Algal Blooms and Cyanotoxins in Jordan Lake, North Carolina
Abstract
:1. Introduction
2. Results
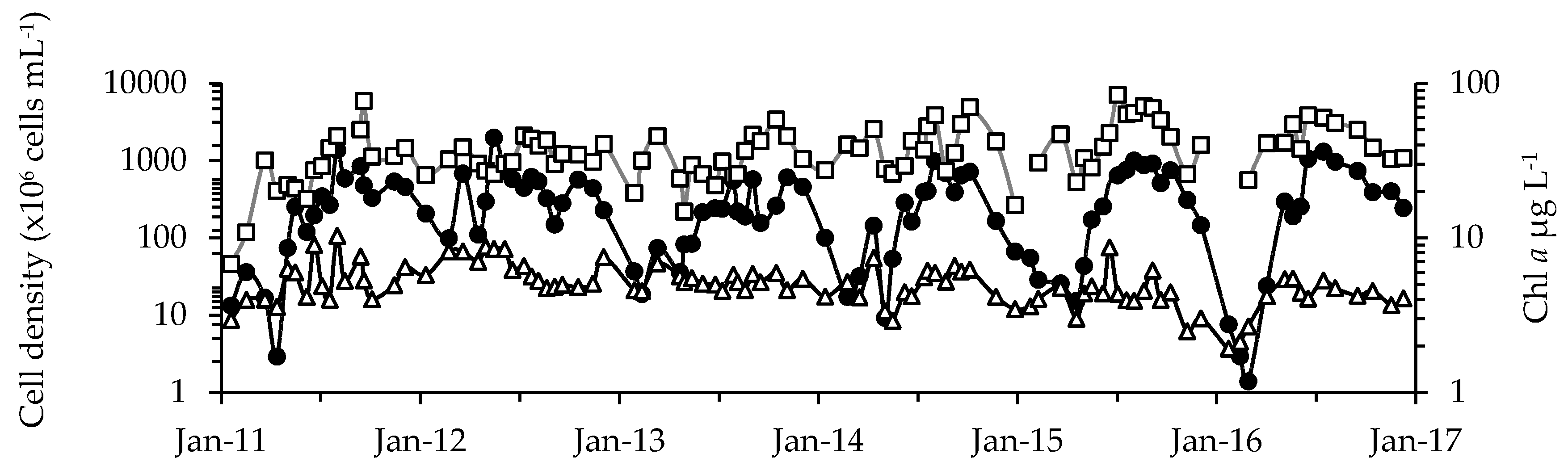
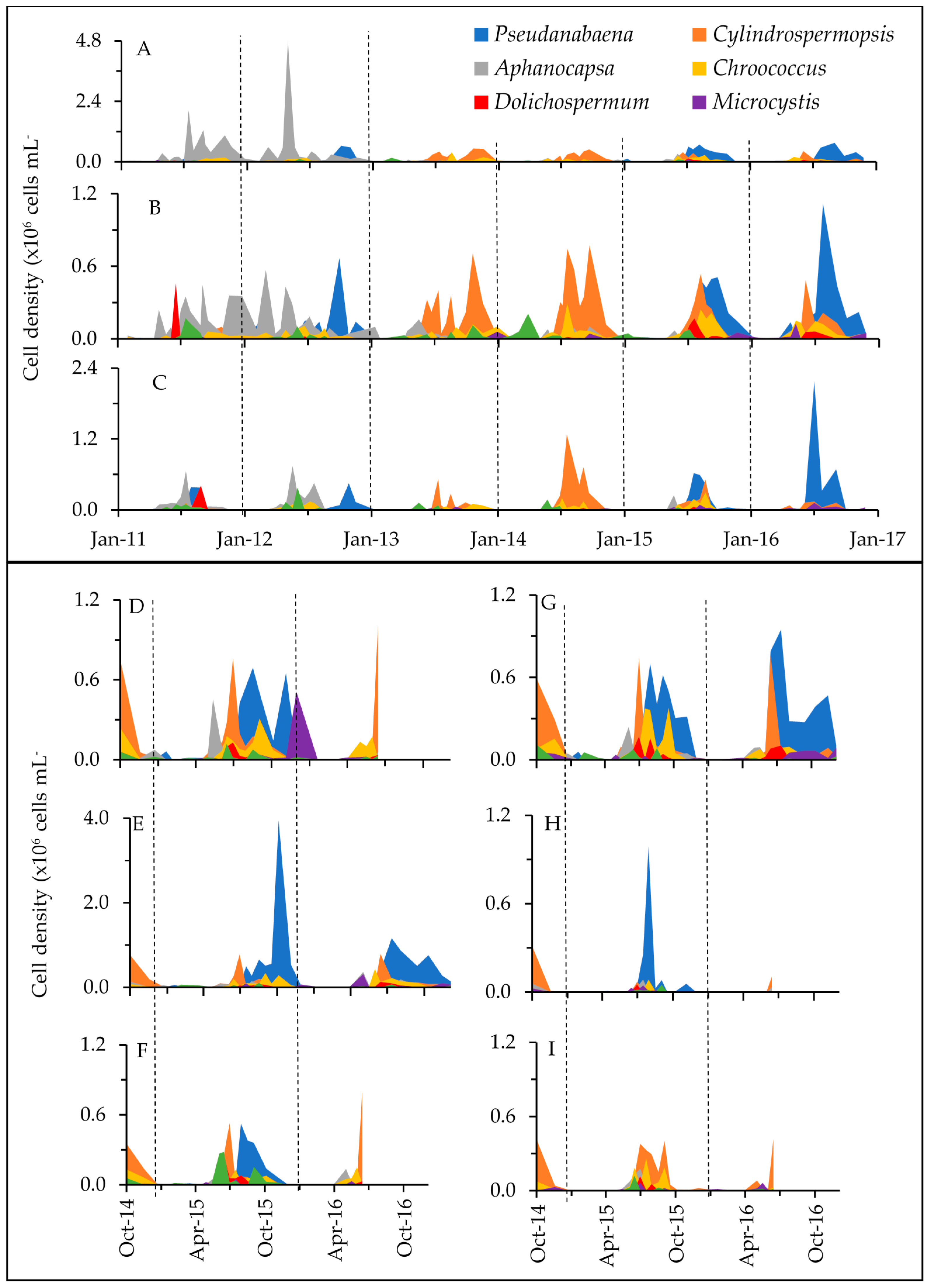
2.1. Phytoplankton Dynamics
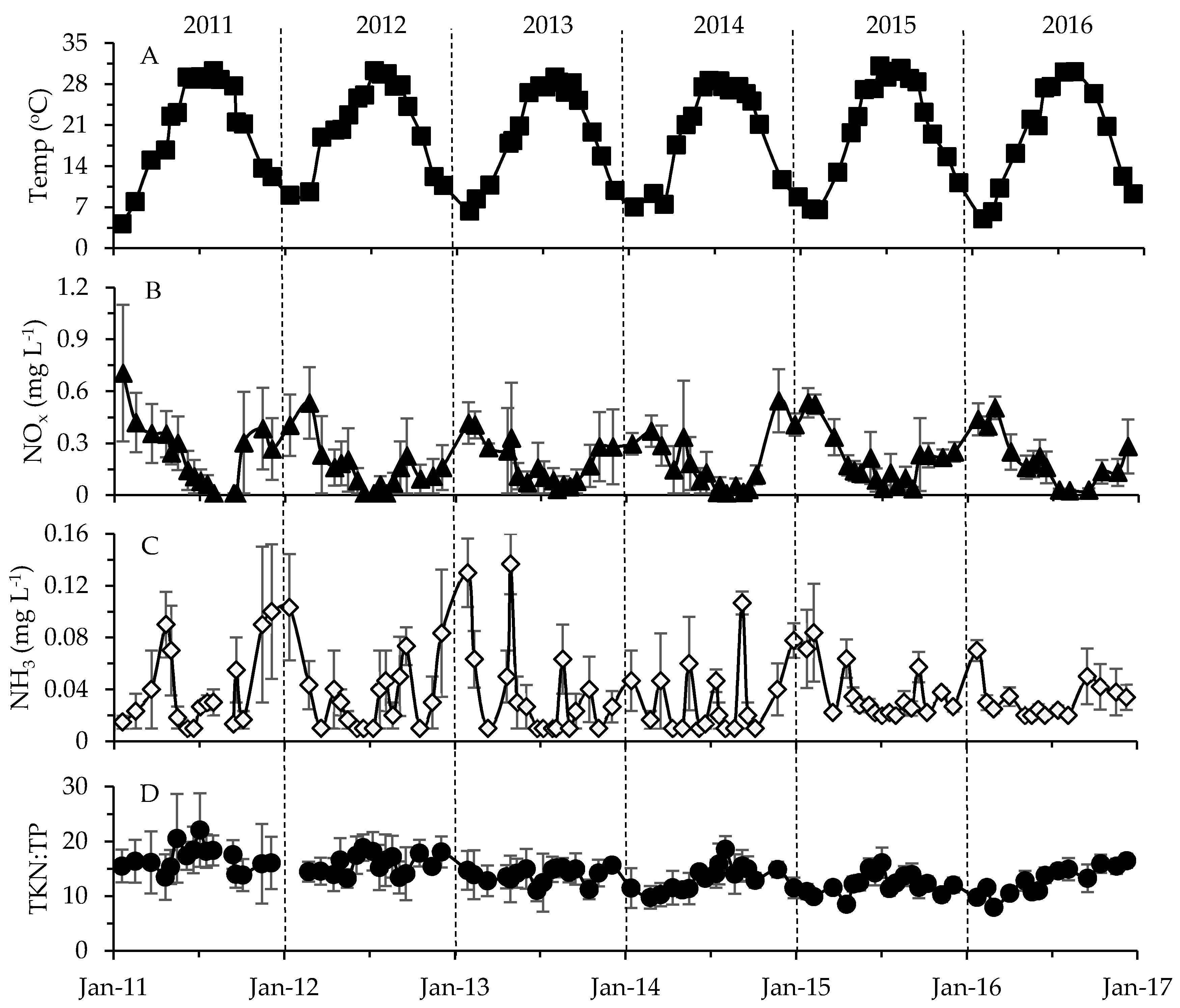
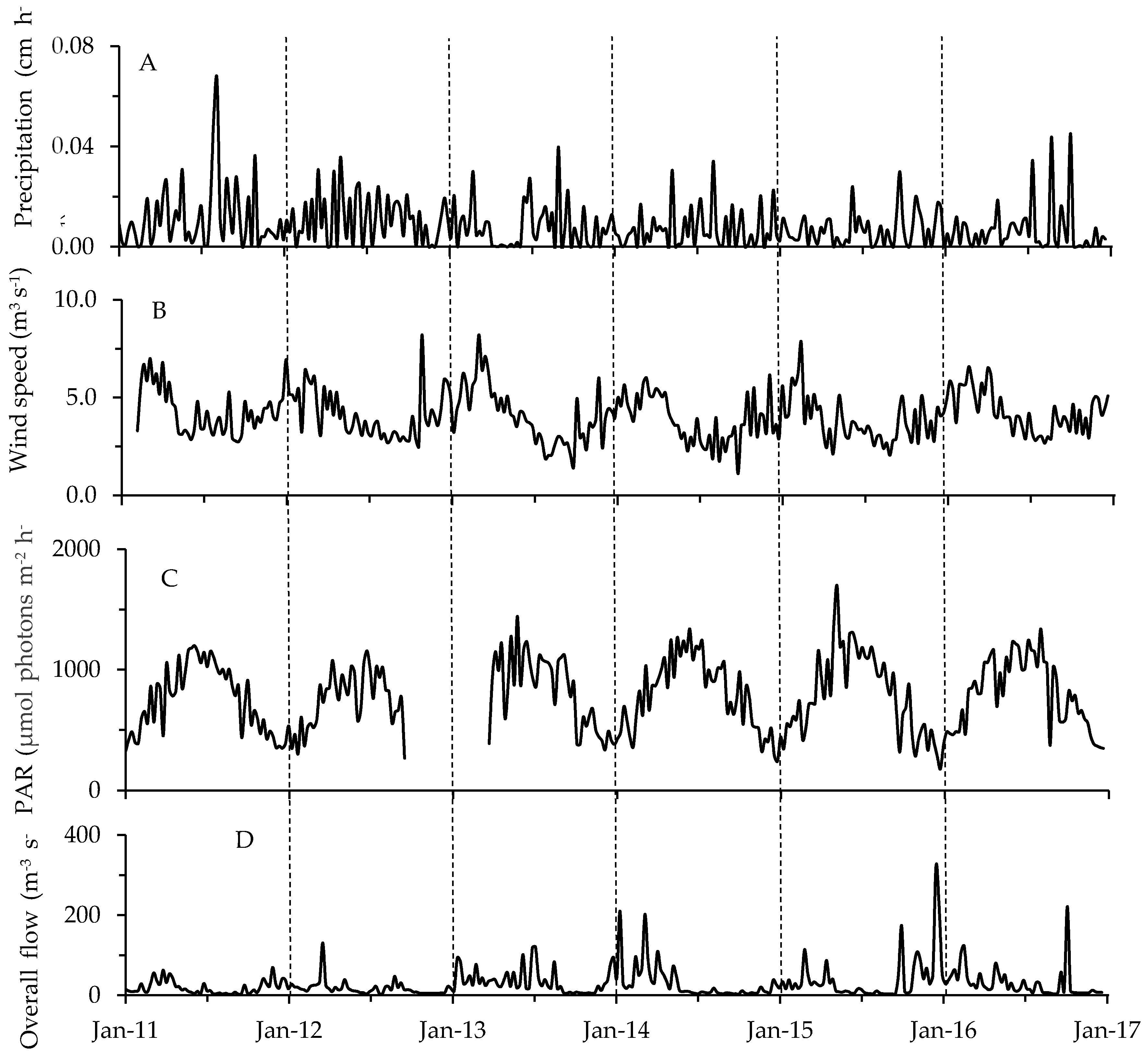
2.2. Physicochemical, Meteorological and Hydrological Parameters
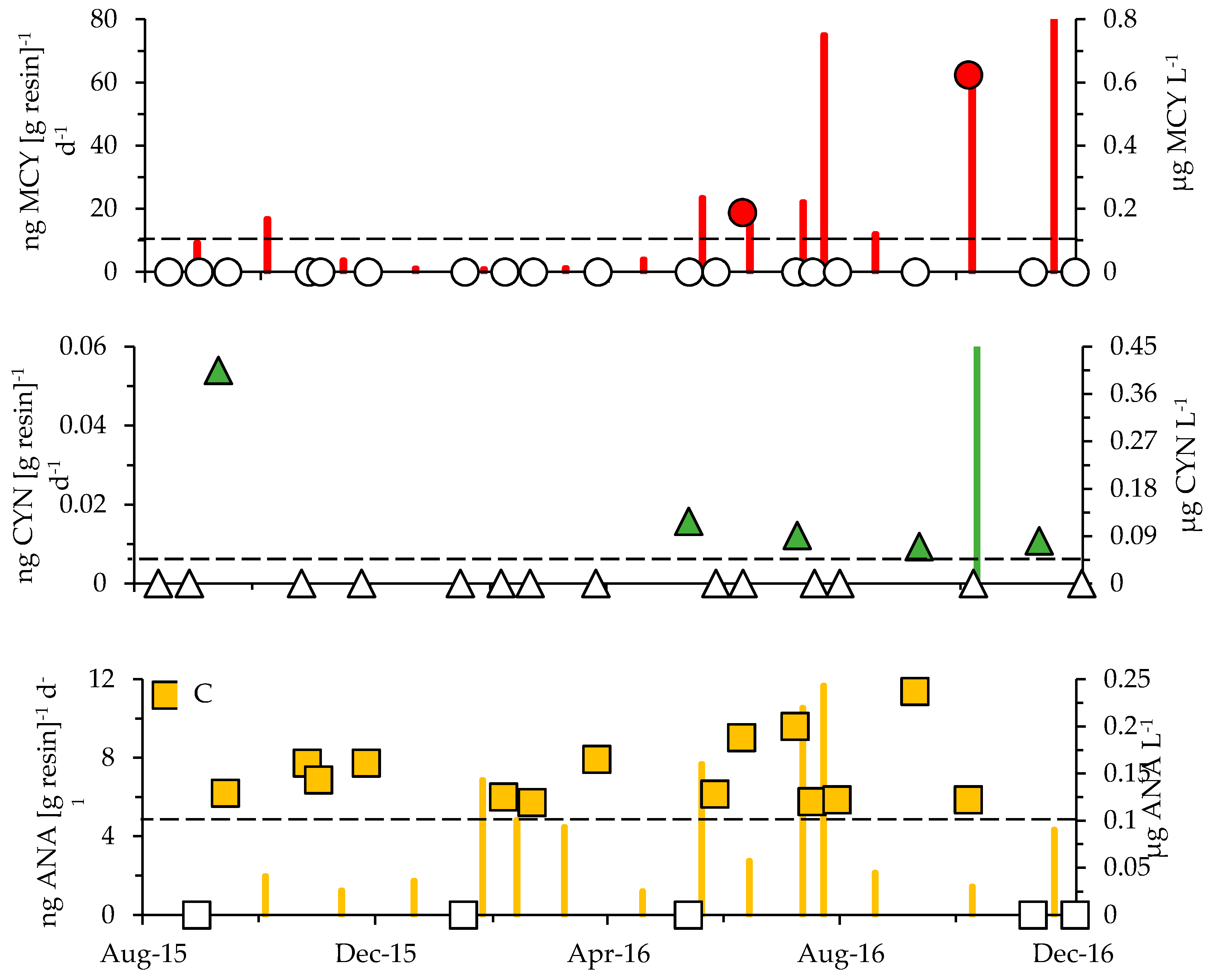
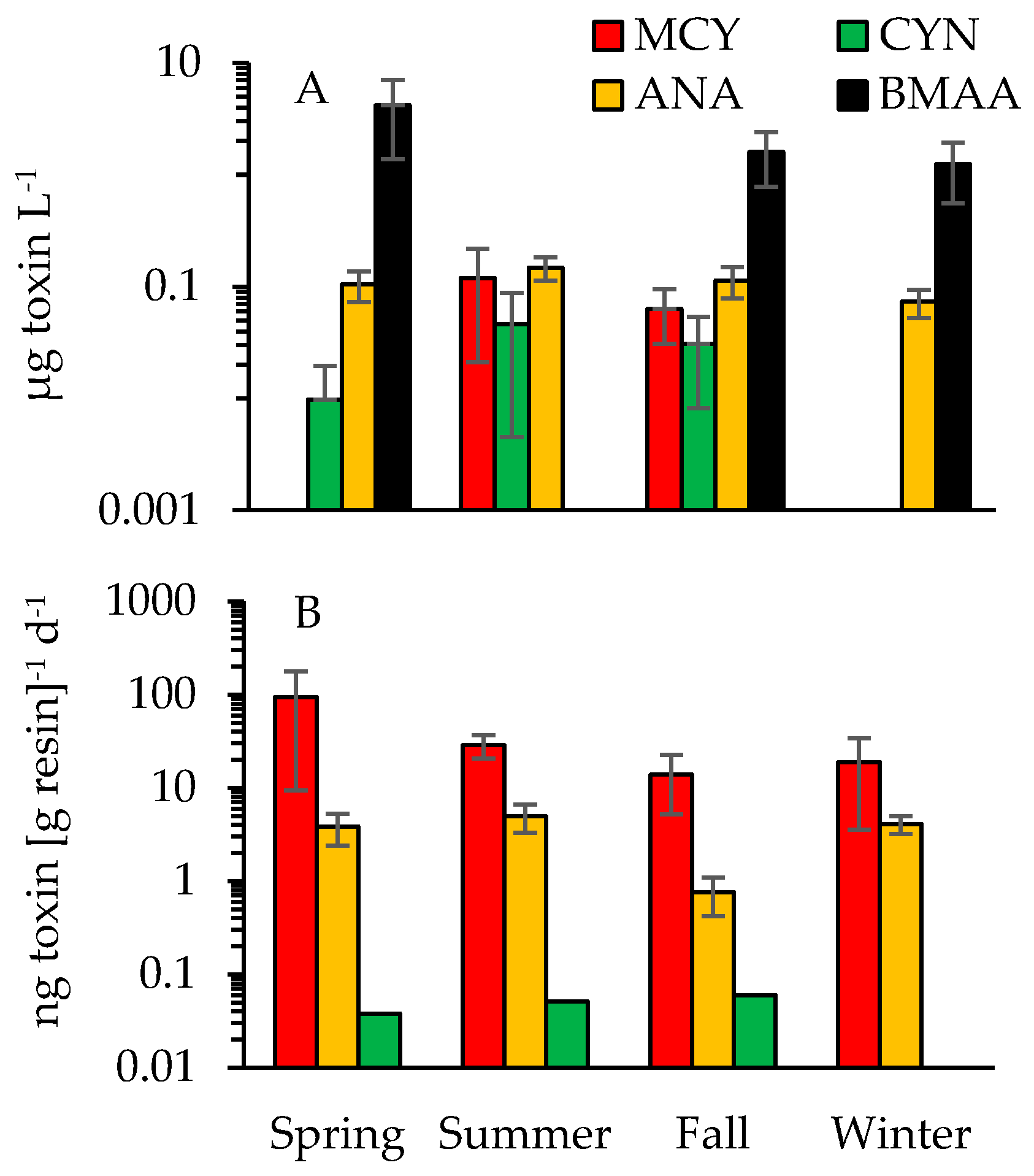
2.3. Cyanotoxins
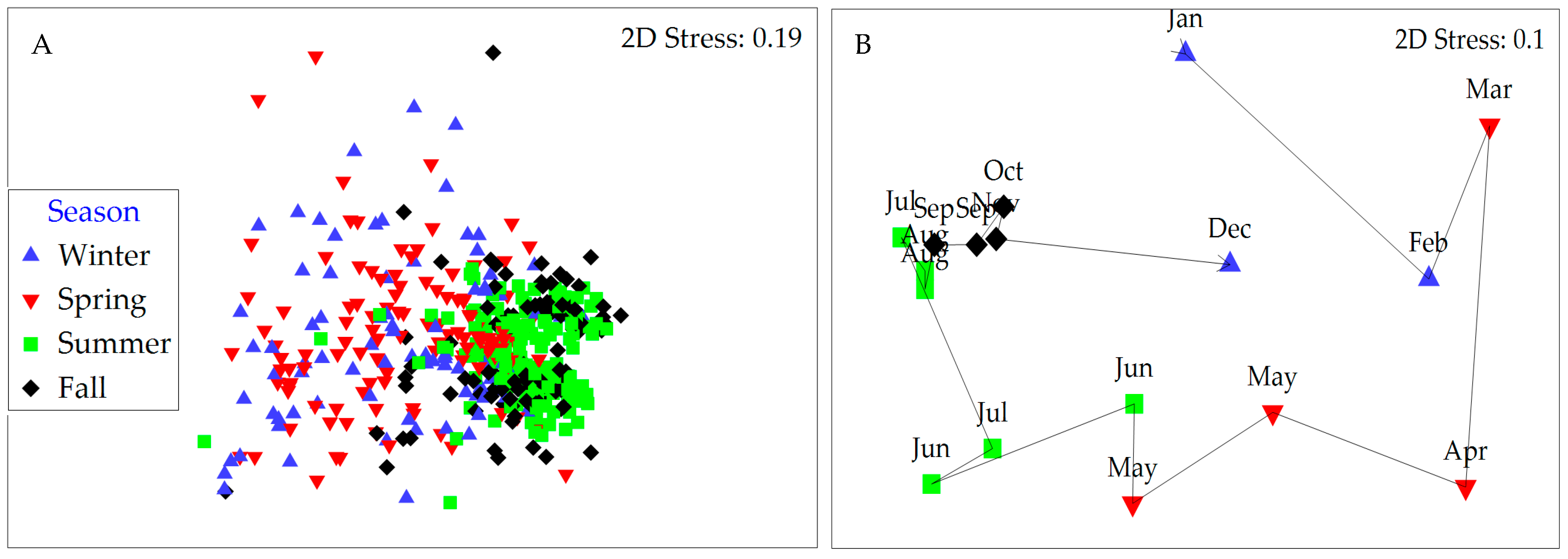
2.4. Linkages between Environmental Factors and Phytoplankton Dynamics
3. Discussion
3.1. Cyanotoxins and Phytoplankton Dynamics in Jordan Lake
3.2. Environmental Factors in Relation to Phytoplankton and Toxin Dynamics
3.3. Conclusions and Recommendations
4. Materials and Methods
4.1. Study Area and Data Collection
4.2. Phytoplankton Data
4.3. Toxin Analyses
4.4. Chlorophyll Analyses
4.5. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selman, M.; Greenhalgh, S.; Diaz, R.; Sugg, Z. Eutrophication and hypoxia in coastal areas: A global assessment of the state of knowledge. World Resour. Inst. 2008, 284, 1–6. [Google Scholar]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Döll, P.; Jimenez, B.; Miller, K.; Oki, T.; Sen, Z.; Shiklomanov, I. The implications of projected climate change for freshwater resources and their management. Hydrol. Sci. J. 2008, 53, 3–10. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justic, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S., 3rd; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Ibelings, B.W.; Chorus, I. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Pinckney, J.L.; Fear, J.M.; Peierls, B.L. Ecosystem responses to internal and watershed organic matter loading: Consequences for hypoxia in the eutrophying Neuse River Estuary, North Carolina, USA. Mar. Ecol. Prog. Ser. 1998, 166, 17–25. [Google Scholar] [CrossRef]
- Watson, S.B.; Miller, C.; Arhonditsis, G.; Boyer, G.L.; Carmichael, W.; Charlton, M.N.; Confesor, R.; Depew, D.C.; Höök, T.O.; Ludsin, S.A. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, D.A. Economic cost of cyanobacterial blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 855–865. [Google Scholar]
- Le, C.; Zha, Y.; Li, Y.; Sun, D.; Lu, H.; Yin, B. Eutrophication of lake waters in China: Cost, causes, and control. Environ. Manag. 2010, 45, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.P.; Wood, S.A.; Dietrich, D.R.; Puddick, J. Costs of harmful blooms of freshwater cyanobacteria. In Cyanobacteria: An Economic Perspective; Sharma, N.K., Rai, A.K., Stal, L.J., Eds.; Wiley: Chichester, UK, 2014; pp. 245–256. [Google Scholar]
- Kratz, W.A.; Myers, J. Nutrition and growth of several blue-green algae. Am. J. Bot. 1955, 42, 282–287. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Havens, K.E.; James, R.T.; East, T.L.; Smith, V.H. N:P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environ. Pollut. 2003, 122, 379–390. [Google Scholar] [CrossRef]
- Paerl, H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 217–237. [Google Scholar]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Lee, T.A.; Rollwagen-Bollens, G.; Bollens, S.M. The influence of water quality variables on cyanobacterial blooms and phytoplankton community composition in a shallow temperate lake. Environ. Monit. Assess. 2015, 187, 315. [Google Scholar] [CrossRef] [PubMed]
- Cloern, J.E. Annual variations in river flow and primary production in the South San Francisco Bay Estuary (USA). Estuar. Coast. 1991, 19, 91–96. [Google Scholar]
- Van Vliet, M.T.H.; Zwolsman, J.J.G. Impact of summer droughts on the water quality of the Meuse river. J. Hydrol. 2008, 353, 1–17. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloglu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.; Brock, T.D. Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L.; Zheng, L.; Dai, G.; Ma, H.; Shan, K.; Wu, H.; Zhou, Q.; Song, L. Patterns of succession between bloom-forming cyanobacteria Aphanizomenon flos-aquae and Microcystis and related environmental factors in large, shallow Dianchi Lake, China. Hydrobiologia 2016, 765, 1–13. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K. Cyanobacterial Toxins. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Elsevier: Oxford, UK, 2009. [Google Scholar]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Kaebernick, M.; Neilan, B.A. Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol. Ecol. 2001, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rapala, J.; Sivonen, K.; Lyra, C.; Niemela, S.I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 1997, 63, 2206–2212. [Google Scholar] [PubMed]
- Gao, K.; Yu, H.; Brown, M.T. Solar PAR and UV radiation affects the physiology and morphology of the cyanobacterium Anabaena sp. PCC 7120. J. Photochem. Photobiol. B 2007, 89, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Råbergh, C.M.I.; Bylund, G.; Eriksson, J.E. Histopathological effects of microcystin-LR, a cyclic peptide toxin from the cyanobacterium (blue-green alga) Microcystis aeruginosa on common carp (Cyprinus carpio L.). Aquat. Toxicol. 1991, 20, 131–145. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.R.; Runnegar, M.T.; Jackson, A.R.; Falconer, I.R. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar] [PubMed]
- Falconer, I.R.; Humpage, A.R. Cyanobacterial (blue-green algal) toxins in water supplies: Cylindrospermopsins. Environ. Toxicol. 2006, 21, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R. Health effects associated with controlled exposures to cyanobacterial toxins. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 607–612. [Google Scholar]
- Acres, J.; Gray, J. Paralytic shellfish poisoning. Can. Med. Assoc. J. 1978, 119, 1195–1197. [Google Scholar] [PubMed]
- Halstead, B.W.; Schantz, E.J. Paralytic Shellfish Poisoning; Report prepared for World Health Organization; World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- Kaas, H.; Henriksen, P. Saxitoxins (PSP toxins) in Danish lakes. Water Res. 2000, 34, 2089–2097. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Kurland, L.T.; Krooth, R.S.; Lessell, S. Parkinsonism-dementia complex, an endemic disease on the island of Guam: I. Clinical features. Brain 1961, 84, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of β-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.A.; Stommel, E.W. The cyanobacteria derived toxin beta-N-methylamino-l-alanine and amyotrophic lateral sclerosis. Toxins 2010, 2, 2837–2850. [Google Scholar] [CrossRef] [PubMed]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Drinking Water Health Advisory for the Cyanobacterial Microcystin Toxins; Report Prepared for Environmental Protection Agency; Environmental Protection Agency: Washington, DC, USA, 2015.
- Drinking Water Health Advisory for the Cyanobacterial Toxin Cylindrospermopsin; Report Prepared for Environmental Protection Agency; Environmental Protection Agency: Washington, DC, USA, 2015.
- Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsins—Draft; Report Prepared for Environmental Protection Agency; Environmental Protection Agency: Washington, DC, USA, 2016.
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Touchette, B.W.; Burkholder, J.M.; Allen, E.H.; Alexander, J.L.; Kinder, C.A.; Brownie, C.; James, J.; Britton, C.H. Eutrophication and cyanobacteria blooms in run-of-river impoundments in North Carolina, USA. Lake Reserv. Manag. 2007, 23, 179–192. [Google Scholar] [CrossRef]
- Grubbs, L.F. Quantification of Select Cyanobacteria and Cyanotoxins in Piedmont North Carolina Lakes Using Real-Time PCR. Master’s Thesis, The University of North Carolina at Greensboro, Greensboro, NC, USA, 2014. [Google Scholar]
- Allender, C.J.; LeCleir, G.R.; Rinta-Kanto, J.M.; Small, R.L.; Satchwell, M.F.; Boyer, G.L.; Wilhelm, S.W. Identifying the source of unknown microcystin genes and predicting microcystin variants by comparing genes within uncultured cyanobacterial cells. Appl. Environ. Microbiol. 2009, 75, 3598–3604. [Google Scholar] [CrossRef] [PubMed]
- Himberg, K.; Keijola, A.M.; Hiisvirta, L.; Pyysalo, H.; Sivonen, K. The effect of water treatment processes on the removal of hepatotoxins from Microcystis and Oscillatoria cyanobacteria: A laboratory study. Water Res. 1989, 23, 979–984. [Google Scholar] [CrossRef]
- Hitzfeld, B.C.; Hoger, S.J.; Dietrich, D.R. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Webb, P.M.; Schluter, P.J.; Fleming, L.E.; Burns, J.W.; Gantar, M.; Backer, L.C.; Shaw, G.R. Epidemiology of recreational exposure to freshwater cyanobacteria—An international prospective cohort study. BMC Public Health 2006, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.C.; Bargu, S.; Dash, P.; Rabalais, N.N.; Sutor, M.; Morrison, W.; Walker, N.D. Evaluating the potential risk of microcystins to blue crab (Callinectes sapidus) fisheries and human health in a eutrophic estuary. Harmful Algae 2010, 9, 134–143. [Google Scholar] [CrossRef]
- Lehman, P.W.; Teh, S.J.; Boyer, G.L.; Nobriga, M.L.; Bass, E.; Hogle, C. Initial impacts of Microcystis aeruginosa blooms on the aquatic food web in the San Francisco Estuary. Hydrobiologia 2010, 637, 229–248. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.N. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- NC Department of Natural and Cultural Resources. Available online: https://www.ncdcr.gov/ (accessed on 22 February 2018).
- Cyanobacterial Toxins: Microcystin-LR in Drinking Water; Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; Report Prepared for World Health Organizaition; World Health Organization: Geneva, Switzerland, 2003.
- Steffen, M.M.; Belisle, B.S.; Watson, S.B.; Boyer, G.L.; Wilhelm, S.W. Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Gt. Lakes Res. 2014, 40, 215–225. [Google Scholar] [CrossRef]
- Howard, M.D.A.; Nagoda, C.; Kudela, R.M.; Hayashi, K.; Tatters, A.; Caron, D.A.; Busse, L.; Brown, J.; Sutula, M.; Stein, E.D. Microcystin prevalence throughout lentic waterbodies in coastal Southern California. Toxins 2017, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Al-Sammak, M.A.; Hoagland, K.D.; Snow, D.D.; Cassada, D. Methods for simultaneous detection of the cyanotoxins BMAA, DABA, and anatoxin-a in environmental samples. Toxicon 2013, 76, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Health Effects Support Document for the Cyanobacterial Toxin Anatoxin-A; Report Prepared for Environmental Protection Agency; Environmental Protection Agency: Washington, DC, USA, 2015.
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid Phase Adsorption Toxin Tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Kudela, R.M. Characterization and deployment of Solid Phase Adsorption Toxin Tracking (SPATT) resin for monitoring of microcystins in fresh and saltwater. Harmful Algae 2011, 11, 117–125. [Google Scholar] [CrossRef]
- Gibble, C.M.; Kudela, R.M. Detection of persistent microcystin toxins at the land–sea interface in Monterey Bay, California. Harmful Algae 2014, 39, 146–153. [Google Scholar] [CrossRef]
- White, S.H.; Duivenvoorden, L.; Fabbro, L.D. A decision-making framework for ecological impacts associated with the accumulation of cyanotoxins (cylindrospermopsin and microcystin). Lakes Reserv. Res. Manag. 2005, 10, 25–37. [Google Scholar] [CrossRef]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Kudela, R.M. Passive sampling for freshwater and marine algal toxins. Compr. Anal. Chem. 2017, 78, 379–409. [Google Scholar]
- Kudela, R.M.; University of California Santa Cruz, Santa Cruz, CA, USA. Personal communication, 2017.
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [PubMed]
- Otten, T.G.; Paerl, H.W. Health effects of toxic cyanobacteria in US drinking and recreational waters: Our current understanding and proposed direction. Curr. Environ. Health Rep. 2015, 2, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Namikoshi, M.; Murakami, T.; Watanabe, M.F.; Oda, T.; Yamada, J.; Tsujimura, S.; Nagai, H.; Oishi, S. Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 2003, 42, 533–538. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Li, R. Cyanobacteria toxins in the Salton Sea. Saline Syst. 2006, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.L.F.F.; do Carmo Calijuri, M.; Lorenzi, A.S.; Honda, R.Y.; Genuário, D.B.; Fiore, M.F. Morphological and molecular characterization of cyanobacteria from a Brazilian facultative wastewater stabilization pond and evaluation of microcystin production. Hydrobiologia 2009, 627, 195–209. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Lake, B. Everett Jordan Watershed Model Report. Available online: ftp://ftp.tjcog.org/pub/planning/water/JordanAllocationModel/TTDataFiles/Documents/Model_Development_Reports/Jordan_Watershed_Model_Report_July2014final.pdf (accessed on 10 December 2017).
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Valladares, A.; Montesinos, M.L.; Herrero, A.; Flores, E. An ABC-type, high-affinity urea permease identified in cyanobacteria. Mol. Microbiol. 2002, 43, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.W.; Harke, M.J.; Marcoval, M.A.; Goleski, J.; Orano-Dawson, C.; Berry, D.L.; Gobler, C.J. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat. Microb. Ecol. 2010, 61, 149–162. [Google Scholar] [CrossRef]
- Huang, W.; Bi, Y.; Hu, Z. Effects of fertilizer-urea on growth, photosynthetic activity and microcystins production of Microcystis aeruginosa isolated from Dianchi lake. Bull. Environ. Contam. Toxicol. 2014, 92, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Xu, H.; Hall, N.S.; Zhu, G.; Qin, B.; Wu, Y.; Rossignol, K.L.; Dong, L.; McCarthy, M.J.; Joyner, A.R. Controlling cyanobacterial blooms in hypertrophic Lake Taihu, China: Will nitrogen reductions cause replacement of non-N2 fixing by N2 fixing taxa? PLoS ONE 2014, 9, e113123. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Waterbury, J.B. The synthesis of nitrogenase by non-heterocystous cyanobacteria. FEMS Microbiol. Lett. 1977, 2, 83–86. [Google Scholar] [CrossRef]
- Bergman, B.; Gallon, J.R.; Rai, A.N.; Stal, L.J. N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol. Rev. 1997, 19, 139–185. [Google Scholar] [CrossRef]
- Farnelid, H.; Bentzon-Tilia, M.; Andersson, A.F.; Bertilsson, S.; Jost, G.; Labrenz, M.; Jürgens, K.; Riemann, L. Active nitrogen-fixing heterotrophic bacteria at and below the chemocline of the central Baltic Sea. ISME J. 2013, 7, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Robson, B.J.; Hamilton, D.P. Summer flow event induces a cyanobacterial bloom in a seasonal Western Australian estuary. Mar. Freshw. Res. 2003, 54, 139–151. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Hardwick, L.; Dorani, F. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J. Plankton Res. 2010, 33, 229–241. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Jha, M.K. Hydrological response due to projected climate variability in Haw River watershed, North Carolina, USA. Hydrol. Sci. J. 2016, 61, 495–506. [Google Scholar] [CrossRef]
- Cape Fear River Basinwide Water Quality Plan; Report Prepared for North Carolina Division of Water Resources; North Carolina Division of Water Resources: Raleigh, NC, USA, 2000.
- DWR Manual for Standard Operating Procedures. Available online: https://deq.nc.gov/about/divisions/water-resources/water-resources-data/water-sciences-home-page/intensive-survey-branch/ambient-lakes-monitoring (accessed on 22 February 2018).
- Intensive Survey Branch Standard Operating Procedures Manual: Physical and Chemical Monitoring; Report Prepared for North Carolina Division of Water Resources; North Carolina Division of Water Resources: Raleigh, NC, USA, 2013.
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Standard Operating Procedures for the Collection and Analysis of Algae; Report Prepared for North Carolina Division of Water Resources; North Carolina Division of Water Resources: Raleigh, NC, USA, 2016.
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. Freshwater Ecology of North America: Ecology and Classification, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Lane, J.Q.; Roddam, C.; Langlois, G.W.; Kudela, R.M. Application of Solid Phase Adsorption Toxin Tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California. Limnol. Oceanogr. Methods 2010, 8, 645–660. [Google Scholar] [CrossRef]
- Method 445.0 In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence; Report Prepared for Environmental Protection Agency; Environmental Protection Agency: Washington, DC, USA, 1997.
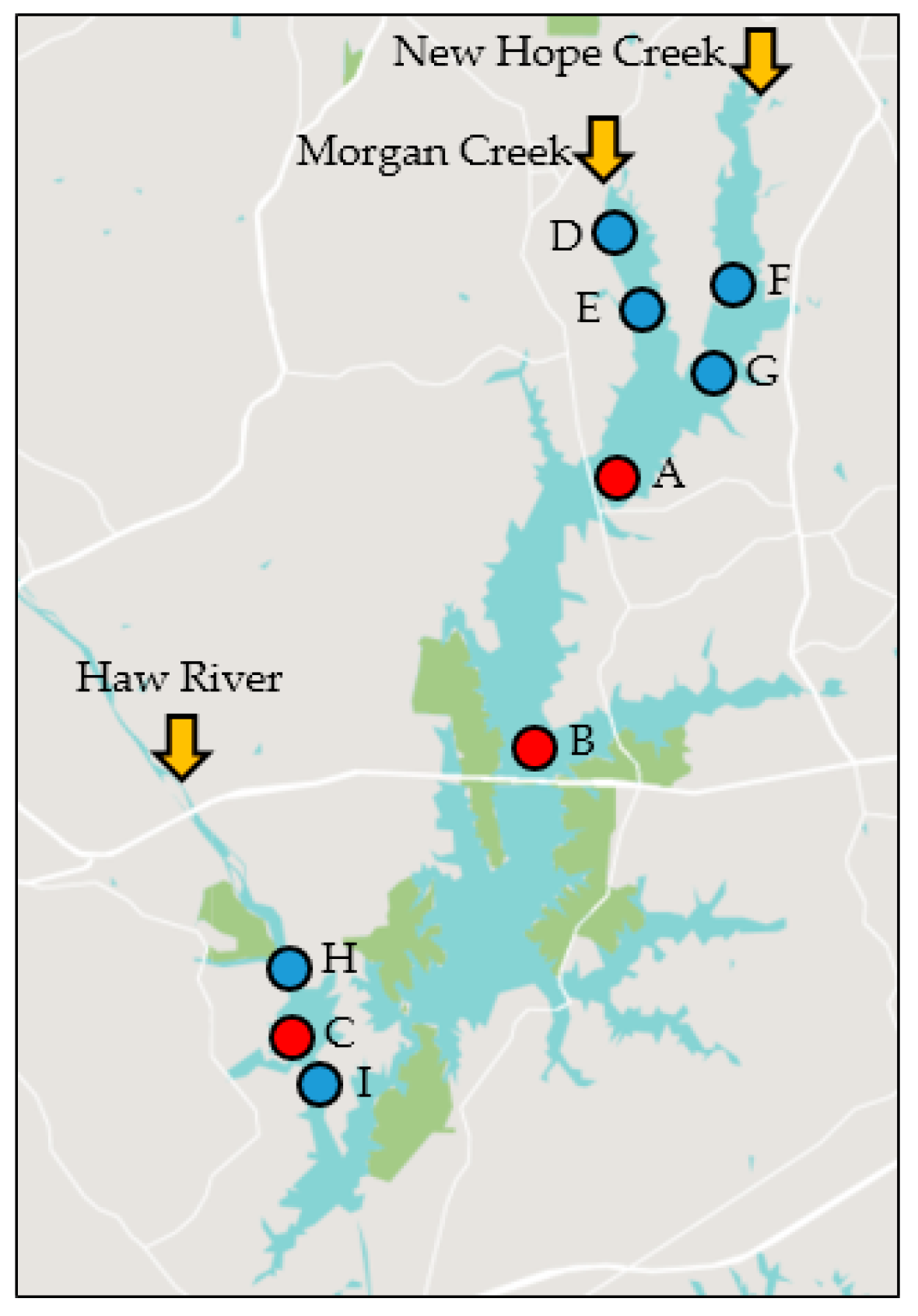

| Site ID | DWR ID | Lat (° N) | Long (° W) | From | To | n | Depth (m) |
|---|---|---|---|---|---|---|---|
| A | CPF086C | 35.794 | 79.004 | January 2011 | December 2016 | 98 | 5.06 |
| B | CPF087D | 35.742 | 79.021 | January 2011 | December 2016 | 96 | 7.67 |
| C | CPF055C | 35.687 | 79.083 | January 2011 | December 2016 | 95 | 5.87 |
| D | CPF086CUPS | 35.837 | 79.001 | October 2014 | June 2016 | 26 | 1.47 |
| E | CPF086C | 35.825 | 78.998 | October 2014 | December 2016 | 34 | 2.92 |
| F | CPF081A1B | 35.836 | 78.976 | October 2014 | June 2016 | 25 | 1.82 |
| G | CPF081A1C | 35.815 | 78.983 | October 2014 | December 2016 | 32 | 3.16 |
| H | CPF055C1 | 35.699 | 79.082 | October 2014 | June 2016 | 27 | 2.25 |
| I | CPF055C6 | 35.682 | 79.078 | October 2014 | June 2016 | 27 | 8.50 |
| Toxin | Sample Type | Ave | Range | Positive (%) | n | LDL |
|---|---|---|---|---|---|---|
| MCY | Diss | 0.37 | BDL—1.98 | 15 | 65 | 0.10 |
| SPATT | 39.49 | BDL—347.45 | 92 | 24 | ||
| ANA | Diss | 0.2 | BDL—0.68 | 57 | 69 | 0.10 |
| SPATT | 3.97 | 0.31—13.28 | 100 | 23 | ||
| CYN | Diss | 0.27 | BDL—0.83 | 10 | 63 | 0.04 |
| SPATT | 0.05 | BDL—0.05 | 13 | 24 | ||
| BMAA | Diss | 10.75 | BDL—23.45 | 14 | 64 | 4.00 |
| STX | Diss | BDL | BDL | 0 | 40 | 0.015 |
| Chl a (µg L−1) | Cyano (Cells mL−1) | Cyano (mm3 m−3) | Microphyto(Cells mL−1) | Microphyto(mm3 m−3) | MCY (ng mL−1) | ANA (ng mL−1) | |
|---|---|---|---|---|---|---|---|
| Cyano (cells mL−1) | 0.555 | ||||||
| Cyano (mm3 m−3) | 0.515 | 0.851 | |||||
| Microphyto (cells mL−1) | 0.358 | 0.367 | 0.314 | ||||
| Microphyto (mm3 m−3) | 0.447 | 0.469 | 0.364 | 0.329 | |||
| Diss MCY (ng mL−1) | 0.101 | 0.272 | 0.313 | 0.408 | 0.055 | ||
| Diss ANA (ng mL−1) | −0.166 | 0.125 | 0.132 | −0.008 | 0.277 | 0.270 | |
| NH3 (mg L−1) | −0.352 | −0.257 | −0.229 | −0.154 | −0.210 | −0.044 | −0.135 |
| NOx (mg L−1) | −0.540 | −0.586 | −0.499 | −0.295 | −0.349 | −0.102 | −0.106 |
| TKN (mg L−1) | 0.750 | 0.498 | 0.492 | 0.314 | 0.282 | 0.147 | −0.016 |
| TP (mg L−1) | 0.217 | −0.022 | 0.061 | 0.049 | −0.031 | −0.023 | −0.183 |
| TKN:TP | 0.110 | 0.306 | 0.229 | 0.089 | 0.158 | 0.200 | 0.326 |
| Turbidity (NTU) | 0.164 | −0.112 | 0.002 | 0.068 | −0.105 | −0.075 | −0.230 |
| Temp (°C) | 0.322 | 0.524 | 0.470 | 0.175 | 0.238 | 0.150 | 0.150 |
| DO (mg L−1) | −0.018 | −0.309 | −0.229 | −0.081 | −0.101 | −0.017 | −0.125 |
| pH | 0.441 | 0.458 | 0.437 | 0.200 | 0.231 | 0.255 | 0.043 |
| Adj. R2 | Parameter 1 | Parameter 2 | Parameter 3 | Parameter 4 | n | |
|---|---|---|---|---|---|---|
| Chl a | 0.680 | NH3 (−8.61) | NOx (−10.50) | TKN (19.99) | DO (5.66) | 427 |
| Cyano (cells mL−1) | 0.521 | NOx (−9.10) | TKN (5.43) | DO (−5.56) | pH (8.62) | 446 |
| Cyano (mm3 m−3) | 0.406 | NOx (−8.79) | TKN (6.31) | pH (6.19) | 452 | |
| Microphyto (cells mL−1) | 0.132 | NOx (−4.42) | TKN (5.05) | 454 | ||
| Microphyto (mm3 m−3) | 0.189 | NH3 (−3.12) | NOx (−3.99) | TKN (5.34) | Turb (−3.71) | 454 |
| Location | Month/Year | Water Body | Toxin | Ave (µg L−1) | Range (µg L−1) | n | Method | Reference |
|---|---|---|---|---|---|---|---|---|
| Apex | August 2015–December 2016 | Jordan Lake | MCY | 0.06 | BDL—1.98 | 65 | ELISA | This Study |
| CYN | 0.02 | BDL—0.83 | 63 | |||||
| ANA | 0.11 | BDL—0.68 | 69 | |||||
| BMAA | 9.32 | BDL—23.45 | 64 | |||||
| Piedmont | June 2002–August 2002 | Jordan Lake | MCY | 0.20 * | ND | 6 | ELISA | [52] |
| Kerr Scott Res | MCY | 0.30 * | ND | 6 | ||||
| Tuckertown Res | MCY | 0.12 * | ND | 6 | ||||
| Oak Hollow Lake | MCY | 0.10 * | ND | 6 | ||||
| Falls Lake | MCY | 0.22 * | ND | 6 | ||||
| Narrows Res | MCY | 0.15 * | ND | 6 | ||||
| Lake Rhodhiss | MCY | 0.25 * | ND | 6 | ||||
| Lake Michie | MCY | 0.15 * | ND | 6 | ||||
| High Rock Lake | MCY | 0.05 * | ND | 6 | ||||
| Lake Tillery | MCY | 0.35 * | ND | 6 | ||||
| High Point Lake | MCY | 0.12 * | ND | 6 | ||||
| Piedmont | June 2011–September 2012 | City Lake | MCY | 0.22 | BDL—0.31 | 6 | ELISA | [53] |
| Oak Hollow Lake | MCY | 0.21 | BDL—0.26 | 4 | ||||
| Randleman Res | MCY | 0.17 | BDL—0.18 | 7 | ||||
| Lake Mackintosh | MCY | 0.17 | BDL—0.17 | 5 | ||||
| Waterville | October 2007 | Waterville Res | MCY | 824.3 | ND | 3 | LC-MS | [54] |
| Statewide | June 2007–July 2007 | Lake Lee | MCY | 0.21 | 0.17—0.24 | 2 | ELISA | [47] |
| Lake Rhodhiss | MCY | 0.14 | BDL—0.14 | 2 | ||||
| STX | 0.03 | BDL—0.03 | 2 | |||||
| Lake Orange | MCY | 0.54 | 0.54 | 1 | ||||
| Lake Fisher | MCY | 0.17 | 0.147 | 1 | ||||
| High Rock Lake | MCY | 0.52 | 0.52 | 1 | ||||
| Lake Townsend | MCY | 0.16 | 0.16 | 1 | ||||
| Falls Lake | MCY | 0.28 | 0.28 | 1 | ||||
| Lake Hickory | MCY | 0.16 | 0.16 | 1 | ||||
| Beaverdam Lake | MCY | 0.23 | 0.23 | 1 | ||||
| Graham-Mebane Lake | MCY | 0.11 | 0.11 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiltsie, D.; Schnetzer, A.; Green, J.; Vander Borgh, M.; Fensin, E. Algal Blooms and Cyanotoxins in Jordan Lake, North Carolina. Toxins 2018, 10, 92. https://doi.org/10.3390/toxins10020092
Wiltsie D, Schnetzer A, Green J, Vander Borgh M, Fensin E. Algal Blooms and Cyanotoxins in Jordan Lake, North Carolina. Toxins. 2018; 10(2):92. https://doi.org/10.3390/toxins10020092
Chicago/Turabian StyleWiltsie, Daniel, Astrid Schnetzer, Jason Green, Mark Vander Borgh, and Elizabeth Fensin. 2018. "Algal Blooms and Cyanotoxins in Jordan Lake, North Carolina" Toxins 10, no. 2: 92. https://doi.org/10.3390/toxins10020092
APA StyleWiltsie, D., Schnetzer, A., Green, J., Vander Borgh, M., & Fensin, E. (2018). Algal Blooms and Cyanotoxins in Jordan Lake, North Carolina. Toxins, 10(2), 92. https://doi.org/10.3390/toxins10020092




