Why is Skeletal Muscle Regeneration Impaired after Myonecrosis Induced by Viperid Snake Venoms?
Abstract
:1. Introduction
2. A Brief Outlook into the Pathogenesis of Myonecrosis Induced by Snake Venoms
3. The Process of Skeletal Muscle Regeneration
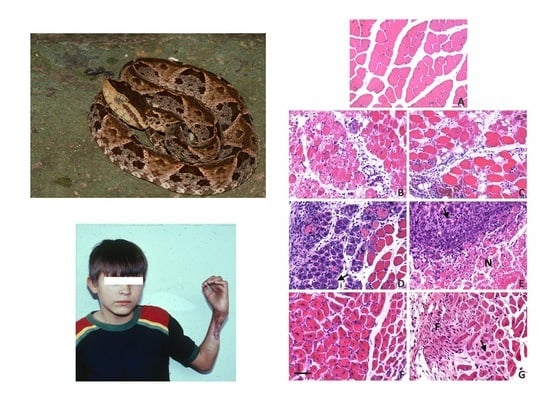
4. Skeletal Muscle Regeneration after Myonecrosis Induced by Snake Venoms at the Clinical Setting
5. Experimental Studies of Muscle Regeneration after Myonecrosis Induced by Snake Venoms and Toxins
5.1. Venoms and Toxins that Induce Myonecrosis without Affecting the Vascular Supply
5.2. Venoms and Toxins that Affect Other Components of Skeletal Muscle in Addition to Causing Myonecrosis
6. The Key Role of Vascular Damage and Its Implications in the Inflammatory Response and Regeneration
7. The Complex Landscape of ECM Alterations in Viperid Venom-Induced Myonecrosis
8. Damage to Intramuscular Nerves
9. Does Residual Venom in the Necrotic Tissue Contribute to the Impairment of Regeneration?
10. Exploring Therapeutic Options to Improve Muscle Regeneration in Viperid Snakebite Envenoming
11. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.L.C.; Franca, F.O.D.S.; Wen, F.H.; Malaque, C.M.S.; Haddad Junior, V. Animais peçonhentos no Brasil: Biologia, clinica e terapêutica dos acidentes; São Paulo Sarvier: Sao Paulo, Brazil, 2009; p. 540. [Google Scholar]
- Warrell, D.A. Clinical toxicology of snakebite in Africa and the Middle East/Arabian peninsula. In Handbook of Clinical Toxicology of Animal Venoms and Poisons; CRC Press: Boca Raton, FL, USA, 1995; pp. 433–492. [Google Scholar]
- Warrell, D.A. Snakebites in Central and South America: Epidemiology, clinical features, and clinical management. In The Venomous Reptiles of the Western Hemisphere; Lamar, W.W, Campbell, J.A., Eds.; Cornell University Press: Ithaca, NY, USA, 2004; pp. 709–761. [Google Scholar]
- White, J. Clinical Toxicology of snakebite in Australia and New Guinea. In Handbook of Clinical Toxicology of Animal Venoms and Poisons; CRC Press: Boca Raton, FL, USA, 1995; pp. 595–617. [Google Scholar]
- Azevedo-Marques, M.M.; Cupo, P.; Coimbra, T.M.; Hering, S.E.; Rossi, M.A.; Laure, C.J. Myonecrosis, myoglobinuria and acute renal failure induced by south american rattlesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon 1985, 23, 631–636. [Google Scholar] [CrossRef]
- Otero-Patiño, R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Lomonte, B.; Angulo, Y.; Fox, J.W. Tissue pathology induced by snake venoms: How to understand a complex pattern of alterations from a systems biology perspective? Toxicon 2010, 55, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev. Proteom. 2011, 8, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A 2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Ownby, C.L.; Fletcher, J.E.; Colberg, T.R. Cardiotoxin 1 from cobra (Naja naja atra) venom causes necrosis of skeletal muscle in vivo. Toxicon 1993, 31, 697–709. [Google Scholar] [CrossRef]
- Ownby, C.L.; Cameron, D.; Tu, A.T. Isolation of myotoxic component from rattlesnake (Crotalus viridis viridis) venom. Electron microscopic analysis of muscle damage. Am. J. Pathol. 1976, 85, 149–166. [Google Scholar] [PubMed]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Schiafino, S.; Partridge, T. Skeletal Muscle Repair and Regeneration. In Skeletal Muscle Repair and Regeneration; Springer: Berlin, Germany, 2008. [Google Scholar]
- Chargé, S.B.P.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Schiaffino, S. Regeneration of Mammalian Skeletal Muscle: Basic Mechanisms and Clinical Implications. Curr. Pharm. Des. 2010, 16, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Karalaki, M.; Fili, S.; Philippou, A.; Koutsilieris, M. Muscle regeneration: Cellular and molecular events. In Vivo 2009, 23, 779–796. [Google Scholar] [PubMed]
- Endo, T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone 2015, 80, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef] [PubMed]
- Ten Broek, R.W.; Grefte, S.; Von den Hoff, J.W. Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol. 2010, 224. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Inflammation in Skeletal Muscle Regeneration. In Skeletal Muscle Repair and Regeneration; Springer: Dordrecht, The Netherlands, 2008; pp. 243–268. [Google Scholar]
- Juban, G.; Chazaud, B. Metabolic regulation of macrophages during tissue repair: Insights from skeletal muscle regeneration. FEBS Lett. 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kharraz, Y.; Guerra, J.; Mann, C.J.; Serrano, A.L.; Muñoz-Cánoves, P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Med. Inflamm. 2013, 2013, 491497. [Google Scholar] [CrossRef] [PubMed]
- Brigitte, M.; Schilte, C.; Plonquet, A.; Baba-Amer, Y.; Henri, A.; Charlier, C.; Tajbakhsh, S.; Albert, M.; Gherardi, R.K.; Chrétien, F. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010, 62, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; McPhee, J.S.; Al-Dabbagh, S.; Stewart, C.E.; Al-Shanti, N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res. Rev. 2016, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Grounds, M.D. The role of tumor necrosis factor-alpha (TNF-alpha) in skeletal muscle regeneration. Studies in TNF-alpha(-/-) and TNF-alpha(-/-)/LT-alpha(-/-) mice. J. Histochem. Cytochem. 2001, 49, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Nguyen, M.-H.; Fantuzzi, G.; Koh, T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2008, 294, C1183–C1191. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. Anatomy of a Discovery: M1 and M2 Macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. Complexity of Extracellular Matrix and Skeletal Muscle Regeneration. In Skeletal Muscle Repair and Regeneration; Springer: Dordrecht, The Netherlands, 2008; pp. 269–302. [Google Scholar]
- Ohtake, Y.; Tojo, H.; Seiki, M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J. Cell Sci. 2006, 119, 3822–3832. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, E.; Moas, M.; Reznick, A.Z.; Coleman, R. Matrix metalloproteinases and skeletal muscle: A brief review. Muscle Nerve 2004, 29, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lluri, G.; Jaworski, D.M. Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve 2005, 32, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Leong, D.; Smith, L.R.; Barton, E.R. Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration. Am. J. Physiol. Cell Physiol. 2013, 305, C529–C538. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, H.; Murray, K.; Jefferson, B.S.; Li, Y. Matrix Metalloproteinase-1 Promotes Muscle Cell Migration and Differentiation. Am. J. Pathol. 2009, 174, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Snyman, C.; Niesler, C.U. MMP-14 in skeletal muscle repair. J. Muscle Res. Cell Motil. 2015, 36, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Chirco, R.; Liu, X.-W.; Jung, K.-K.; Kim, H.-R.C. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006, 25, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Muñoz-Cánoves, P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp. Cell Res. 2010, 316, 3050–3058. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Vracko, R.; Benditt, E.P. Basal lamina: The scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J. Cell Biol. 1972, 55, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.R.; Schiaffino, S. Innervation of Regenerating Muscle. In Skeletal Muscle Repair and Regeneration; Springer: Dordrecht, The Netherlands, 2008; pp. 303–334. [Google Scholar]
- França, F.O.S.; Málaque, C.M. Acidente botrópico. In Animais Peçonhentos no Brasil. Biologia, Clínica e Terapêutica dos Acidentes; Sarvier: São Paulo, Brazil, 2009; pp. 81–95. [Google Scholar]
- White, J. Envenomation. Prevention and treatment in Australia. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010; pp. 423–451. [Google Scholar]
- Sitprija, V.; Sitprija, S. Renal effects and injury induced by animal toxins. Toxicon 2012, 60, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Kosuge, T.; Okonogi, T.; Hattori, Z.; Sawai, Y. A histopathological study on arterial lesions caused by Habu (Trimeresurus flavoviridis) venom. Jpn. J. Exp. Med. 1967, 37, 323–336. [Google Scholar] [PubMed]
- Rivel, M.; Solano, D.; Herrera, M.; Vargas, M.; Villalta, M.; Segura, Á.; Arias, A.S.; León, G.; Gutiérrez, J.M. Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: An experimental study in mice. Toxicon 2016, 119, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Gutiérrez, J.; Beatriz Mesa, M.; Duque, E.; Rodríguez, O.; Luis Arango, J.; Gómez, F.; Toro, A.; Cano, F.; María Rodríguez, L.; et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef]
- Dart, R.C.; McNally, J.T.; Spaite, D.W.; Gustafson, R. The sequelae of pitviper poisoning in the United States. In Biology of the Pitvipers; Selva: Tyler, TX, USA, 1992; pp. 395–404. [Google Scholar]
- Jayawardana, S.; Gnanathasan, A.; Arambepola, C.; Chang, T. Chronic Musculoskeletal Disabilities following Snake Envenoming in Sri Lanka: A Population-Based Study. PLoS Negl. Trop. Dis. 2016, 10, e0005103. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Johnson, M.A. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharmacol. Physiol. 1978, 5, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Maltin, C.A. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br. J. Pharmacol. 1982, 76, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.A.; Harris, J.B.; Cullen, M.J. Regeneration of mammalian skeletal muscle following the injection of the snake-venom toxin, taipoxin. Cell Tissue Res. 1983, 232, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon 2003, 42, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Head, S.I.; Houweling, P.J.; Chan, S.; Chen, G.; Hardeman, E.C. Properties of regenerated mouse extensor digitorum longus muscle following notexin injury. Exp. Physiol. 2014, 99, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Duchen, L.W.; Excell, B.J.; Patel, R.; Smith, B. Changes in motor end-plates resulting from muscle fibre necrosis and regeneration. A light and electron microscopic study of the effects of the depolarizing fraction (cardiotoxin) of Dendroaspis jamesoni venom. J. Neurol. Sci. 1974, 21, 391–417. [Google Scholar] [CrossRef]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thépenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.M.; et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS ONE 2016, 11, e0147198. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, O.; Andolfi, G.; Tirone, M.; Iavarone, F.; Brunelli, S.; Minchiotti, G. Induction of Acute Skeletal Muscle Regeneration by Cardiotoxin Injection. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ownby, C.L.; Odell, G.V. Skeletal muscle regeneration after myonecrosis induced by crude venom and a myotoxin from the snake Bothrops asper (Fer-de-Lance). Toxicon 1984, 22, 719–731. [Google Scholar] [CrossRef]
- Hernández, R.; Cabalceta, C.; Saravia-Otten, P.; Chaves, A.; Gutiérrez, J.M.; Rucavado, A. Poor regenerative outcome after skeletal muscle necrosis induced by Bothrops asper venom: Alterations in microvasculature and nerves. PLoS ONE 2011, 6, e19834. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Núñez, J.; Díaz, C.; Cintra, A.C.; Homsi-Brandeburgo, M.I.; Giglio, J.R. Skeletal muscle degeneration and regeneration after injection of bothropstoxin-II, a phospholipase A2 isolated from the venom of the snake Bothrops jararacussu. Exp. Mol. Pathol. 1991, 55, 217–229. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Chaves, F.; Gené, J.A.; Lomonte, B.; Camacho, Z.; Schosinsky, K. Myonecrosis induced in mice by a basic myotoxin isolated from the venom of the snake Bothrops nummifer (jumping viper) from Costa Rica. Toxicon 1989, 27, 735–745. [Google Scholar] [CrossRef]
- Salvini, T.F.; Morini, C.C.; Selistre de Araújo, H.S.; Ownby, C.L. Long-term regeneration of fast and slow murine skeletal muscles after induced injury by ACL myotoxin isolated from Agkistrodon contortrix laticinctus (broad-banded copperhead) venom. Anat. Rec. 1999, 254, 521–533. [Google Scholar] [CrossRef]
- Conte, T.C.; Franco, D.V.; Baptista, I.L.; Bueno, C.R.; Selistre-de-Araújo, H.S.; Brum, P.C.; Moriscot, A.S.; Miyabara, E.H. Radicicol improves regeneration of skeletal muscle previously damaged by crotoxin in mice. Toxicon 2008, 52, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Arce, V.; Brenes, F.; Chaves, F. Changes in myofibrillar components after skeletal muscle necrosis induced by a myotoxin isolated from the venom of the snake Bothrops asper. Exp. Mol. Pathol. 1990, 52, 25–36. [Google Scholar] [CrossRef]
- Rucavado, A.; Escalante, T.; Teixeira, C.F.P.; Fernándes, C.M.; Diaz, C.; Gutiérrez, J.M. Increments in cytokines and matrix metalloproteinases in skeletal muscle after injection of tissue-damaging toxins from the venom of the snake Bothrops asper. Med. Inflamm. 2002, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Nicolau, C.A.; Escalante, T.; Kim, J.; Herrera, C.; Gutiérrez, J.M.; Fox, J.W. Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway. Toxins 2016, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.D.F.; Lopes, D.D.S.; Mendes, M.M.; Homsi-Brandeburgo, M.I.; Hamaguchi, A.; de Alcântara, T.M.; Clissa, P.B.; Rodrigues, V.D.M. Insights of local tissue damage and regeneration induced by BnSP-7, a myotoxin isolated from Bothrops (neuwiedi) pauloensis snake venom. Toxicon 2009, 53, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Cury, Y.; Moreira, V.; Picolo, G.; Chaves, F. Inflammation induced by Bothrops asper venom. Toxicon 2009, 54, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Macêdo, J.K.A.; Feoli, A.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Muscle Tissue Damage Induced by the Venom of Bothrops asper: Identification of Early and Late Pathological Events through Proteomic Analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004599. [Google Scholar] [CrossRef] [PubMed]
- Saravia-Otten, P.; Robledo, B.; Escalante, T.; Bonilla, L.; Rucavado, A.; Lomonte, B.; Hernández, R.; Flock, J.I.; Gutiérrez, J.M.; Gastaldello, S. Homogenates of skeletal muscle injected with snake venom inhibit myogenic differentiation in cell culture. Muscle Nerve 2013, 47, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Klein-Ogus, C.; Harris, J.B. Preliminary observations of satellite cells in undamaged fibres of the rat soleus muscle assaulted by a snake-venom toxin. Cell Tissue Res. 1983, 230, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Morini, C.C.; Pereira, E.C.; Selistre de Araújo, H.S.; Ownby, C.L.; Salvini, T.F. Injury and recovery of fast and slow skeletal muscle fibers affected by ACL myotoxin isolated from Agkistrodon contortrix laticinctus (Broad-Banded copperhead) venom. Toxicon 1998, 36, 1007–1024. [Google Scholar] [CrossRef]
- Vignaud, A.; Hourdé, C.; Torres, S.; Caruelle, J.P.; Martelly, I.; Keller, A.; Ferry, A. Functional, cellular and molecular aspects of skeletal muscle recovery after injury induced by snake venom from Notechis scutatus scutatus. Toxicon 2005, 45, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Couteaux, R.; Mira, J.C.; d’Albis, A. Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol. Cell 1988, 62, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Arce, V.; Brenes, F.; Gutiérrez, J.M. Degenerative and regenerative changes in murine skeletal muscle after injection of venom from the snake Bothrops asper: A histochemical and immunocytochemical study. Int. J. Exp. Pathol. 1991, 72, 211–226. [Google Scholar] [PubMed]
- Queiróz, L.S.; Marques, M.J.; Santo Neto, H. Acute local nerve lesions induced by Bothrops jararacussu snake venom. Toxicon 2002, 40, 1483–1486. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Santo Neto, H.; Rodrigues-Simioni, L.; Prado-Franceschi, J. Muscle necrosis and regeneration after envenomation by Bothrops jararacussu snake venom. Toxicon 1984, 22, 339–346. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Petta, C.A. Histopathological changes caused by venom of urutu snake (Bothrops alternatus) in mouse skeletal muscle. Rev. Inst. Med. Trop. Sao Paulo 1984, 26, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Teibler, P.; Acosta de Pérez, O.; Maruñak, S.; Sanchez Negrette, M.; Ortega, H. Muscular regeneration after myonecrosis induced by Bothrops jararacussu snake venom from Argentina. Biocell 2001, 25, 257–264. [Google Scholar] [PubMed]
- Santo Neto, H.; Vomero, V.U.; Marques, M.J. Insights into the loss of muscle mass following B. jararacussu venom in mice. Toxicon 2004, 44, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: Biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteomics 2014, 105, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Fox, J.W.; Gutiérrez, J.M. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteomics 2011, 74, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A Comprehensive View of the Structural and Functional Alterations of Extracellular Matrix by Snake Venom Metalloproteinases (SVMPs): Novel Perspectives on the Pathophysiology of Envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Escalante, T.; Gutiérrez, J.M.; Rucavado, A. Skin Pathology Induced by Snake Venom Metalloproteinase: Acute Damage, Revascularization, and Re-epithelization in a Mouse Ear Model. J. Investig. Dermatol. 2008, 128, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Lomonte, B.; Ovadia, M.; Gutiérrez, J.M. Local Tissue Damage Induced by BaP1, a Metalloproteinase Isolated from Bothrops asper (Terciopelo) Snake Venom. Exp. Mol. Pathol. 1995, 63, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Ownby, C.L.; Kainer, R.A.; Tu, A.T. Pathogenesis of hemorrhage induced by rattlesnake venom. An electron microscopic study. Am. J. Pathol. 1974, 76, 401–414. [Google Scholar] [PubMed]
- Queiroz, L.S.; Santo Neto, H.; Assakura, M.T.; Reichl, A.P.; Mandelbaum, F.R. Pathological changes in muscle caused by haemorrhagic and proteolytic factors from Bothrops jararaca snake venom. Toxicon 1985, 23, 341–345. [Google Scholar] [CrossRef]
- Nagaraju, S.; Kemparaju, K.; Girish, K. Hyaluronidases, a Neglected Class of Glycosidases from Snake Venom. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2009; pp. 237–258. [Google Scholar]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Santo Neto, H.; Marques, M.J. Microvessel damage by B. jararacussu snake venom: Pathogenesis and influence on muscle regeneration. Toxicon 2005, 46, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Pinto, A.F.M.; Terra, R.M.S.; Gutiérrez, J.M.; Fox, J.W. Wound exudate as a proteomic window to reveal different mechanisms of tissue damage by snake venom toxins. J. Proteome Res. 2009, 8, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Ortiz, N.; Rucavado, A.; Sanchez, E.F.; Richardson, M.; Fox, J.W.; Gutiérrez, J.M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Tu, A.T. Morphology of local tissue damage in experimental snake envenomation. Br. J. Exp. Pathol. 1971, 52, 538–542. [Google Scholar] [PubMed]
- Grounds, M.D. Towards Understanding Skeletal Muscle Regeneration. Pathol. Res. Pract. 1991, 187, 1–22. [Google Scholar] [CrossRef]
- Chaillou, T.; Koulmann, N.; Meunier, A.; Chapot, R.; Serrurier, B.; Beaudry, M.; Bigard, X. Effect of hypoxia exposure on the recovery of skeletal muscle phenotype during regeneration. Mol. Cell. Biochem. 2014, 390, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.P.; Zamunér, S.R.; Zuliani, J.P.; Fernandes, C.M.; Cruz-Hofling, M.A.; Fernandes, I.; Chaves, F.; Gutiérrez, J.M. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 2003, 28, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Romero, M.; Núñez, J.; Chaves, F.; Borkow, G.; Ovadia, M. Skeletal muscle necrosis and regeneration after injection of BaH1, a hemorrhagic metalloproteinase isolated from the venom of the snake Bothrops asper (Terciopelo). Exp. Mol. Pathol. 1995, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Tontonoz, P.; Spiegelman, B.M. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc. Natl. Acad. Sci. USA 1995, 92, 9856–9860. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Foster, W.; Deasy, B.M.; Chan, Y.; Prisk, V.; Tang, Y.; Cummins, J.; Huard, J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. Am. J. Pathol. 2004, 164, 1007–1019. [Google Scholar] [CrossRef]
- Rucavado, A.; Escalante, T.; Shannon, J.; Gutiérrez, J.M.; Fox, J.W. Proteomics of wound exudate in snake venom-induced pathology: Search for biomarkers to assess tissue damage and therapeutic success. J. Proteom. Res. 2011, 10, 1987–2005. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, G.; Shi, D.; Zhu, R.; Zeng, H.; Cao, B.; Huang, M.; Liao, H. Effects of nitric oxide on notexin-induced muscle inflammatory responses. Int. J. Biol. Sci. 2015, 11, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Neto, H.S.; Vomero, V.U.; Marques, M.J. l-arginine enhances muscle regeneration after experimental envenomation by B. jararacussu: A future for nitric oxide-based therapy? Toxicon 2006, 48, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Vomero, V.U.; Marques, M.J.; Neto, H.S. Treatment with an anti-inflammatory drug is detrimental for muscle regeneration at Bothrops jararacussu envenoming: An experimental study. Toxicon 2009, 54, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Lesault, P.-F.; Theret, M.; Magnan, M.; Cuvellier, S.; Niu, Y.; Gherardi, R.K.; Tremblay, J.P.; Hittinger, L.; Chazaud, B. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS ONE 2012, 7, e46698. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.W.A.; Gonçalves, W.; Garrido Cavalcante, W.L.; Pai-Silva, M.D.; Gallacci, M. Nandrolone stimulates MyoD expression during muscle regeneration in the condition of myonecrosis induced by Bothrops jararacussu venom poisoning. J. Toxicol. Environ. Health. A 2010, 73, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Tangherlini, F.; Rudy, S.; Lee, D.; Toraldo, G.; Sandor, N.L.; Zhang, A.; Jasuja, R.; Bhasin, S. Testosterone improves the regeneration of old and young mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakamura, T.; Fujihara, S.; Tanaka, E. Ultrasound modulates the inflammatory response and promotes muscle regeneration in injured muscles. Ann. Biomed. Eng. 2013, 41, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Saturnino-Oliveira, J.; Tomaz, M.A.; Fonseca, T.F.; Gaban, G.A.; Monteiro-Machado, M.; Strauch, M.A.; Cons, B.L.; Calil-Elias, S.; Martinez, A.M.B.; Melo, P.A. Pulsed ultrasound therapy accelerates the recovery of skeletal muscle damage induced by Bothrops jararacussu venom. Br. J. Med. Biol. Res. 2012, 45, 488–496. [Google Scholar] [CrossRef]
- Silva, L.H.; Silva, M.T.; Gutierrez, R.M.; Conte, T.C.; Toledo, C.A.; Aoki, M.S.; Liebano, R.E.; Miyabara, E.H. GaAs 904-nm laser irradiation improves myofiber mass recovery during regeneration of skeletal muscle previously damaged by crotoxin. Lasers Med. Sci. 2012, 27, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Aranha de Sousa, E.; Bittencourt, J.A.H.M.; Seabra de Oliveira, N.K.; Correia Henriques, S.V.; dos Santos Picanço, L.C.; Lobato, C.P.; Ribeiro, J.R.; Pereira, W.L.A.; Carvalho, J.C.T.; da Silva, J.O. Effects of a low-level semiconductor gallium arsenide laser on local pathological alterations induced by Bothrops moojeni snake venom. Photochem. Photobiol. Sci. 2013, 12, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Li, Y.; Zhu, J.; Ambrosio, F.; Uehara, K.; Fu, F.H.; Huard, J. Improved Muscle Healing after Contusion Injury by the Inhibitory Effect of Suramin on Myostatin, a Negative Regulator of Muscle Growth. Am. J. Sports Med. 2008, 36, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Thind, G.S.; Agrawal, P.R.; Hirsh, B.; Saravolatz, L.; Chen-Scarabelli, C.; Narula, J.; Scarabelli, T.M. Mechanisms of myocardial ischemia-reperfusion injury and the cytoprotective role of minocycline: Scope and limitations. Future Cardiol. 2015, 11, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, X.; He, B.; Xu, C.; Wu, L.; Cui, B.; Wen, H.; Lu, Z.; Jiang, H. Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur. J. Pharmacol. 2010, 638, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Lee, O.-H.; Lee, B.-Y. Low-molecular-weight fucoidan regulates myogenic differentiation through the mitogen-activated protein kinase pathway in C2C12 cells. Br. J. Nutr. 2011, 106, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.E.; Meddahi-Pellé, A.; Ho-Tin-Noe, B.; Colliec-Jouault, S.; Guezennec, J.; Louedec, L.; Prats, H.; Jacob, M.P.; Osborne-Pellegrin, M.; Letourneur, D.; et al. Low-Molecular-Weight Fucoidan Promotes Therapeutic Revascularization in a Rat Model of Critical Hindlimb Ischemia. J. Pharmacol. Exp. Ther. 2003, 305, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.R.; Drucker, N.A.; Khaneki, S.; Ferkowicz, M.J.; Yoder, M.C.; DeLeon, E.R.; Olson, K.R.; Markel, T.A. Hydrogen Sulfide. Shock 2017, 48, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.M.; Van Goor, H.; Joles, J.A.; Whiteman, M.; Leuvenink, H.G.D. Hydrogen sulfide: Physiological properties and therapeutic potential in ischaemia. Br. J. Pharmacol. 2015, 172, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ota, S.; Terada, S.; Kawakami, Y.; Otsuka, T.; Fu, F.H.; Huard, J. The Combined Use of Losartan and Muscle-Derived Stem Cells Significantly Improves the Functional Recovery of Muscle in a Young Mouse Model of Contusion Injuries. Am. J. Sports Med. 2016, 44, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Fakhfakh, R.; Lamarre, Y.; Skuk, D.; Tremblay, J.P. Losartan enhances the success of myoblast transplantation. Cell Transplant. 2012, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.E.; Knapp, J.R.; Hodges, B.L.; Wuebbles, R.D.; Burkin, D.J. Laminin-111 protein therapy reduces muscle pathology and improves viability of a mouse model of merosin-deficient congenital muscular dystrophy. Am. J. Pathol. 2012, 180, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Riederer, I.; Bonomo, A.C.; Mouly, V.; Savino, W. Laminin therapy for the promotion of muscle regeneration. FEBS Lett. 2015, 589, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, B.; Chun-Lansinger, Y.; Hagen, T.; Ingham, S.J.; Wright, V.; Fu, F.; Huard, J. Biological approaches to improve skeletal muscle healing after injury and disease. Birth Defects Res. C Embryo Today. 2015, 96, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.; Morgan, J.E. Recent progress in satellite cell/myoblast engraftment—Relevance for therapy. FEBS J. 2013, 280, 4281–4293. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Ota, S.; Kobayashi, M.; Kobayashi, T.; Mifune, Y.; Takayama, K.; Witt, M.; Vadalà, G.; Oyster, N.; Otsuka, T.; et al. Use of an Antifibrotic Agent Improves the Effect of Platelet-Rich Plasma on Muscle Healing After Injury. J. Bone Jt. Surg. Am. Vol. 2013, 95, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.S.A.; Yusof, A.; Mohamed Ali, M.R. Platelet-rich plasma (PRP) for acute muscle injury: A systematic review. PLoS ONE 2014, 9, e90538. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; León, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]





| Agents | Selected Actions for Improving Regeneration | References |
|---|---|---|
| Suramin | Antagonist of transforming growth factor-β (TGF-β). Stimulates myoblast differentiation and inhibits myostatin expression. | [114,115] |
| Minocycline | Protects against ischemia-reperfusion injury. Exerts anti-inflammatory effects. | [116,117,118] |
| Fucoidan | Promotes revascularization after ischemia. Regulates myogenic differentiation in vitro. | [119,120] |
| NaHS | H2S donor. Has pro-angiogenic properties after ischemia. Increases the expression of angiogenic factors. | [121,122] |
| Losartan | Inhibits TGF-β1 and reduces deposition of fibrotic tissue. | [123,124] |
| Laminin 111 | Increases the expression of α7β1 integrin-type laminin receptor. Systemic administration in merosin-deficient congenital muscular dystrophy type 1A (MDC1A) prevents muscle pathology. | [125,126] |
| Stem cells/myoblast transplant | Repair of damaged skeletal muscle fibers by directly differentiating into myofibers and secretion of paracrine factors that promote tissue repair. | [127,128] |
| Platelet-rich plasma | Combination therapy with an anti-fibrotic agent improves skeletal muscle healing. | [129,130] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, J.M.; Escalante, T.; Hernández, R.; Gastaldello, S.; Saravia-Otten, P.; Rucavado, A. Why is Skeletal Muscle Regeneration Impaired after Myonecrosis Induced by Viperid Snake Venoms? Toxins 2018, 10, 182. https://doi.org/10.3390/toxins10050182
Gutiérrez JM, Escalante T, Hernández R, Gastaldello S, Saravia-Otten P, Rucavado A. Why is Skeletal Muscle Regeneration Impaired after Myonecrosis Induced by Viperid Snake Venoms? Toxins. 2018; 10(5):182. https://doi.org/10.3390/toxins10050182
Chicago/Turabian StyleGutiérrez, José María, Teresa Escalante, Rosario Hernández, Stefano Gastaldello, Patricia Saravia-Otten, and Alexandra Rucavado. 2018. "Why is Skeletal Muscle Regeneration Impaired after Myonecrosis Induced by Viperid Snake Venoms?" Toxins 10, no. 5: 182. https://doi.org/10.3390/toxins10050182
APA StyleGutiérrez, J. M., Escalante, T., Hernández, R., Gastaldello, S., Saravia-Otten, P., & Rucavado, A. (2018). Why is Skeletal Muscle Regeneration Impaired after Myonecrosis Induced by Viperid Snake Venoms? Toxins, 10(5), 182. https://doi.org/10.3390/toxins10050182