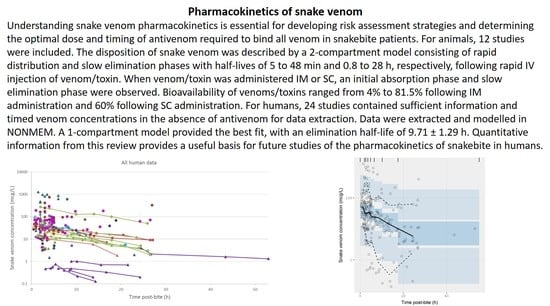
Pharmacokinetics of Snake Venom
Abstract
:1. Introduction
2. Results
2.1. Pharmacokinetic Studies in Animals
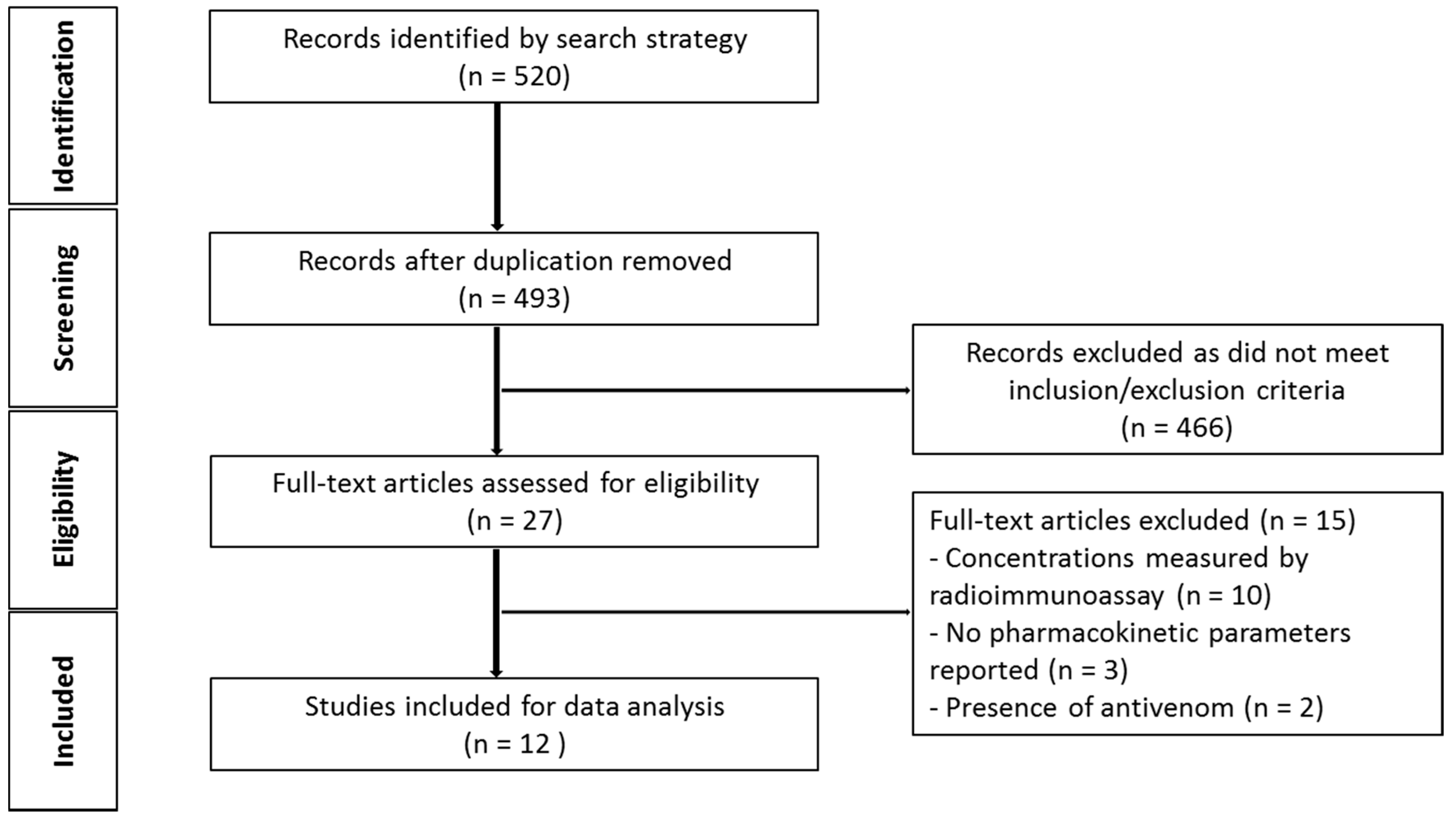
2.1.1. Literature Search
2.1.2. Demographics of Snake Venom Pharmacokinetic Studies in Animals
2.1.3. Pharmacokinetic Parameters of Snake Venoms and Toxins Following Intravenous Administration
2.1.4. Pharmacokinetic Parameters of Snake Venoms and Toxins Following Intramuscular or Subcutaneous Administration
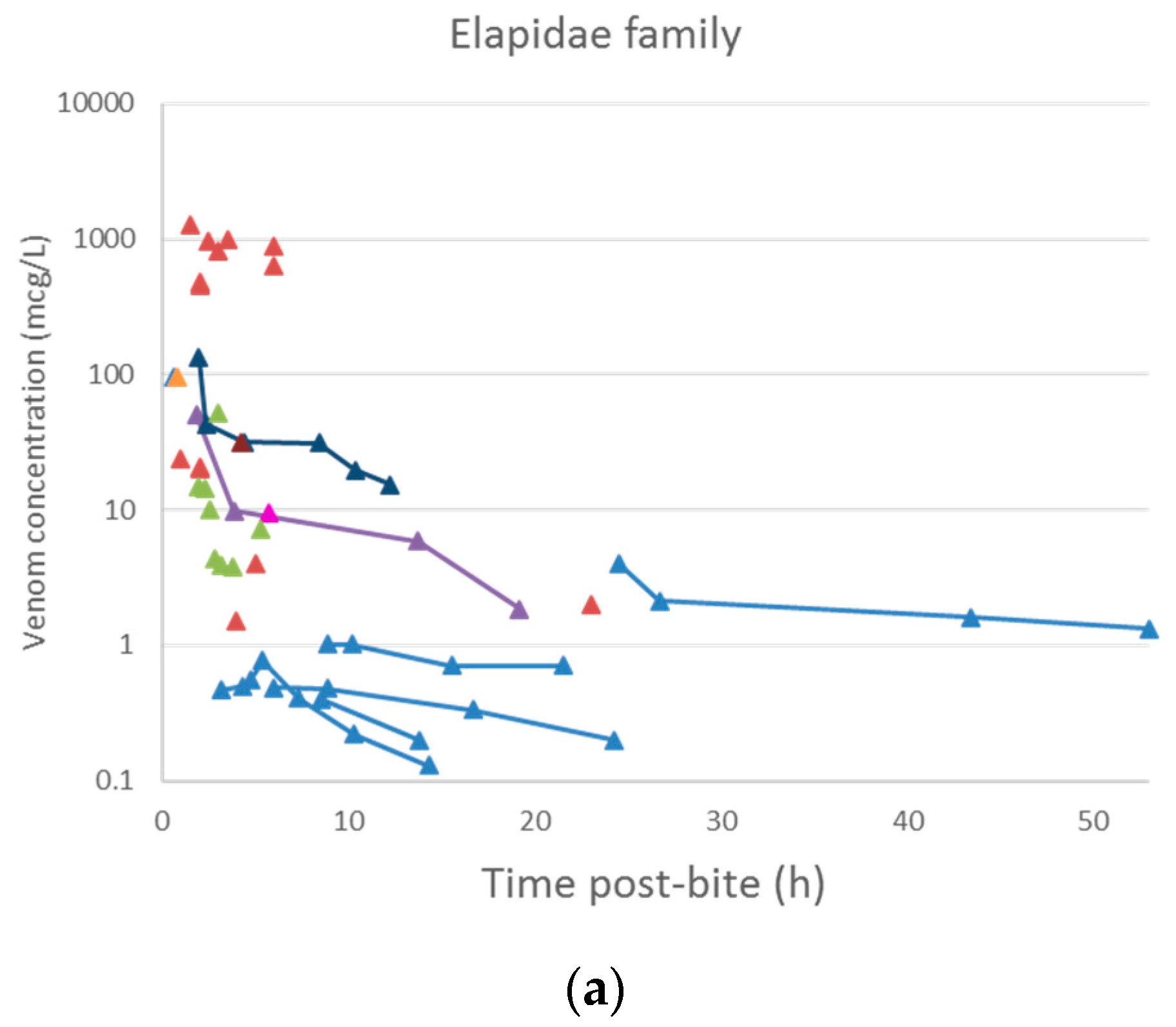
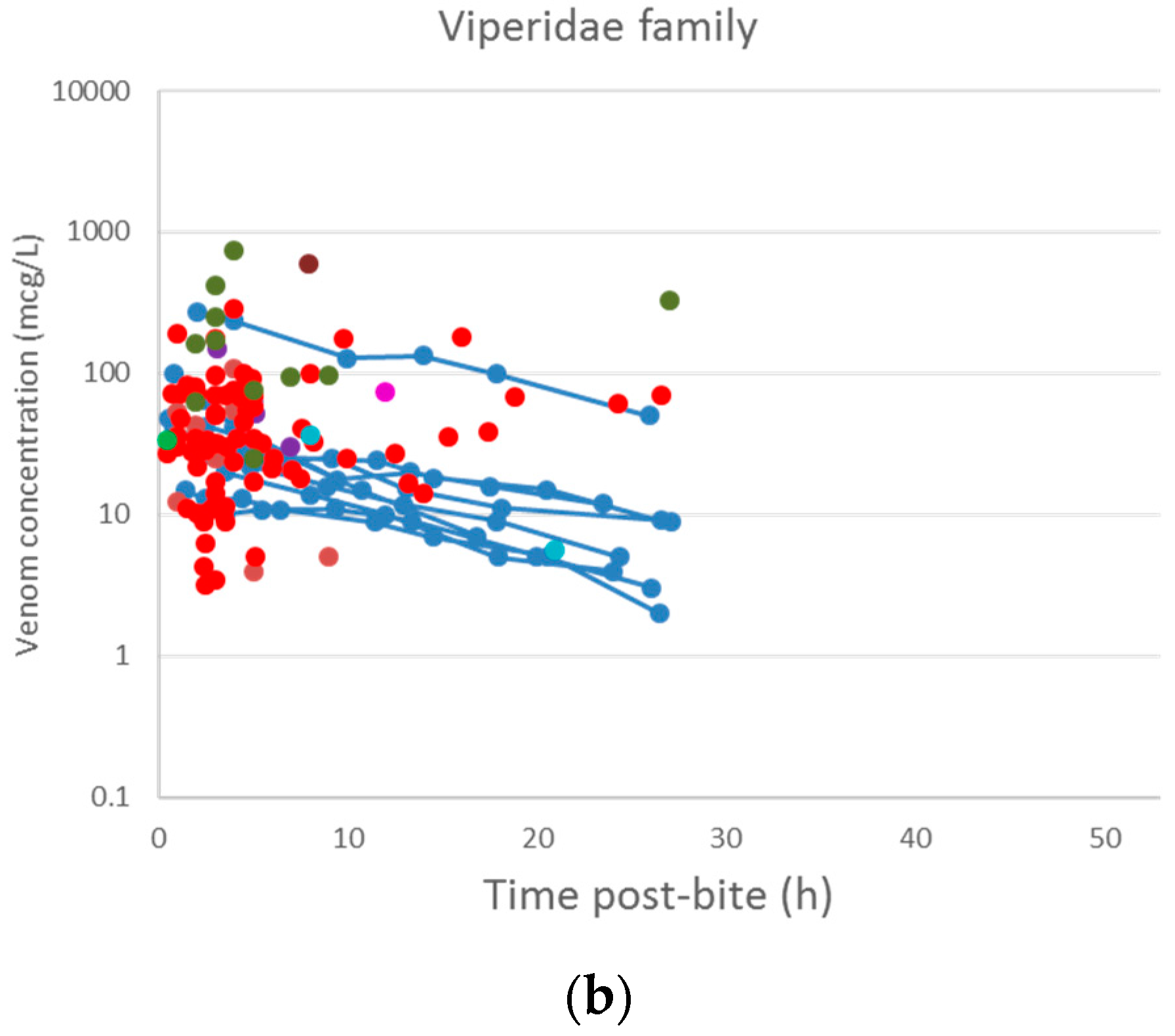
2.2. Pharmacokinetic Studies in Humans
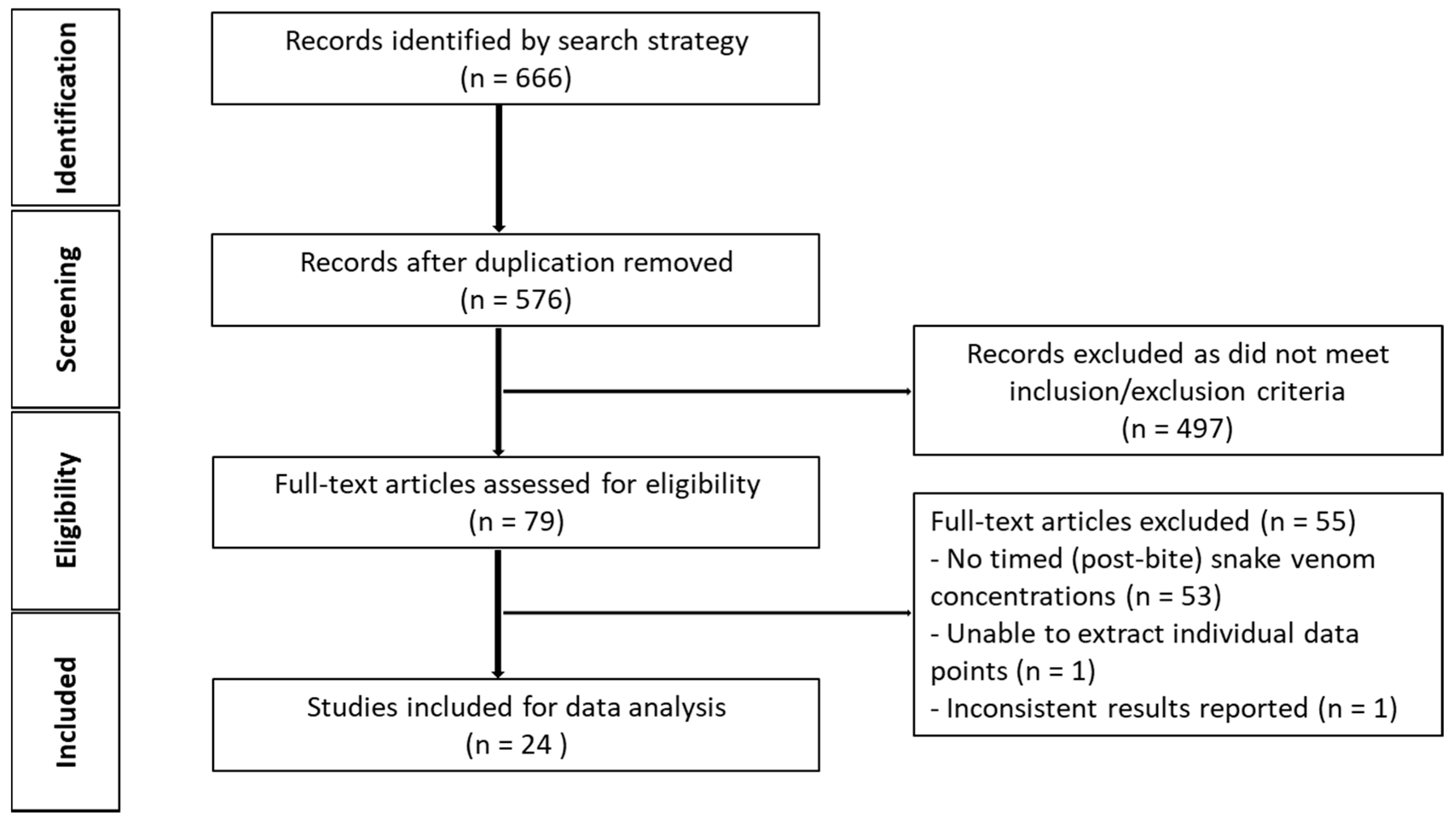
2.2.1. Literature Search
2.2.2. Data Extraction
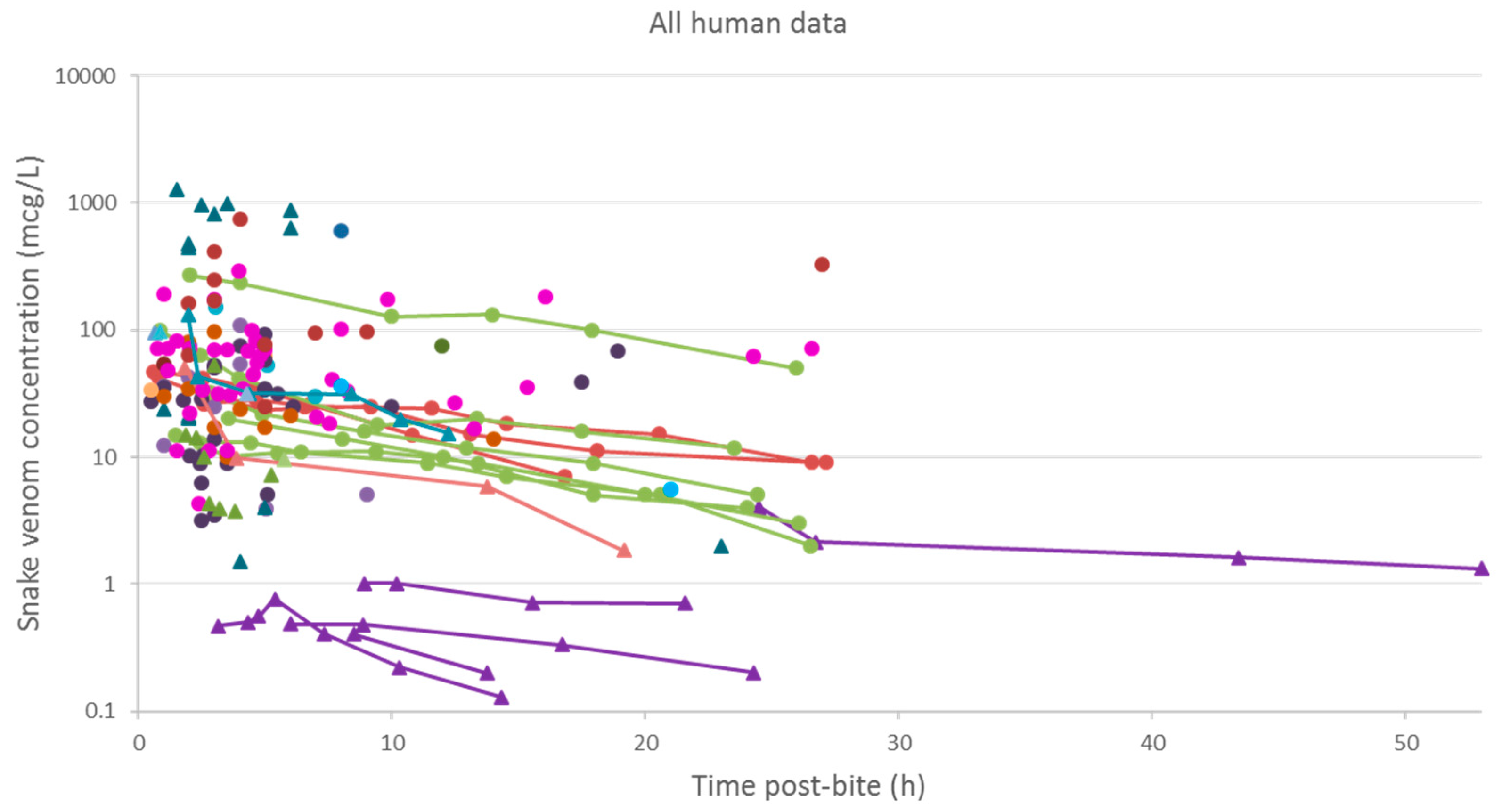
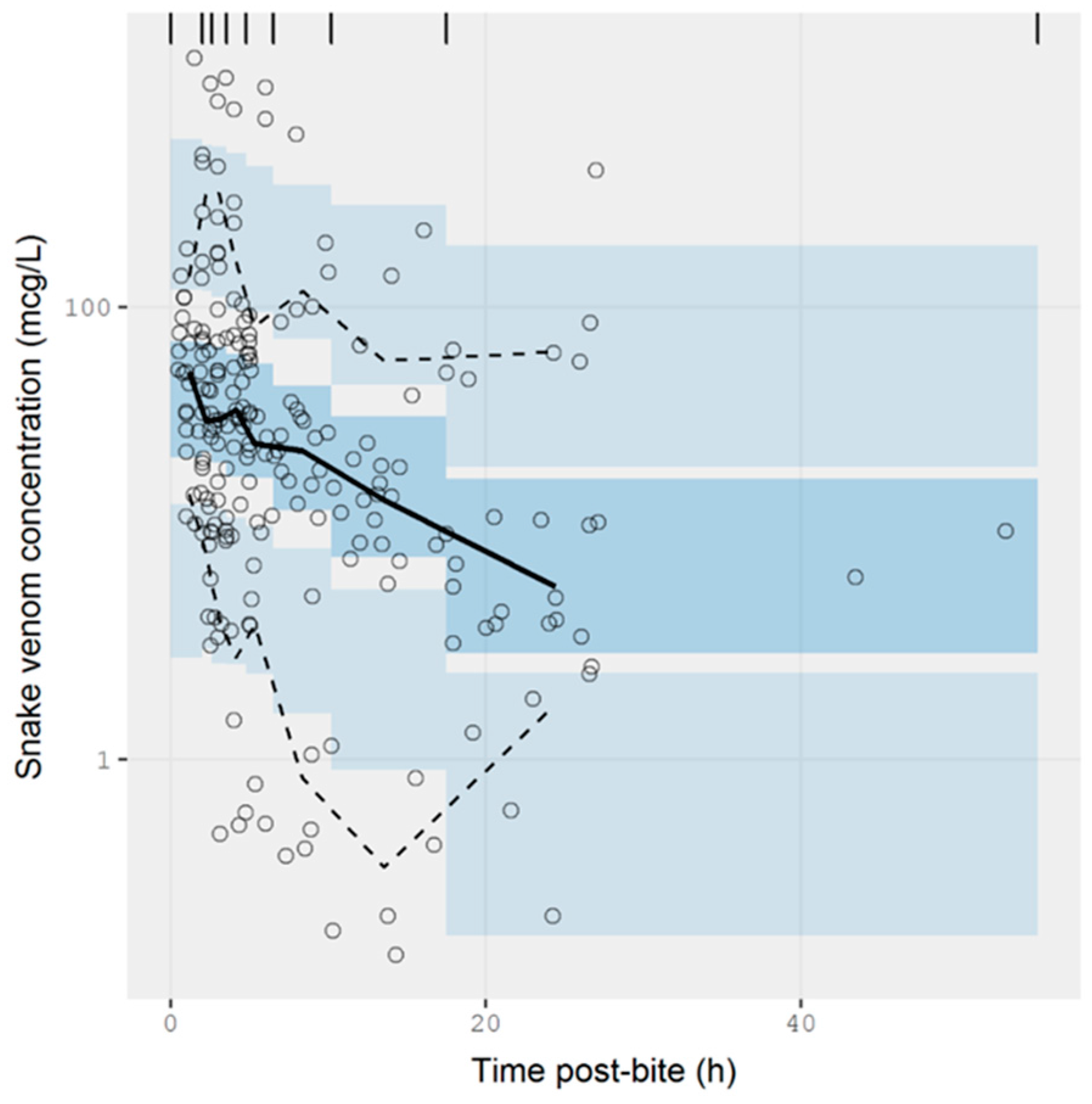
2.2.3. Data Analysis
3. Discussion
3.1. Pharmacokinetic Studies in Animals
3.2. Pharmacokinetic Studies in Humans
4. Conclusions
5. Methods
5.1. Pharmacokinetic Studies in Animals
5.1.1. Literature Search Strategy
5.1.2. Inclusion and Exclusion Criteria
5.1.3. Data Extraction and Creation of a Summary Table
5.2. Pharmacokinetic Studies in Humans
5.2.1. Literature Search Strategy
5.2.2. Data Extraction and Synthesis
5.2.3. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Parameters | Parameter Estimates (RSE%) | |
|---|---|---|
| Base Model | Covariate Model | |
| CL (L/h) | 15.2 (11%) | 13.3 (14%) |
| V (L) | 215 (9%) | 184 (10%) |
| D1 (h) | 1 FIX | 1 FIX |
| F1 (Viperidae) | 1 FIX | 1 FIX |
| F1 (Elapidae) | 1 FIX | 0.569 (43%) |
| Between subject variability | ||
| CL (CV %) | 52.6% (30%) | 43.7% (52%) |
| V (CV %) | 15.5% (237%) | 29.8% (101%) |
| D1 (CV %) | 44.1% (21%) | 44.1% (17%) |
| F1 (CV %) | 286.5% (7%) | 275.4% (7%) |
| Residual error | ||
| Proportional error | 0.047 (25%) | 0.047 (25%) |
References
- Warrell, D.A. Guidelines for the Management of Snake-Bites; World Health Organization: Geneva, Switzerland, 2010; p. 162. [Google Scholar]
- Gutiérrez, J.M.; Theakston, R.D.G.; Warrell, D.A. Confronting the neglected problem of snake bite envenoming: The need for a global partnership. PLoS Med. 2006, 3, e150. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Warrell, D.A.; Gutiérrez, J.M.; Organization, W.H. Rabies and Envenomings: A Neglected Public Health Issue: Report of a Consultative Meeting; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Tasoulis, T.; Isbister, K.G. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; van der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the toxicofera reptile venom system. J. Proteom. 2009, 72, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N. Colubroid systematics: Evidence for an early appearance of the venom apparatus followed by extensive evolutionary tinkering. J. Toxicol. Toxin Rev. 2002, 21, 21–41. [Google Scholar] [CrossRef]
- Bieber, A.L. Metal and nonprotein constituents in snake venoms. In Snake Venoms; Lee, C.-Y., Ed.; Springer: Berlin, Heidelberg, 1979; pp. 295–306. [Google Scholar]
- Bon, C. Pharmacokinetics of venom toxins and their modification by antivenom therapy. J. Toxicol. Toxin Rev. 2003, 22, 129–138. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Isbister, G.K.; Brown, S.G.A.; Page, C.B.; McCoubrie, D.L.; Greene, S.L.; Buckley, N.A. Snakebite in australia: A practical approach to diagnosis and treatment. Med. J. Aust. 2013, 199, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Sashindran, V.K. Clinical features and management of snake bite. Med. J. Armed Forces India 2002, 58, 247–249. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: Mechanisms of action and pharmacological relevance. J. Clin. Toxicol. 2014, 4, 1000181. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases:Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Isbister, G.K. Snakebite doesn’t cause disseminated intravascular coagulation: Coagulopathy and thrombotic microangiopathy in snake envenoming. Semin. Thromb. Hemost. 2010, 36, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, U.K.; Lalloo, D.G.; de Silva, H.J. Neurotoxicity in snakebite—The limits of our knowledge. PLoS Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.; Laing, G.D. Diagnosis of snakebite and the importance of immunological tests in venom research. Toxins 2014, 6, 1667–1695. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.; Lloyd-Jones, M.J.; Reid, H.A. Micro-elisa for detecting and assaying snake venom and venom-antibody. Lancet 1977, 2, 639–641. [Google Scholar] [CrossRef]
- Theakston, R.D.G. The application of immunoassay techniques, including enzyme-linked immunosorbent assay (elisa), to snake venom research. Toxicon 1983, 21, 341–352. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; Menon, J.C.; Joseph, J.K.; Raveendran, D.K.; Oommen, O.V. Snake venom detection kit (svdk): Update on current aspects and challenges. In Clinical Toxinology in Asia Pacific and Africa; Gopalakrishnakone, P., Faiz, A., Fernando, R., Gnanathasan, C.A., Habib, A.G., Yang, C.-C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 379–400. [Google Scholar]
- Selvanayagam, Z.E.; Gopalakrishnakone, P. Tests for detection of snake venoms, toxins and venom antibodies: Review on recent trends (1987–1997). Toxicon 1999, 37, 565–586. [Google Scholar] [CrossRef]
- Allen, G.E.; Brown, S.G.A.; Buckley, N.A.; O’Leary, M.A.; Page, C.B.; Currie, B.J.; White, J.; Isbister, G.K. Clinical effects and antivenom dosing in brown snake (pseudonaja spp.) envenoming—Australian snakebite project (asp-14). PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Audebert, F.; Grosselet, O.; Sabouraud, A.; Bon, C. Quantitation of venom antigens from european vipers in human serum or urine by elisa. J. Anal. Toxicol. 1993, 17, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.P.; Wang, Q.C.; Liu, G.F. Pharmacokinetics of cytotoxin from chinese cobra (naja naja atra) venom. Toxicon 1993, 31, 339–343. [Google Scholar] [CrossRef]
- Hanvivatvong, O.; Mahasandana, S.; Karnchanachetanee, C. Kinetic study of russell’s viper venom in envenomed patients. Am. J. Trop. Med. Hyg. 1997, 57, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Khin Ohn, L.; Aye Aye, M.; Tun, P.; Theingie, N.; Min, N. Russell’s viper venom levels in serum of snake bite victims in burma. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 165–168. [Google Scholar]
- Sano-Martins, I.S.; Tomy, S.C.; Campolina, D.; Dias, M.B.; de Castro, S.C.; de Sousa-e-Silva, M.C.; Amaral, C.F.; Rezende, N.A.; Kamiguti, A.S.; Warrell, D.A.; et al. Coagulopathy following lethal and non-lethal envenoming of humans by the south american rattlesnake (crotalus durissus) in brazil. QJM 2001, 94, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Barral-Netto, M.; Schriefer, A.; Barral, A.; Almeida, A.R.P.; Mangabeira, A. Serum levels of bothropic venom in patients without antivenom intervention. Am. J. Trop. Med. Hyg. 1991, 45, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Kulawickrama, S.; O’Leary, M.A.; Hodgson, W.C.; Brown, S.G.; Jacoby, T.; Davern, K.; Isbister, G.K. Development of a sensitive enzyme immunoassay for measuring taipan venom in serum. Toxicon 2010, 55, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Isbister, G.K.; Schneider, J.J.; Brown, S.G.A.; Currie, B.J. Enzyme immunoassays in brown snake (pseudonaja spp.) envenoming: Detecting venom, antivenom and venom-antivenom complexes. Toxicon 2006, 48, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Karlson-Stiber, C.; Persson, H.; Al-Abdulla, I.H.; Smith, D.C. Development and clinical application of immunoassays for european adder (vipera berus berus) venom and antivenom. Toxicon 1996, 34, 91–98. [Google Scholar] [CrossRef]
- Audebert, F.; Urtizberea, M.; Sabouraud, A.; Scherrmann, J.M.; Bon, C. Pharmacokinetics of vipera aspis venom after experimental envenomation in rabbits. J. Pharmacol. Exp. Ther. 1994, 268, 1512–1517. [Google Scholar] [PubMed]
- Minton, S.A. Present tests for detection of snake venom: Clinical applications. Ann. Emerg. Med. 1987, 16, 932–937. [Google Scholar] [CrossRef]
- Toyama, M.H.; Soares, A.M.; Wen-Hwa, L.; Polikarpov, I.; Giglio, J.R.; Marangoni, S. Amino acid sequence of piratoxin-ii, a myotoxic lys49 phospholipase A2 homologue from bothrops pirajai venom. Biochimie 2000, 82, 245–250. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Arni, R.K.; Ward, R.J. Phospholipase A2—A structural review. Toxicon 1996, 34, 827–841. [Google Scholar] [CrossRef]
- Bjarnason, J.B.; Fox, J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994, 62, 325–372. [Google Scholar] [CrossRef]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian adam/adamts family proteins. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. Substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Du, X.-Y.; Clemetson, K.J. Snake venom l-amino acid oxidases. Toxicon 2002, 40, 659–665. [Google Scholar] [CrossRef]
- Fox, J.W. A brief review of the scientific history of several lesser-known snake venom proteins: L-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon 2013, 62, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, S.; Yao, Y.; Zhang, Q.; Sun, M.-Z. Past decade study of snake venom l-amino acid oxidase. Toxicon 2012, 60, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. The field of reptile toxinology. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2009; pp. 3–23. [Google Scholar]
- Iwanaga, S.; Suzuki, T. Enzymes in snake venom. In Snake Venoms; Lee, C.-Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 61–158. [Google Scholar]
- Ouyang, C.; Huang, T.-F. Inhibition of platelet aggregation by 5′-nucleotidase purified from trimeresurus gramineus snake venom. Toxicon 1983, 21, 491–501. [Google Scholar] [CrossRef]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Frobert, Y.; Créminon, C.; Cousin, X.; Rémy, M.-H.; Chatel, J.-M.; Bon, S.; Bon, C.; Grassi, J. Acetylcholinesterases from elapidae snake venoms: Biochemical, immunological and enzymatic characterization. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1997, 1339, 253–267. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Gwee, M.C.E. Three-finger α-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Willmott, N.; Gaffney, P.; Masci, P.; Whitaker, A. A novel serine protease inhibitor from the australian brown snake, pseudonaja textilis textilis: Inhibition kinetics. Fibrinolysis 1995, 9, 1–8. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Mackessy, S.P.; Dutta, S. Characterization of a kunitz-type protease inhibitor peptide (rusvikunin) purified from daboia russelii russelii venom. Int. J. Biol. Macromol. 2014, 67, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Structures and functions of snake venom clps (c-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon 2005, 45, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of c-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Zuzel, M.; Theakston, R.D.G. Snake venom metalloproteinases and disintegrins: Interactions with cells. Braz. J. Med. Biol. Res. 1998, 31, 853–862. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, L.; Flight, S.; Masci, P.P.; Hanchard, K.J.; Lewis, R.J.; Alewood, P.F.; de Jersey, J.; Lavin, M.F. Cloning and characterisation of natriuretic peptides from the venom glands of australian elapids. Biochimie 2006, 88, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [PubMed]
- Gutierrez, J.M.; Leon, G.; Lomonte, B.; Angulo, Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets 2011, 10, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Maduwage, K.; Silva, A.; O’Leary, M.A.; Hodgson, W.C.; Isbister, G.K. Efficacy of indian polyvalent snake antivenoms against sri lankan snake venoms: Lethality studies or clinically focussed in vitro studies. Sci. Rep. 2016, 6, 26778. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Hodgson, W.C.; O’Leary, M.; Isbister, G.K. Pharmacokinetics and pharmacodynamics of the myotoxic venom of pseudechis australis (mulga snake) in the anesthetised rat. Clin. Toxicol. 2014, 52, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; Jimenez, L.; Romero, C.; Vergara, I.; Calderon, A.; Benard, M.; Bernas, M.J.; Rilo, H.; De Roodt, A.; D’Suze, G.; et al. Lymphatic route of transport and pharmacokinetics of micrurus fulvius (coral snake) venom in sheep. Lymphology 2012, 45, 144–153. [Google Scholar] [PubMed]
- Tan, C.H.; Sim, S.M.; Gnanathasan, C.A.; Fung, S.Y.; Tan, N.H. Pharmacokinetics of the sri lankan hump-nosed pit viper (hypnale hypnale) venom following intravenous and intramuscular injections of the venom into rabbits. Toxicon 2014, 79, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zheng, J.; Jiang, Z. Pharmacokinetics of thrombin-like enzyme from venom of agkistrodon halys ussuriensis emelianov determined by elisa in the rat. Toxicon 2001, 39, 1821–1826. [Google Scholar] [CrossRef]
- Nakamura, M.; Kinjoh, K.; Miyagi, C.; Oka, U.; Sunagawa, M.; Yamashita, S.; Kosugi, T. Pharmacokinetics of habutobin in rabbits. Toxicon 1995, 33, 1201–1206. [Google Scholar] [CrossRef]
- Yap, M.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Tan, C.H. Pharmacokinetics of naja sumatrana (equatorial spitting cobra) venom and its major toxins in experimentally envenomed rabbits. PLoS Negl. Trop. Dis. 2014, 8, e2890. [Google Scholar] [CrossRef] [PubMed]
- Mello, S.M.; Linardi, A.; Renno, A.L.; Tarsitano, C.A.B.; Pereira, E.M.; Hyslop, S. Renal kinetics of bothrops alternatus (urutu) snake venom in rats. Toxicon 2010, 55, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Riviere, G.; Choumet, V.; Saliou, B.; Debray, M.; Bon, C. Absorption and elimination of viper venom after antivenom administration. J. Pharmacol. Exp. Ther. 1998, 285, 490–495. [Google Scholar] [PubMed]
- Yap, M.K.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y. Toxicokinetics of naja sputatrix (javan spitting cobra) venom following intramuscular and intravenous administrations of the venom into rabbits. Toxicon 2013, 68, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.M.; Saremi, K.; Tan, N.H.; Fung, S.Y. Pharmacokinetics of cryptelytrops purpureomaculatus (mangrove pit viper) venom following intravenous and intramuscular injections in rabbits. Int. Immunopharmacol. 2013, 17, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Audebert, F.; Sorkine, M.; Robbe-Vincent, A.; Bon, C. Viper bites in france: Clinical and biological evaluation; kinetics of envenomations. Hum. Exp. Toxicol. 1994, 13, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Barraviera, B.; Sartori, A.; Pereira Da Silva, M.F.; Kaneno, R.; Peracoli, M.T.S. Use of an elisa assay to evaluate venom, antivenom, igg and igm human antibody levels in serum and cerebrospinal fluid from patients bitten by crotalus durissus terrificus in brazil. J. Venom. Anim. Toxins 1996, 2, 14–27. [Google Scholar] [CrossRef]
- Brvar, M.; Kurtovic, T.; Grenc, D.; Lang Balija, M.; Krizaj, I.; Halassy, B. Vipera ammodytes bites treated with antivenom viperatab: A case series with pharmacokinetic evaluation. Clin. Toxicol. 2017, 55, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; Hyslop, S.; Mello, S.M.; Vieira, R.J. Bothrops snakebite on the head: Case report and review of the literature. Ann. Trop. Med. Parasitol. 2007, 101, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; Capitani, E.M.D.; Hyslop, S.; Mello, S.M.; Madureira, P.R.; Zanardi, V.; Ferreira, D.M.; Meirelles, G.V.; Fernandes, L.C.R. Compartment syndrome after bothrops jararaca snakebite: Monitoring, treatment, and outcome. Clin. Toxicol. 2010, 48, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; De Capitani, E.M.; Hyslop, S.; Mello, S.M.; Fernandes, C.B.; Bergo, F.; Nascimento, F.B.P. Compartment syndrome after south american rattlesnake (crotalus durissus terrificus) envenomation. Clin. Toxicol. 2014, 52, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Bucher, B.; Canonge, D.; Robbe-Vincent, A.; Choumet, V.; Bon, C.; Lang, J.; Biao, T.; Brunod, M.; Garnier, D.; Guerin, J.F.; et al. Clinical indicators of envenoming and serum levels of venom antigens in patients bitten by bothrops lanceolatus in martinique. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 186–190. [Google Scholar] [CrossRef]
- Hung, D.Z.; Liau, M.Y.; Lin-Shiau, S.Y. The clinical significance of venom detection in patients of cobra snakebite. Toxicon 2003, 41, 409–415. [Google Scholar] [CrossRef]
- Hung, D.Z.; Yu, Y.J.; Hsu, C.L.; Lin, T.J. Antivenom treatment and renal dysfunction in russell’s viper snakebite in taiwan: A case series. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; O’Leary, M.A.; Schneider, J.J.; Brown, S.G.A.; Currie, B.J. Efficacy of antivenom against the procoagulant effect of australian brown snake (pseudonaja sp.) venom: In vivo and in vitro studies. Toxicon 2007, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Halkidis, L.; O’Leary, M.A.; Whitaker, R.; Cullen, P.; Mulcahy, R.; Bonnin, R.; Brown, S.G.A. Human anti-snake venom igg antibodies in a previously bitten snake-handler, but no protection against local envenoming. Toxicon 2010, 55, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Gault, A.; Tasoulis, T.; O’Leary, M.A. A definite bite by the ornamental snake (denisonia maculata) causing mild envenoming. Clin. Toxicol. 2016, 54, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.Y.L.; Singh, P.N.; Isbister, G.K. Severe rhabdomyolysis from red-bellied black snake (pseudechis porphyriacus) envenoming despite antivenom. Toxicon 2016, 117, 46–48. [Google Scholar] [CrossRef] [PubMed]
- McNally, T.; Conway, G.S.; Jackson, L.; Theakston, R.D.G.; Marsh, N.A.; Warrell, D.A.; Young, L.; Mackie, I.J.; Machin, S.J. Accidental envenoming by a gaboon viper (bitis gabonica): The haemostatic disturbances observed and investigation of in vitro haemostatic properties of whole venom. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 66–70. [Google Scholar] [CrossRef]
- Phillips, R.E.; Theakston, R.D.G.; Warrell, D.A.; Galigedara, Y.; Abeysekera, D.T.D.J.; Dissanayaka, P.; Hutton, R.A.; Aloysius, D.J. Paralysis, rhabdomyolysis and haemolysis caused by bites of russell’s viper (vipera russelli pulchella) in sri lanka: Failure of indian (haffkine) antivenom. Q. J. Med. 1988, 68, 691–716. [Google Scholar] [PubMed]
- Schneemann, M.; Cathomas, R.; Laidlaw, S.T.; El Nahas, A.M.; Theakston, R.D.G.; Warrell, D.A. Life-threatening envenoming by the saharan horned viper (cerastes cerastes) causing micro-angiopathic haemolysis, coagulopathy and acute renal failure: Clinical cases and review. QJM Mon. J. Assoc. Phys. 2004, 97, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.A.; Johnston, C.; Siribaddana, S.; Isbister, G.K. Neuromuscular effects of common krait (bungarus caeruleus) envenoming in sri lanka. PLoS Negl. Trop. Dis. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Tun, P.; Aye Aye, M.; Warrell, D.A.; Tin, M. King cobra (ophiophagus hannah) bites in myanmar: Venom antigen levels and development of venom antibodies. Toxicon 1995, 33, 379–382. [Google Scholar] [CrossRef]
- Warrell, D.A.; Hudson, B.J.; Lalloo, D.G.; Trevett, A.J.; Whitehead, P.; Bamler, P.R.; Ranaivoson, M.; Wiyono, A.; Richie, T.L.; Fryauff, D.J.; et al. The emerging syndrome of envenoming by the new guinea small-eyed snake micropechis ikaheka. QJM Mon. J. Assoc. Phys. 1996, 89, 523–530. [Google Scholar] [CrossRef]
- Ismail, M.; Abd-Elsalam, M.A.; Al-Ahaidib, M.S. Pharmacokinetics of 125i-labelled walterinnesia aegyptia venom and its distribution of the venom and its toxin versus slow absorption and distribution of igg, f(ab’)2 and f(ab) of the antivenin. Toxicon 1998, 36, 93–114. [Google Scholar] [CrossRef]
- Ismail, M.; Aly, M.H.M.; Abd-Elsalam, M.A.; Morad, A.M. A three-compartment open pharmacokinetic model can explain variable toxicities of cobra venoms and their alpha toxins. Toxicon 1996, 34, 1011–1026. [Google Scholar] [CrossRef]
- Maung Maung, T.; Khin Mee, M.; Mi Mi, K.; Thein, T. Kinetics of envenomation with russell’s viper (vipera russelli) venom and of antivenom use in mice. Toxicon 1988, 26, 373–378. [Google Scholar] [CrossRef]
- Al-Sallami, H.S.; Cheah, S.L.; Han, S.Y.; Liew, J.; Lim, J.; Ng, M.A.; Solanki, H.; Soo, R.J.; Tan, V.; Duffull, S.B. Between-subject variability: Should high be the new normal? Eur. J. Clin. Pharmacol. 2014, 70, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Krifi, M.N.; Savin, S.; Debray, M.; Bon, C.; El Ayeb, M.; Choumet, V. Pharmacokinetic studies of scorpion venom before and after antivenom immunotherapy. Toxicon 2005, 45, 187–198. [Google Scholar] [CrossRef] [PubMed]
| Enzymatic Components | Approximate Molecular Mass (kDa) | Mechanism of Action | Examples of Biological Effects | Snake Families |
|---|---|---|---|---|
| Phospholipase A2 (PLA2) | 12–14 [37] | Hydrolyses the ester bond at sn-2 position of phospholipids producing free fatty acids and lysophospholipid. Toxic effects can result from this enzymatic action or may be the results of non-enzymatic activity [38]. | Myotoxicity, oedema formation, anticoagulant effects, hypotension, presynaptic neurotoxicity [16,39] | Elapidae (type I PLA2); Viperidae (type II PLA2) [7] |
| Snake-venom metalloproteases (SVMP) | Classified into 3 groups based on domain organisation [40]; P-I: 20–30; P-II: 30–60; P-III: 60–100 | Proteolytic activities leading to degradation of protein structures e.g., basement membranes of blood vessel and extracellular matrix components [17,41]. Disintegrin-like domain of SVMP may also contribute to the haemorrhagic effects [17]. | Induce local and systemic bleeding and disrupt haemostasis through its pro-/anticoagulation properties. Extravasation of blood, inflammation and tissue necrosis [17,41] | Major protein family in viper venoms, but less abundant in elapid venom [7] |
| Serine proteases (SVSP) e.g., thrombin-like enzymes | 26–67 [42] | Hydrolyse peptide bonds mainly in pro-enzymes in the coagulation cascade, causing procoagulant, fibrinolytic and/or fibrinogenolytic activities. Some SVSPs have kallikrein-like activity leading to release of bradykinin [42,43]. | Disruption of haemostasis and hypotension [42] | Almost all Viperidae, uncommon in Elapidae except Australian snakes [7] |
| l-amino acid oxidases (LAAO) | 110–150 when measured by gel-filtration method under non-denaturing conditions; 50–70 when measured by SDS/PAGE method under reducing and non-reducing conditions [44] | Catalyse stereospecific oxidative deamination of l-amino acid, resulting in production of α-keto acid, ammonia and hydrogen peroxide [45]. | Effects on platelet aggregation, inducing cell apoptosis, and antimicrobial activities [46] | Both Elapidae and Viperidae. Most common in Crotalinae [7]. |
| 5′-Nucleotidases | 53–82 [47] | Hydrolyse phosphate monoester linked to 5′-position of DNA and RNA [48]. | Platelet aggregation inhibition [49,50] | Both Elapidae and Viperidae [48] |
| Acetylcholinesterases | 55–60 [47] | Hydrolyse acetylcholine to choline and acetate group [51]. | Termination of neurotransmission by acetylcholine [51,52] | Elapidae except Dendroaspis genus [52] |
| Hyaluronidases | 33–110 [45] | Hydrolyse hyaluronan into oligosaccharides and N-acetylglucosamine [45]. | “Spreading factor” alters the structural, rheological, and chemical properties of the extracellular matrix [45] | Both Elapidae and Viperidae [48] |
| Non-Enzymatic Components | Approximate Molecular Mass (kDa) | Mechanism of Action | Examples of Biological Effects | Snake Families |
|---|---|---|---|---|
| Three-finger toxins (3FTx) e.g., α-neurotoxins | 6–9 [47] | Inhibit postsynaptic nicotinic acetylcholine receptors in neuromuscular junction and interfere with neuromuscular transmission [53,54]. Other activities include cardiotoxins, L-type calcium channel blockage, inhibition of platelet aggregation [53]. | Postsynaptic neurotoxicity | Elapidae and very rare in Viperidae [7] |
| Kunitz peptides (KUN) | 7 [55,56] | Inhibit serine protease (e.g., trypsin and plasmin) activities, interfering with blood coagulation and fibrinolysis [55,56]. Other activities include ion channel blockade and inflammation [56]. | Disruption of haemostasis | Elapidae and Viperinae (absent in Crotalinae) [7] |
| Cysteine-rich secretory proteins (CRiSP) | 20–30 [57] | L-type calcium and cyclic nucleotide-gated (CNG) channel blockade [57]. | Inhibit smooth muscle contraction [57] | More common and abundant in Viperidae [7] |
| C-type lectins (CTL) | Composed of two subunits [58]; α (A chain): 14–15; β (B chain): 13–14 | Bind to, inhibit, or activate specific platelet membrane receptors or blood coagulation factors [59]. | Anticoagulation, promote or inhibit platelet aggregation [59] | More abundant in Viperidae [7] |
| Disintegrins (DIS) | 5–10 [60] | Bind to glycoprotein IIb/IIIa (αIIbβ3 integrin) expressed on activated platelet to prevent interaction with fibrinogen [60]. | Inhibit platelet aggregation [60] | Viperidae, absent in Elapidae [7] |
| Natriuretic peptides (NP) | 3.5–4 [61] | Interaction between NPs and guanylyl cyclase receptors leads to an increase in cGMP levels and subsequent signalling cascade [62]. NPs can also affect renin-angiotensin system by inhibiting angiotensin-converting enzyme [61]. | Vasodilation, diuresis, and natriuresis leading to hypotension, and promote sodium and water excretion [62] | Both Elapidae (atrial-type and brain-type) and Viperidae (C-type) [5]. More common and abundant in Viperidae than Elapidae [7] |
| Snake Species | Animal Model | No. | Dose (mcg·kg−1) | t1/2α (h) | t1/2β (h) | Vss (L·kg−1) | CL (L·h−1·kg−1) | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Isolated Toxin | |||||||||
| Rats | |||||||||
| Agkistrodon halys ussuriensis Emelianov | Sprague-Dawley rats of either sex (180–200 g) | 5 | 50 (thrombin-like enzyme) | 0.3 (±0.12 *) | 3.9 (±1.63 *) | 1.8 (±1.03 *) | 0.324 (±0.067 *) | [68] | |
| Rabbits | |||||||||
| Naja naja atra | New Zealand rabbits of either sex (1.82 ± 0.09 kg) | 6 | 200 (cytotoxin) | 0.097 (±0.01) | 3.5 (±0.2) | 1.7 a (±0.3) | 0.185 b | [27] | |
| Trimeresurus flavoviridis | Japanese white rabbits (3.2–4.4 kg) | 5 | 50 (habutobin) | 0.074 (±0.021 **) | 0.84 (±0.13 **) | 0.031 b,c, 0.021 b,d | 0.061 b | [69] | |
| Naja sumatrana | New Zealand white rabbits (approx. 2 kg) | 3 | 50 (PLA2) | 0.7 (±0.03) | 11.7 (±0.8) | 0.25 b,c, 0.45 b,d | 0.048 b | [70] | |
| 50 (neurotoxin) | 0.5 (±0.1) | 8.8 (±0.9) | 0.45 b,c, 0.5 b,d | 0.082 b | |||||
| 50 (cardiotoxin) | 0.6 (±0.1) | 8.6 (±0.1) | 0.5 b,c, 0.55 b,d | 0.087 b | |||||
| 100 (cardiotoxin in whole venom) | 0.5 (±0.01) | 11.0 (±0.2) | 0.4 b,c, 0.5 b,d | 0.060 b | |||||
| Whole Venom | |||||||||
| Rats | |||||||||
| Bothrops alternatus | Male Wistar rats (200–250 g) | 6 | 800 | 0.38 (±0.03) | 12.1 (±6.4) | 0.50 (±0.12) | 0.033 (±0.011) | [71] | |
| Pseudechis australis | Male Sprague-Dawley rats (320–420 g) | 8 | 100 | - | 0.27 *** | - | - | [65] | |
| Rabbits | |||||||||
| Vipera aspis | Charles de Bouscat HY rabbits (2.5–3 kg) | 5 | 250 | 0.71 (±0.2 *) | 12 (±2.24 *) | 1.2 (±0.089 *) | 0.084 (±0.013 *) | [35] | |
| Vipera aspis | New Zealand rabbits (2.75–3 kg) | 5 | 250 | 0.53 (±0.31 *) | 14.2 (±2.68 *) | 0.7 (±0.11 *) | 0.040 (±0.002 *) | [72] | |
| Naja sputatrix | New Zealand rabbits (2 kg) | 3 | 90 | 0.5 (±0.3) | 15.4 (±2.5) | 0.8 b | 0.034 b | [73] | |
| Cryptelytrops purpureomaculatus | Male New Zealand rabbits (1.7–2.1 kg) | 3 | 200 | 0.25 (±0.01) | 27.7 (±0.0) | 0.39 c (±0.01), 1.80 d (±0.11) | 0.055 (±0.003) | [74] | |
| Naja sumatrana | New Zealand white rabbits (approx. 2 kg) | 3 | 100 | 0.8 (±0.3) | 13.6 (±1.1) | 0.5 b,c, 0.4 b,d | 0.046 b | [70] | |
| Hypnale hypnale | New Zealand white rabbits (1.95 ± 0.05 kg) | 3 | 10 | 0.8 (±0.17 *) | 19.3 (±3.29 *) | 0.13 b | 0.007 (±0.001 *) | [67] | |
| Sheep | |||||||||
| Micrurus fulvius | Sheep (36–60 kg) | 4 | 1000 mcg | - | 0.42 (±0.11 *) | 0.12 b | 0.093 b | [66] | |
| Snake Species | Animal Model | No. | Dose (mcg·kg−1) | t1/2ka (h) | F (%) | t1/2α (h) | t1/2β (h) | Vss (L·kg−1) | CL (L·h−1·kg−1) | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated Toxin | |||||||||||
| Rats | |||||||||||
| Naja naja atra | New Zealand rabbits of either sex (1.82 ± 0.09 kg) | 6 | 500 (cytotoxin) | 0.077 (±0.018) | - | 0.37 (±0.12) | 5.9 (±0.9) | 9 a (±4) | 0.56 b | [27] | |
| Rabbits | |||||||||||
| Naja sumatrana | New Zealand white rabbits (approx. 2 kg) | 3 | 100 (PLA2) | - | 68.6 (±0.8) | - | 10.18 (±1.18) | - | 0.048 b | [70] | |
| 70 (neurotoxin) | - | 81.5 (±0.6) | - | 8.6 (±0.5) | - | 0.082 b | |||||
| 150 (cardiotoxin) | - | 45.6 (±0.1) | - | 8.2 (±0.1) | - | 0.087 b | |||||
| 500 (cardiotoxin in whole venom) | - | 39.5 (±1.1) | - | 11.6 (±0.9) | - | 0.061 b | |||||
| Whole Venom | |||||||||||
| Rabbits | |||||||||||
| Vipera aspis | Charles de Bouscat HY rabbits (2.5–3 kg) | 5 | 300 | - | 63 (±17.89 *) | - | 32 (±8.94 *) | - | - | [35] | |
| 500 | - | 67 (±11.18 *) | - | 36 (±8.94 *) | - | - | |||||
| 700 | - | 63 (±38.01 *) | - | 29 (±4.47 *) | - | - | |||||
| Naja sputatrix | New Zealand rabbits (2 kg) | 3 | 500 | - | 41.7 ** | - | 18.9 (±4.6) | - | 0.034 b | [73] | |
| Cryptelytrops purpureomaculatus | Male New Zealand rabbits (1.7–2.1 kg) | 3 | 500 | - | 41.6 (±3.0) | - | 27 (±0.6) | - | 0.055 (±0.004) | [74] | |
| Naja sumatrana | New Zealand white rabbits (approx. 2 kg) | 3 | 500 | - | 41.9 (±0.2) | - | 12.5 (±0.9) | - | 0.047 b | [70] | |
| Hypnale hypnale | New Zealand white rabbits (2.03 ± 0.06 kg) | 3 | 1000 | - | 4 ** | - | 19.3 (±1.21 *) | - | 0.007 (±0.003 *) | [67] | |
| Snake Species | Animal Model | No. | Dose (mcg·kg−1) | t1/2ka (h) | F (%) | t1/2α (h) | t1/2β (h) | Vss (L·kg−1) | CL (L·h−1·kg−1) | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated Toxin | |||||||||||
| Rats | |||||||||||
| Agkistrodon halys ussuriensis Emelianov | Sprague-Dawley rats of either sex (180–200 g) | 6 | 750 (thrombin-like enzyme) | 2.5 (±0.73 *) | - | 4.8 (±4.16 *) | 125 (±181.26 *) | 19 (±49 *) | 0.294 (±0.103 *) | [68] | |
| Whole Venom | |||||||||||
| Rabbits | |||||||||||
| Micrurus fulvius | Sheep (36–60 kg) | 4 | 5000 mcg | - | 60 (±10 *) | - | 4.35 (±1.83 *) | 0.56 b | 0.084 b | [66] | |
| Snake Species | Country | Number of Patients with Timed Concentration Post-Bite for Data Retrieval | Ref |
|---|---|---|---|
| Pseudonaja spp. | Australia | 5 | [25] |
| Vipera aspis, Vipera berus, and Vipera ammodytes | France | 3 | [26] |
| Vipera aspis and Vipera berus | France | 6 | [75] |
| Crotalus durissus terrificus | Brazil | 9 | [76] |
| Vipera ammodytes | Slovenia | 3 | [77] |
| Bothrops jararaca | Brazil | 1 | [78] |
| Bothrops jararaca | Brazil | 1 | [79] |
| Crotalus durissus terrificus | Brazil | 1 | [80] |
| Bothrops lanceolatus | Martinique | 1 | [81] |
| Daboia russelli siamensis | Thailand | 24 | [28] |
| Naja atra | Taiwan | 14 | [82] |
| Daboia russelli siamensis | Taiwan | 10 | [83] |
| Pseudonaja spp. | Australia | 1 | [84] |
| Acanthophis spp. | Australia | 1 | [85] |
| Denisonia maculata | Australia | 1 | [86] |
| Vipera russelli | Myanmar | 38 | [29] |
| Pseudechis porphyriacus | Australia | 1 | [87] |
| Bitis gabonica | UK | 1 | [88] |
| Vipera russelli pulchella | Sri Lanka | 1 | [89] |
| Crotalus durissus | Brazil | 11 | [30] |
| Cerastes cerastes mutila | Switzerland & UK | 2 | [90] |
| Bungarus caeruleus | Sri Lanka | 8 | [91] |
| Ophiophagus Hannah | Myanmar | 1 | [92] |
| Micropechis ikaheka | Papua New Guinea | 1 | [93] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of Snake Venom. Toxins 2018, 10, 73. https://doi.org/10.3390/toxins10020073
Sanhajariya S, Duffull SB, Isbister GK. Pharmacokinetics of Snake Venom. Toxins. 2018; 10(2):73. https://doi.org/10.3390/toxins10020073
Chicago/Turabian StyleSanhajariya, Suchaya, Stephen B. Duffull, and Geoffrey K. Isbister. 2018. "Pharmacokinetics of Snake Venom" Toxins 10, no. 2: 73. https://doi.org/10.3390/toxins10020073
APA StyleSanhajariya, S., Duffull, S. B., & Isbister, G. K. (2018). Pharmacokinetics of Snake Venom. Toxins, 10(2), 73. https://doi.org/10.3390/toxins10020073