Staphylococcal Enterotoxin A Induces Intestinal Barrier Dysfunction and Activates NLRP3 Inflammasome via NF-κB/MAPK Signaling Pathways in Mice
Abstract
:1. Introduction
2. Results
2.1. Preparation of SEA Protein
2.2. SEA Induced Histological Injury in the Small Intestine of Mice
2.3. SEA Suppressed the Expression of Tight Junction Proteins of Small Intestine in Mice
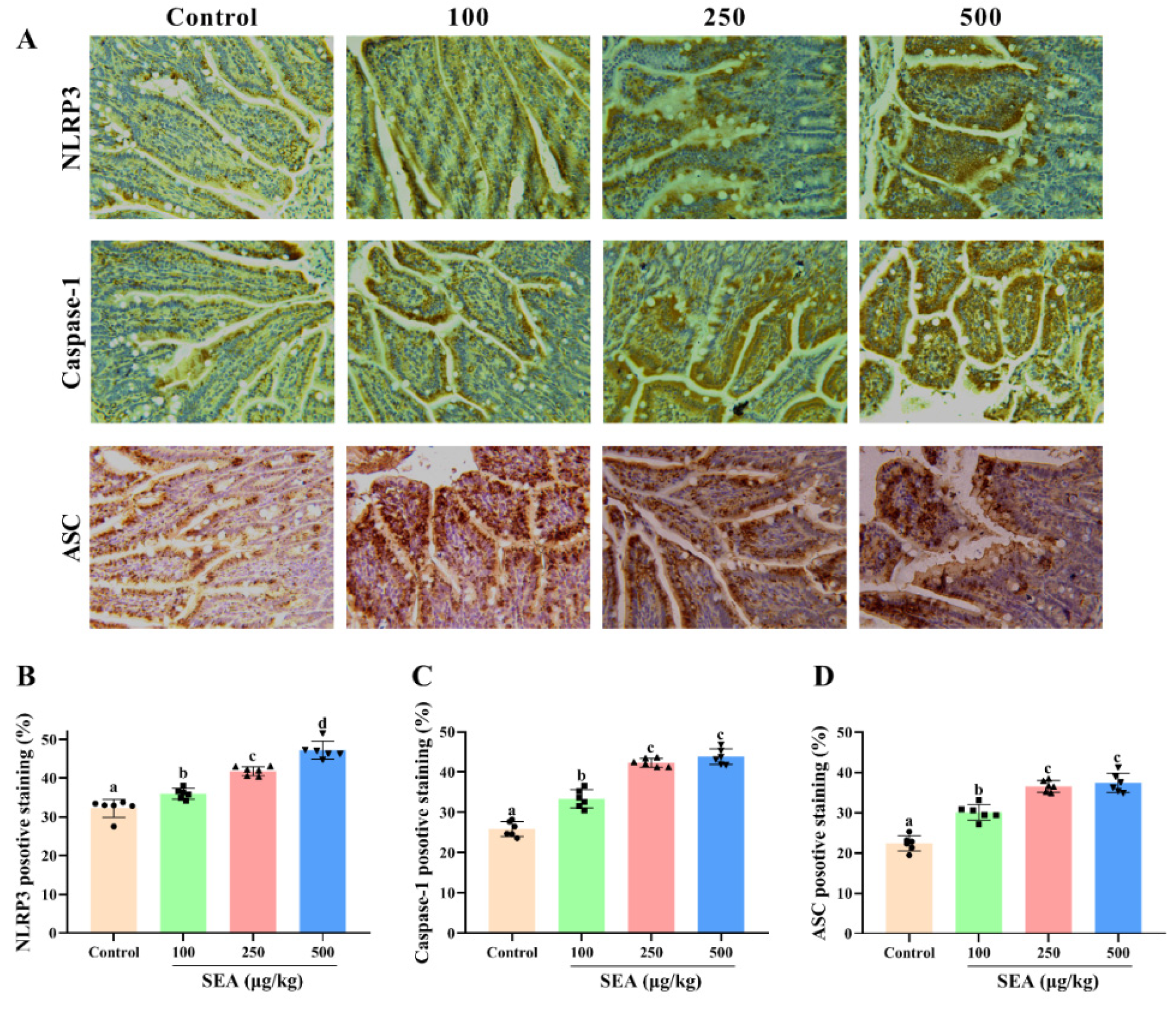
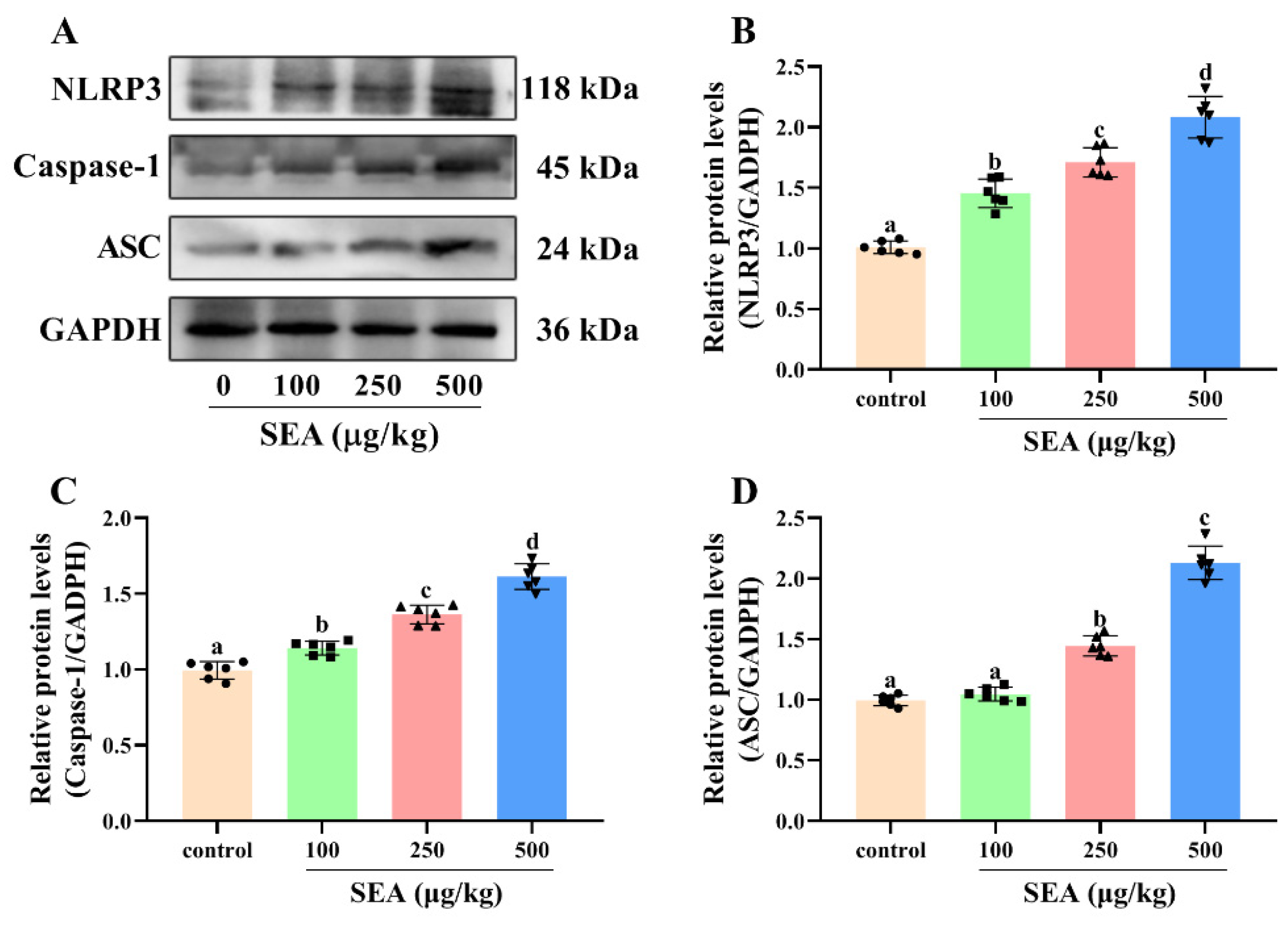
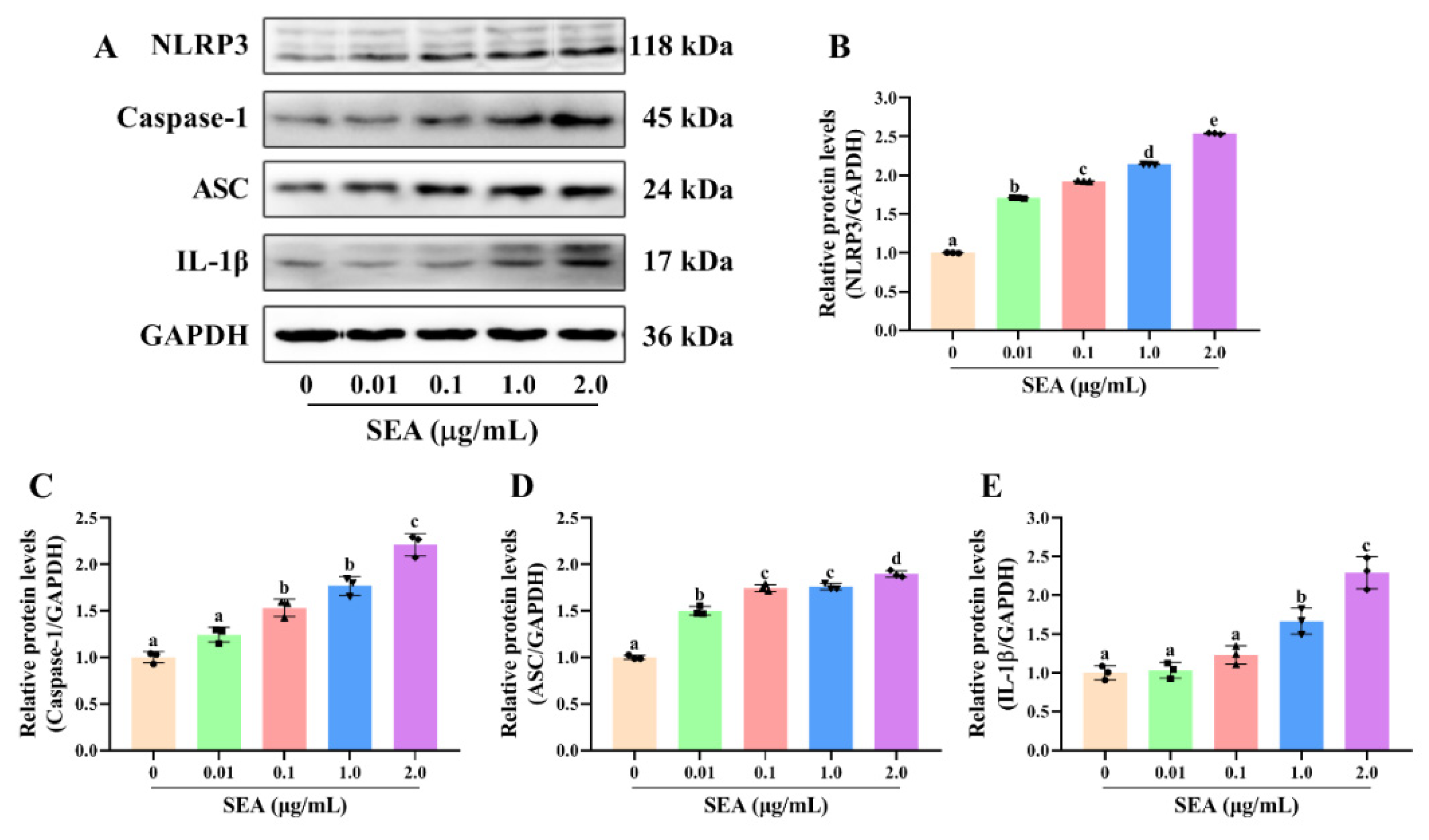
2.4. SEA Activated NLRP3 Inflammasome in Jejunum Tissues
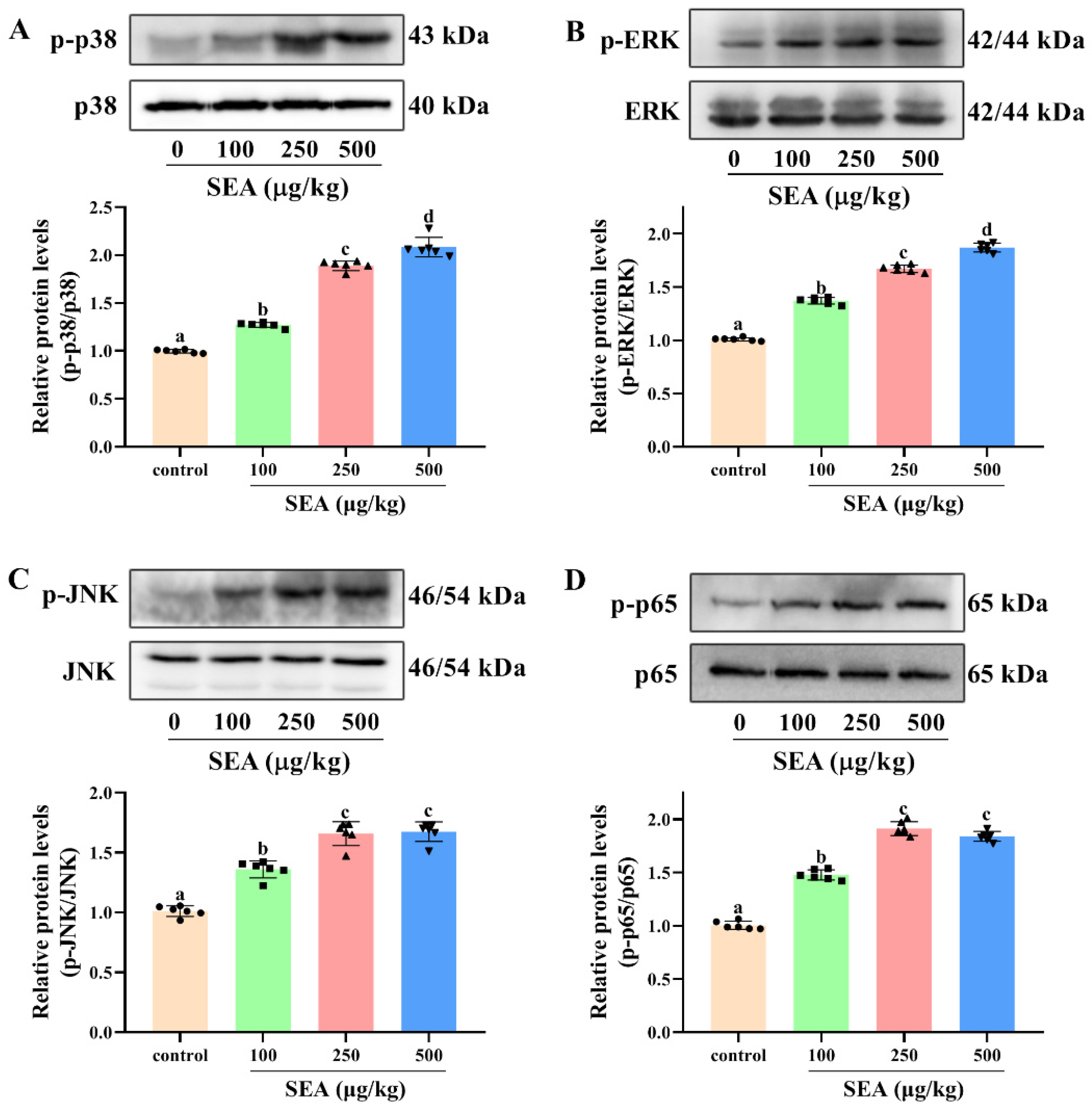
2.5. SEA Induced the Phosphorylation of NF-κB p65 and Activation of the MAPK Pathway in Jejunum Tissues
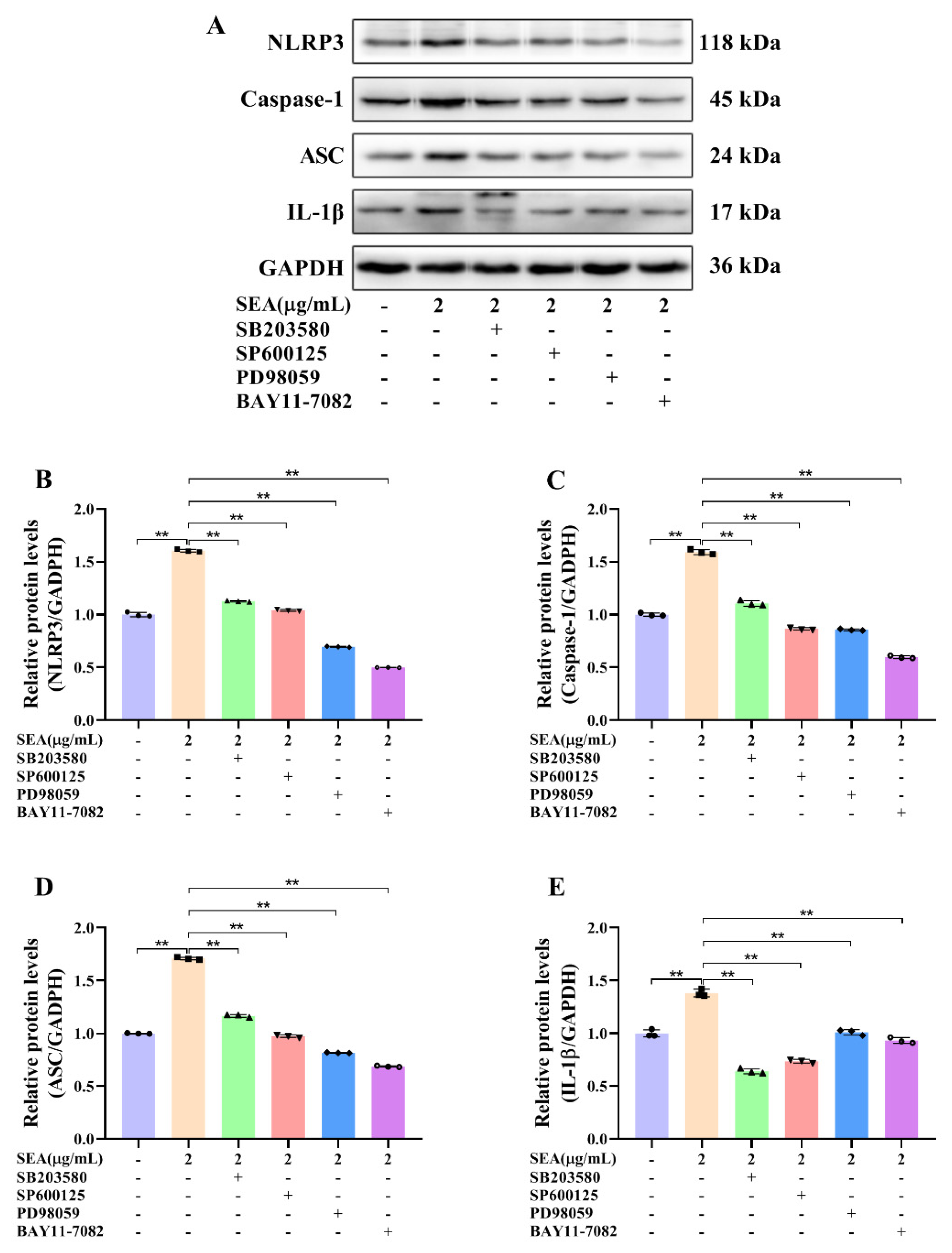
2.6. SEA Activated NLRP3 Inflammasome via the NF-κB/MAPK Signaling Pathways
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Expression and Purification of SEA
5.3. Animals and Experimental Design
5.4. Histopathological Analysis
5.5. Immunohistochemical (IHC) Analysis
5.6. Western Blot Analysis
5.7. ELISA Assay
5.8. Cell Culture
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grispoldi, L.; Karama, M.; Armani, A.; Hadjicharalambous, C.; Cenci-Goga, B.T. Staphylococcus aureus enterotoxin in food of animal origin and staphylococcal food poisoning risk assessment from farm to table. Ital. J. Anim. Sci. 2021, 20, 677–690. [Google Scholar] [CrossRef]
- Hirose, S.; Ono, H.K.; Omoe, K.; Hu, D.L.; Asano, K.; Yamamoto, Y.; Nakane, A. Goblet cells are involved in translocation of staphylococcal enterotoxin A in the intestinal tissue of house musk shrew (Suncus murinus). J. Appl. Microbiol. 2016, 120, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. Biomed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [Green Version]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. Fems. Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bencardino, D.; Amagliani, G.; Brandi, G. Carriage of Staphylococcus aureus among food handlers: An ongoing challenge in public health. Food Control 2021, 130, 108362. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. GMR 2003, 2, 63–76. [Google Scholar] [PubMed]
- Tarisse, C.F.; Goulard-Huet, C.; Nia, Y.; Devilliers, K.; Marce, D.; Dambrune, C.; Lefebvre, D.; Hennekinne, J.A.; Simon, S. Highly sensitive and specific detection of staphylococcal enterotoxins SEA, SEG, SEH, and SEI by immunoassay. Toxins 2021, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Argudin, M.A.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.K.; Hirose, S.; Narita, K.; Sugiyama, M.; Asano, K.; Hu, D.L.; Nakane, A. Histamine release from intestinal mast cells induced by staphylococcal enterotoxin A (SEA) evokes vomiting reflex in common marmoset. PLoS Pathog. 2019, 15, e1007803. [Google Scholar] [CrossRef] [Green Version]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Shimamura, Y.; Noaki, R.; Kurokawa, A.; Utsumi, M.; Hirai, C.; Kan, T.; Masuda, S. Effect of (−)-epigallocatechin gallate on activation of JAK/STAT signaling pathway by staphylococcal enterotoxin A. Toxins 2021, 13, 609. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Yue, D.; Wang, Z.; Yang, Y.; Hu, Z.; Luo, G.; Wang, F. EZH2 inhibitor GSK343 inhibits sepsis-induced intestinal disorders. Exp. Ther. Med. 2021, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, T.N.; El-Maadawy, W.H.; Kandil, Z.A.; Khalil, H.; El-Fiky, N.M.; El Alfy, T. Canna x generalis L.H. Bailey rhizome extract ameliorates dextran sulfate sodium-induced colitis via modulating intestinal mucosal dysfunction, oxidative stress, inflammation, and TLR4/ NF-B and NLRP3 inflammasome pathways. J. Ethnopharmacol. 2021, 269, 113670. [Google Scholar] [CrossRef]
- Serreli, G.; Melis, M.P.; Zodio, S.; Naitza, M.R.; Casula, E.; Penalver, P.; Lucas, R.; Loi, R.; Morales, J.C.; Deiana, M. Altered paracellular permeability in intestinal cell monolayer challenged with lipopolysaccharide: Modulatory effects of pterostilbene metabolites. Food Chem. Toxicol. 2020, 145, 111729. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, B.R.; Zhang, Q.; Zhao, X.H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef]
- Kim, T.I. The role of barrier dysfunction and change of claudin expression in inflammatory bowel disease. Gut Liver 2015, 9, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Yu, R.; Zhou, Q.; Liu, P.; Liu, Z.; Bian, Y. Naringenin prevents TNF-alpha-induced gut-vascular barrier disruption associated with inhibiting the NF-kappaB-mediated MLCK/p-MLC and NLRP3 pathways. Food Funct. 2021, 12, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Ma, P.; Zhang, Y.; Mi, Y.; Fan, D.D. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway. Int. Immunopharmacol. 2020, 85, 106645. [Google Scholar] [CrossRef]
- Lee, P.Y.; Liu, C.C.; Wang, S.C.; Chen, K.Y.; Lin, T.C.; Liu, P.L.; Chiu, C.C.; Chen, I.C.; Lai, Y.H.; Cheng, W.C.; et al. Mycotoxin zearalenone attenuates innate immune responses and suppresses NLRP3 inflammasome activation in LPS-activated macrophages. Toxins 2021, 13, 593. [Google Scholar] [CrossRef]
- Zhao, W.M.; Ma, L.; Cai, C.; Gong, X.H. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-kappa B and A2aR signaling in LPS-induced THP-1 macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-kappaB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.-J. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, J. Staphylococcal enterotoxin M causes intestine dysfunction via activating inflammation. J. Food Saf. 2018, 38, e12465. [Google Scholar] [CrossRef]
- Larcombe, S.; Jiang, J.H.; Hutton, M.L.; Abud, H.E.; Peleg, A.Y.; Lyras, D. A mouse model of Staphylococcus aureus small intestinal infection. J. Med. Microbiol. 2020, 69, 290–297. [Google Scholar] [CrossRef]
- Cui, Y.J.; Okyere, S.K.; Gao, P.; Wen, J.; Cao, S.Z.; Wang, Y.; Deng, J.L.; Hu, Y.C. Ageratina adenophora disrupts the intestinal structure and immune barrier integrity in rats. Toxins 2021, 13, 651. [Google Scholar] [CrossRef]
- Colucci, R.; Pellegrini, C.; Fornai, M.; Tirotta, E.; Antonioli, L.; Renzulli, C.; Ghelardi, E.; Piccoli, E.; Gentile, D.; Benvenuti, L.; et al. Pathophysiology of NSAID-associated intestinal lesions in the rat: Luminal bacteria and mucosal inflammation as targets for prevention. Front. Pharmacol. 2018, 9, 1340. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, D.; Liao, P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-kappaB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, Y.; Tian, L.; Yang, M.; He, S.; Liu, Y.; Khan, A.; Li, Y.; Cao, J.; Cheng, G. Anneslea fragrans Wall. ameliorates ulcerative colitis via inhibiting NF-kappaB and MAPK activation and mediating intestinal barrier integrity. J. Ethnopharmacol. 2021, 278, 114304. [Google Scholar] [CrossRef]
- Zhong, G.; Wan, F.; Lan, J.; Jiang, X.; Wu, S.; Pan, J.; Tang, Z.; Hu, L. Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci. Total Environ. 2021, 788, 147780. [Google Scholar] [CrossRef]
- Zhao, L.; Geng, T.; Sun, K.; Su, S.; Zhao, Y.; Bao, N.; Pan, L.; Sun, H.; Li, M. Proteomic analysis reveals the molecular mechanism of Hippophae rhamnoides polysaccharide intervention in LPS-induced inflammation of IPEC-J2 cells in piglets. Int. J. Biol. Macromol. 2020, 164, 3294–3304. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Zhong, J.; Zhou, Q.; Zhong, Y.; Liu, P.; Liu, Z.J. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int. Immunopharmacol. 2019, 71, 321–327. [Google Scholar] [CrossRef]
- Zmora, N.; Levy, M.; Pevsner-Fischer, M.; Elinav, E. Inflammasomes and intestinal inflammation. Mucosal Immunol. 2017, 10, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Lin, Z.; Le, G.; Hou, L.; Mao, X.; Liu, S.; Liu, D.; Gan, F.; Huang, K. Nontoxic-dose deoxynivalenol aggravates lipopolysaccharides-induced inflammation and tight junction disorder in IPEC-J2 cells through activation of NF-kappaB and LC3B. Food Chem. Toxicol. 2020, 145, 111712. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Yang, L.; Park, H.J.; Li, J. Dietary intake of Lycium ruthenicum Murray ethanol extract inhibits colonic inflammation in dextran sulfate sodium-induced murine experimental colitis. Food Funct. 2020, 11, 2924–2937. [Google Scholar] [CrossRef]
- Kebaier, C.; Chamberland, R.R.; Allen, I.C.; Gao, X.; Broglie, P.M.; Hall, J.D.; Jania, C.; Doerschuk, C.M.; Tilley, S.L.; Duncan, J.A. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 2012, 205, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.C.; Jiang, J.L.; Chen, T.T.; Xu, D.Y.; Hou, F.Q.; Huang, Q.Y.; Peng, Y.Y.; Ye, C.; Hu, D.L.; Fang, R.D. Toxic Shock Syndrome Toxin 1 Induces Immune Response via the Activation of NLRP3 Inflammasome. Toxins 2021, 13, 68. [Google Scholar] [CrossRef]
- Liao, P.; Li, Y.H.; Li, M.J.; Chen, X.F.; Yuan, D.X.; Tang, M.; Xu, K. Baicalin alleviates deoxynivalenol-induced intestinal inflammation and oxidative stress damage by inhibiting NF-kappa B and increasing mTOR signaling pathways in piglets. Food Chem. Toxicol. 2020, 140, 111326. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cheng, J.Z.; Zhu, S.G.; Zhao, J.; Ye, Q.Y.; Xu, Y.Y.; Dong, H.L.; Zheng, X.H. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Xie, M.H.; Chen, G.J.; Dai, Z.Q.; Hu, B.; Zeng, X.; Sun, Y. Anti-inflammatory effects of dicaffeoylquinic acids from Ilex kudingcha on lipopolysaccharide-treated RAW264.7 macrophages and potential mechanisms. Food Chem. Toxicol. 2019, 126, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Chi, D.H.; Bae, C.H.; Kim, Y.D. Staphylococcus enterotoxin A induces MUC5B expression via Toll-like receptor 2, extracellular signal-regulated kinase 1/2, and p38 mitogen-activated protein kinase in human airway epithelial cells. Am. J. Rhinol. Allergy 2014, 28, E25–E30. [Google Scholar] [CrossRef]
- Shao, D.Z.; Lee, J.J.; Huang, W.T.; Liao, J.F.; Lin, M.T. Inhibition of nuclear factor-kappa B prevents staphylococcal enterotoxin A-induced fever. Mol. Cell. Biochem. 2004, 262, 177–185. [Google Scholar] [CrossRef]
- Mudili, V.; Makam, S.S.; Sundararaj, N.; Siddaiah, C.; Gupta, V.K.; Rao, P.V.L. A novel IgY-Aptamer hybrid system for cost-effective detection of SEB and its evaluation on food and clinical samples. Sci. Rep. 2015, 5, 15151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.N.; Yuan, F.; Luo, Y.J.; Wang, J.F.; Zhang, C.B.; Zhou, W.E.; Ren, Z.Q.; Wu, W.J.; Zhang, F. Biosynthesis of staphylococcal enterotoxin A by genetic engineering technology and determination of staphylococcal enterotoxin A in water by HPLC-ESI-TOF. Environ. Sci. Pollut. Res. Int. 2017, 24, 19375–19385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chi, K.; Yang, M.; Guo, N. Staphylococcal Enterotoxin A Induces Intestinal Barrier Dysfunction and Activates NLRP3 Inflammasome via NF-κB/MAPK Signaling Pathways in Mice. Toxins 2022, 14, 29. https://doi.org/10.3390/toxins14010029
Liu C, Chi K, Yang M, Guo N. Staphylococcal Enterotoxin A Induces Intestinal Barrier Dysfunction and Activates NLRP3 Inflammasome via NF-κB/MAPK Signaling Pathways in Mice. Toxins. 2022; 14(1):29. https://doi.org/10.3390/toxins14010029
Chicago/Turabian StyleLiu, Chunmei, Kunmei Chi, Meng Yang, and Na Guo. 2022. "Staphylococcal Enterotoxin A Induces Intestinal Barrier Dysfunction and Activates NLRP3 Inflammasome via NF-κB/MAPK Signaling Pathways in Mice" Toxins 14, no. 1: 29. https://doi.org/10.3390/toxins14010029



