspergillus flavus Link:Fr vegetative compatibility groups in preventing aflatoxin contamination in maize (Zea mays L.). Biol. Control 2014, 72, 62–70. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Ortega-Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.; Kaptoge, L.; Senghor, A.L.; Adhikari, B.N.; Cotty, P.J. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016, 9, 771–789. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bayman, P. Competitive exclusion of a toxigenic strain of Aspergillus flavus by an atoxigenic strain. Phytopathology 1993, 83, 1283–1287. [Google Scholar] [CrossRef]
- Cotty, P.J. Biocompetitive exclusion of toxigenic fungi. In The Mycotoxin Factbook: Food and Feed Topics; Barug, D., Bhatnagar, D., van Egmond, H.P., van der Kamp, J.W., van Osenbruggen, W.A., Visconti, A., Eds.; Wageningen Academic Publisher: Wageningen, The Netherlands, 2006; pp. 179–197. ISBN 978-90-8686-006-7. [Google Scholar]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. 2008, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W. Biological control of aflatoxin contamination in corn using a nontoxigenic strain of Aspergillus flavus. J. Food Prot. 2009, 72, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of aflatoxin in peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Horn, B.W. Separate and combined applications of nontoxigenic Aspergillus flavus and A. parasiticus for biocontrol of aflatoxin in peanuts. Mycopathologia 2007, 163, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.; Atehnkeng, J.; Sikora, R.A.; Bandyopadhyay, R.; Cotty, P.J. Distribution of Aspergillus section flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol. Biochem. 2009, 41, 37–44. [Google Scholar] [CrossRef]
- Klueken, A.M.; Borgemeister, C.; Hau, B. Field release of a non-toxigenic Aspergillus flavus L strain in central Benin. J. Plant Dis. Prot. 2009, 116, 17–22. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Horn, B.W.; Phillips, N.A.; Johnson, B.J.; Jin, X.; Abel, C.A. Comparison of major biocontrol strains of non-aflatoxigenic Aspergillus flavus for the reduction of aflatoxins and cyclopiazonic acid in maize. Food Addit. Contam. 2011, 28, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Alaniz Zanon, M.S.; Chiotta, M.L.; Giaj-Merlera, G.; Barros, G.; Chulze, S. Evaluation of potential biocontrol agent for aflatoxin in Argentinean peanuts. Int. J. Food Microbiol. 2013, 162, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J. Effect of atoxigenic strains of Aspergillus flavus on aflatoxin contamination of developing cottonseed. Plant Dis. 1990, 74, 233–235. [Google Scholar] [CrossRef]
- Cotty, P.J. Influence of field application of an atoxigenic strain of Aspergillus flavus on populations of A. flavus infecting cotton bolls and on the aflatoxin content of cottonseed. Phytopathology 1994, 84, 1270–1277. [Google Scholar] [CrossRef]
- Brown, R.L.; Cotty, P.J.; Cleveland, T.E. Reduction in aflatoxin content of maize by atoxigenic strains of Aspergillus flavus. J. Food Prot. 1991, 54, 623–626. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Bruns, H.A.; Abel, C.A. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci. Technol. 2006, 16, 437–449. [Google Scholar] [CrossRef]
- Accinelli, C.; Saccà, M.L.; Abbas, H.K.; Zablotowicz, R.M.; Wilkinson, J.R. Use of a granular bioplastic formulation for carrying conidia of a non-aflatoxigenic strain of Aspergillus flavus. Bioresour. Technol. 2009, 100, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
- Papa, K.E. Heterokaryon incompatibility in Aspergillus flavus. Mycologia 1986, 78, 98–101. [Google Scholar] [CrossRef]
- Leslie, J.F. Fungal vegetative compatibility. Annu. Rev. Phytopathol. 1993, 31, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Bayman, P.; Cotty, P.J. Vegetative compatibility and genetic diversity in the Aspergillus flavus population of a single field. Can. J. Bot. 1991, 69, 1707–1711. [Google Scholar] [CrossRef]
- Barros, G.G.; Torres, A.M.; Rodriguez, M.I.; Chulze, S.N. Genetic diversity within Aspergillus flavus strains isolated from peanut-cropped soils in Argentina. Soil Biol. Biochem. 2006, 38, 145–152. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E.; Kaller, M.D. Comparison of soil and corn kernel Aspergillus flavus populations: Evidence for niche specialization. Phytopathology 2011, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Battilani, P.; Callicott, K.A.; Giorni, P.; Pietri, A.; Cotty, P.J. Structure of an Aspergillus flavus population from maize kernels in northern Italy. Int. J. Food Microbiol. 2013, 162, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Camardo Leggieri, M.; Rossi, V.; Giorni, P. AFLA-maize, a predictive model for Aspegillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Mauro, A.; Battilani, P.; Cotty, P.J. Selection of atoxigenic Aspergillus flavus isolates endemic to Italy for the biocontrol of aflatoxins in maize. Biol. Control 2015, 60, 125–134. [Google Scholar] [CrossRef]
- Jaime-Garcia, R.; Cotty, P.J. Aspergillus flavus in soils and corncobs in South Texas: Implications for management of aflatoxins in corn–cotton rotations. Plant Dis. 2004, 88, 1366–1371. [Google Scholar] [CrossRef]
- Antilla, L.; Cotty, P.J. Advances in the utilization of atoxigenic strain technology to manage aflatoxin in commercial cotton. Mycopathologia 2004, 157, 448. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, C.; Sebok, F.; Varga, J.; Kocsube, S.; Szigeti, G.; Baranyi, N.; Szecsi, A.; Toth, B.; Varga, M.; Kriszt, B.; et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in Hungary. Acta Aliment. 2013, 42, 451–459. [Google Scholar] [CrossRef]
- Levic, J.; Gosic-Dondo, S.; Ivanovic, D.; Stankovic, S.; Krnjaja, V.; Bocarov-Stancic, A.; Stepanic, A. An outbreak of Aspergillus species in response to environmental conditions in Serbia. Pestic. Fitomed. 2013, 28, 167–179. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Barbano, C.; Scandolara, A.; Bertuzzi, T.; Marocco, A. Logistic regression modelling of cropping systems to predict fumonisin contamination in maize. J. Agric. Food Chem. 2008, 56, 10433–10438. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Formenti, S.; Rossi, V.; Ramponi, C. Maize hybrids and fumonisin contamination in kernels. J. Cereal Sci. 2011, 54, 467–472. [Google Scholar] [CrossRef]
- King, S.B.; Scott, G.E. Field inoculation technique to evaluate maize for reaction to kernel infection by Aspergillus flavus. Phytopathology 1982, 72, 782–785. [Google Scholar] [CrossRef]
- Probst, C.; Bandyopadhyay, R.; Price, L.E.; Cotty, P.J. Identification of atoxigenic Aspergillus flavus isolates to reduce aflatoxin contamination of maize in Eastern Kenya. Plant Dis. 2011, 95, 212–218. [Google Scholar] [CrossRef]
- Callicott, K.A.; Cotty, P.J. Method for monitoring deletions in the aflatoxin biosynthesis gene cluster of Aspergillus flavus with multiplex PCR. Lett. Appl. Microbiol. 2015, 60, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Bleiholder, H. Erläuterungen zu den BBCH-Dezimal-Codes für die Entwicklungsstadien von Mais, Raps, Faba-Bohne, Sonnenblume und Erbse-mit Abbildungen. Gesunde Pflanz. 1990, 42, 308–321. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Langelüddecke, P.; Stauss, R.; Van Den Boom, T.; Weber, E.; Witzen-Berger, A. An uniformdecimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Cotty, P.J. Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathologia 1994, 125, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Adye, J.; Matales, R.I. Incorporation of labeled compounds into aflatoxins. Biochim. Biophys. Acta 1964, 86, 418–420. [Google Scholar] [CrossRef]
- Cove, D.J. Chlorate toxicity in Aspergillus nidulans. Studies of mutants altered in nitrate assimilation. Mol. Gen. Genet. 1976, 146, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J.; Taylor, D.R. Influence of complementation medium on vegetative compatibility analyses of Aspergillus flavus. Phytopathology 2003, 93, S18. [Google Scholar]
- Stroka, J.; Petz, M.; Joerissen, U.; Anklam, E. Investigation of various extractants for the analysis of aflatoxin B1 in different food and feed matrices. Food Addit. Contam. 1999, 16, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Pietri, A.; Zanetti, M.; Bertuzzi, T. Distribution of aflatoxins and fumonisins in dry-milled maize fractions. Food Addit. Contam. 2009, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Solfrizzo, M.; De Girolamo, A. Determination of fumonisins B1 and B2 in corn and corn flakes by liquid chromatography with immunoaffinity column cleanup: Collaborative study. J. AOAC Int. 2001, 84, 1828–1837. [Google Scholar] [PubMed]
- Ramirez-Prado, J.H.; Moore, G.G.; Horn, B.H.; Carbone, I. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 2008, 45, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Grubisha, L.C.; Cotty, P.J. Twenty-four microsatellite markers for the aflatoxin-producing fungus Aspergillus flavus. Mol. Ecol. Resour. 2009, 9, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Lamb, M.C. Development and commercial use of afla–guard, an aflatoxin biocontrol agent. Mycotoxin Res. 2006, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Abbas, H.K.; Weaver, M.A.; Ehrlich, K.C.; Scharfenstein, L.L.; Cotty, P.J. Identification of genetic defects in the atoxigenic biocontrol strain Aspergillus flavus K49 reveals the presence of a competitive recombinant group in field populations. Int. J. Food Microbiol. 2012, 154, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Olarte, R.A.; Horn, B.W.; Dorner, J.W.; Monacell, J.T.; Singh, R.; Stone, E.A.; Carbone, I. Effect of sexual recombination on population diversity in aflatoxin production by Aspergillus flavus and evidence for cryptic heterokaryosis. Mol. Ecol. 2012, 21, 1453–1476. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Gauvreau, K. Principles of Biostatistics, 2nd ed.; Duxbury/Thomson Learning: Pacific Grove, CA, USA, 2000; ISBN 978-0534229023. [Google Scholar]
| Inoculum a | Year 2012 | Year 2013 | ||||
|---|---|---|---|---|---|---|
| Aflatoxin B1 (μg/kg) f | Transformed (ln + 1) | Reduction (%) h | Aflatoxin B1 (μg/kg) f | Transformed (ln + 1) | Reduction (%) h | |
| Unwounded b | 0.7 | 0.53 | 0.6A | 0.45 | ||
| A2092 c | 1415.4 | 7.10 | 132.9 | 4.87 | ||
| A2085 d | 0.9 | 0.57 | 1.4 | 0.78 | ||
| A2321 d | 0.0 | 0.00 | 3.8 | 1.55 | ||
| A2092 + A2085 e | 96.2 | 4.57 | 93.2 | 2.3 | 1.05 | 98.3 |
| A2092 + A2321 e | 1381.9 | 7.03 | ns J | 176.7 | 4.99 | ns |
| Not inoculated g | ND i | ND | 1.2 | 0.67 | ||
| Location a | District b | CFU/g c | VCGs (%) e | ||||
|---|---|---|---|---|---|---|---|
| Control | Treated | ||||||
| Control d | Treated d | IT006 | IT019 | IT006 | IT019 | ||
| Canaro (1) | Rovigo (RO1) | 6.60 | 6.74 | 56.7 | 26.7 | 76.7 | 20.0 |
| Dosolo | Mantova (MN1) | 6.67 | 6.64 | 60.0 | 33.3 | 66.7 | 16.7 |
| Moglia | Mantova (MN2) | 4.82 | 5.95 | 53.3 | 16.7 | 60.0 | 30.0 |
| Piano di Rivà | Rovigo (RO3) | 5.09 | 5.52 | 73.3 | 16.7 | 40.0 | 46.7 |
| Canaro (2) | Rovigo (RO2) | 6.68 | 6.73 | 46.7 | 33.3 | 73.3 | 23.3 |
| Fidenza | Parma (PR) | 6.06 | 6.38 | 35.0 | 35.0 | 55.0 | 40.0 |
| Legnago (1) | Verona (VR1) | 6.82 | 7.18 | 60.0 | 17.7 | 56.7 | 33.3 |
| Legnago (2) | Verona (VR2) | 6.57 | 6.93 | 43.3 | 23.3 | 63.3 | 30.0 |
| Average | 6.16 | 6.51 | 53.5 | 25.2 | 61.9 | 30.0 | |
| Location a | District b | Fumonisin B1 + B2 (mg/kg) c | Aflatoxin B1 (μg/kg) c | Aflatoxin B1 Reduction (%) d | ||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | |||
| Canaro (1) | Rovigo (RO1) | 0.1 | 0.1 | <1.0 | <1.0 | - |
| Dosolo | Mantova (MN1) | 5.0 | 5.0 | <1.0 | <1.0 | - |
| Moglia | Mantova (MN2) | 0.1 | 0.2 | <1.0 | <1.0 | - |
| Piano di Rivà | Rovigo (RO3) | 0.6 | 1.4 | <1.0 | <1.0 | - |
| Canaro (2) | Rovigo (RO2) | 0.1 | 0.1 | 37.9 | 0.2 | 94.8 |
| Fidenza | Parma (PR) | 7.9 | 11.1 | 8.6 | 1.5 | 83.7 |
| Legnago (1) | Verona (VR1) | 1.8 | 4.2 | 87.2 | 6.6 | 92.8 |
| Legnago (2) | Verona (VR2) | 2.1 | 2.2 | 150.7 | 8.4 | 94.5 |
| Average | 2.2 | 3.0 | 71.1 | 4.2 | 92.3 | |
| Strain a | Locus Name | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF8 | AF11 | AF13 | AF16 | AF17 | AF22 | AF28 | AF31 | AF42 | AF43 | AF53 | AF54 | AF55 | AF63 | AF64 | AF66 | |
| A2085 | 166 b | 135 | 141 | 169 | 367 | 144 | 119 | 312 | 150 | 399 | 131 | 161 | 181 | 127 | 161 | 271 |
| A2321 | 168 | 126 | 128 | 169 | 364 | 144 | 119 | 312 | 143 | 399 | 131 | 161 | 172 | 127 | 161 | 269 |
| NRRL18543 | 177 | 162 | 161 | 191 | 353 | 188 | 119 | 309 | 162 | 385 | 134 | 169 | 174 | 135 | 211 | 269 |
| NRRL21882 | 168 | 138 | 141 | 169 | 353 | 144 | 119 | 312 | 146 | 402 | 131 | 161 | 181 | 127 | 161 | 267 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, A.; Garcia-Cela, E.; Pietri, A.; Cotty, P.J.; Battilani, P. Biological Control Products for Aflatoxin Prevention in Italy: Commercial Field Evaluation of Atoxigenic Aspergillus flavus Active Ingredients. Toxins 2018, 10, 30. https://doi.org/10.3390/toxins10010030
Mauro A, Garcia-Cela E, Pietri A, Cotty PJ, Battilani P. Biological Control Products for Aflatoxin Prevention in Italy: Commercial Field Evaluation of Atoxigenic Aspergillus flavus Active Ingredients. Toxins. 2018; 10(1):30. https://doi.org/10.3390/toxins10010030
Chicago/Turabian StyleMauro, Antonio, Esther Garcia-Cela, Amedeo Pietri, Peter J. Cotty, and Paola Battilani. 2018. "Biological Control Products for Aflatoxin Prevention in Italy: Commercial Field Evaluation of Atoxigenic Aspergillus flavus Active Ingredients" Toxins 10, no. 1: 30. https://doi.org/10.3390/toxins10010030
APA StyleMauro, A., Garcia-Cela, E., Pietri, A., Cotty, P. J., & Battilani, P. (2018). Biological Control Products for Aflatoxin Prevention in Italy: Commercial Field Evaluation of Atoxigenic Aspergillus flavus Active Ingredients. Toxins, 10(1), 30. https://doi.org/10.3390/toxins10010030







