Long-Term Exposure to Microcystin-LR Induces Gastric Toxicity by Activating the Mitogen-Activated Protein Kinase Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Characteristics
2.2. MC-LR Concentration in Gastric Tissue
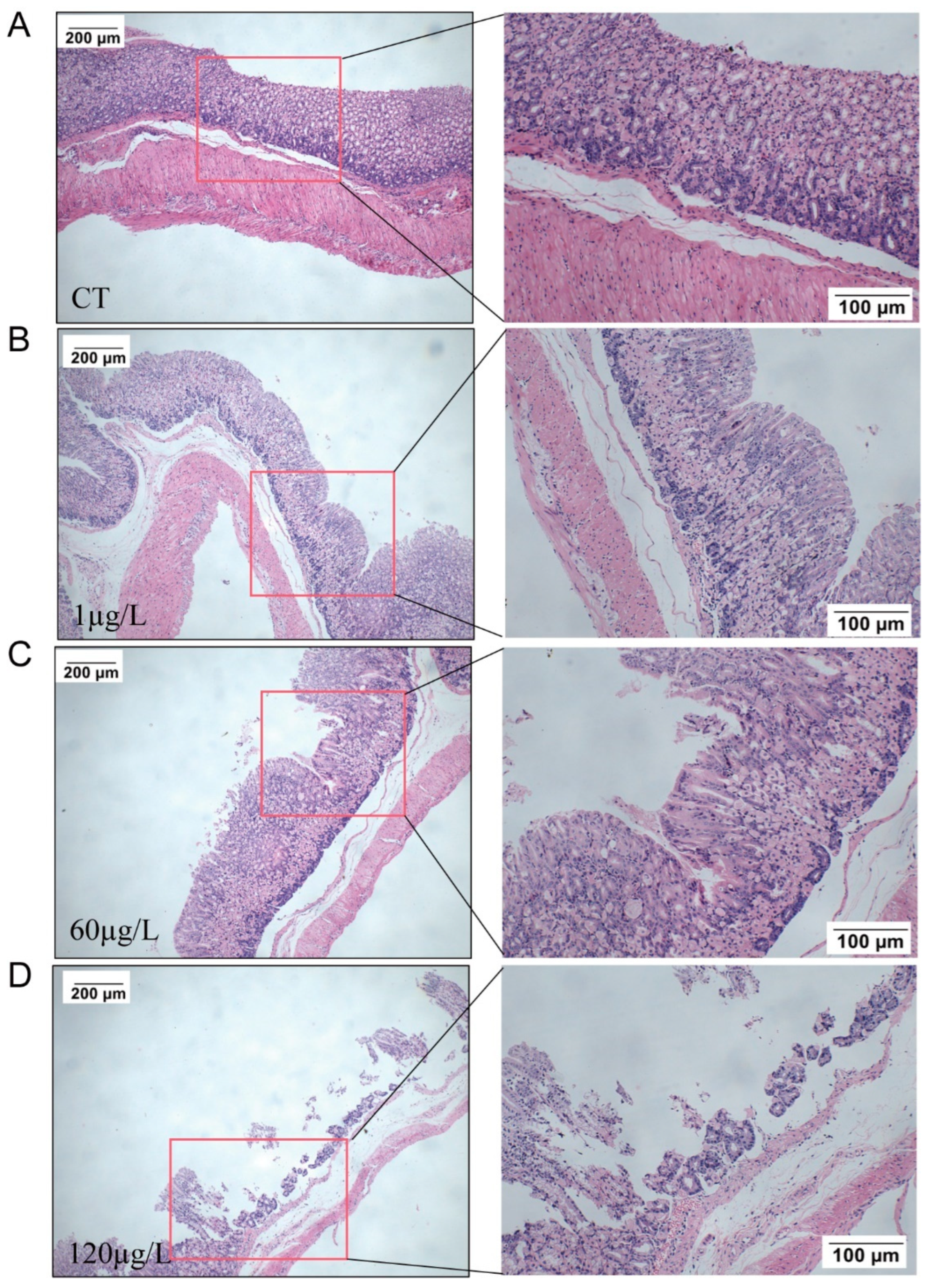
2.3. Histopathology in the Stomach
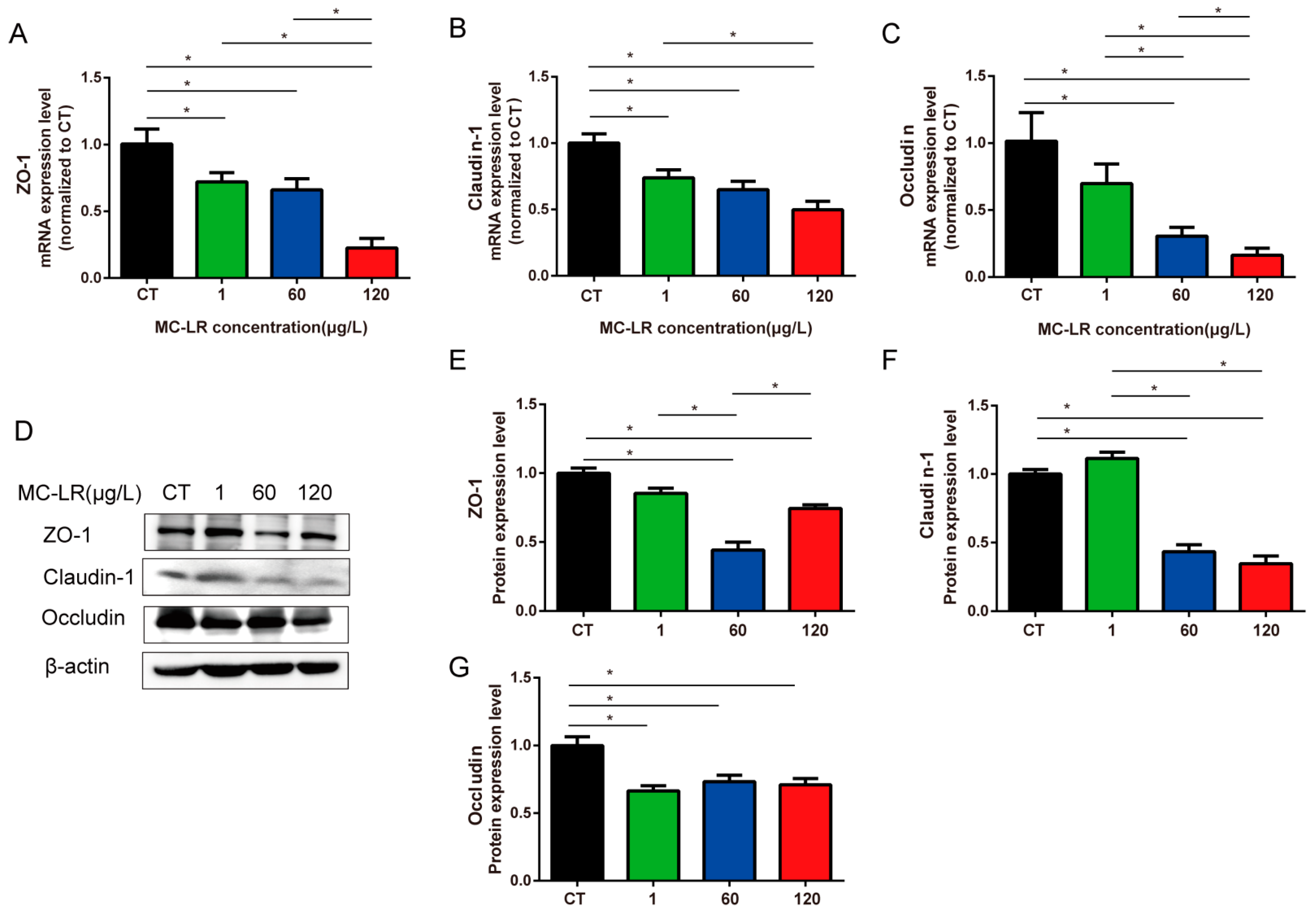
2.4. Tight-Junction-Related Genes and Protein Expression of Gastric Tissue
2.5. Inflammatory Cytokine mRNA Expression in Gastric Tissue of Mice
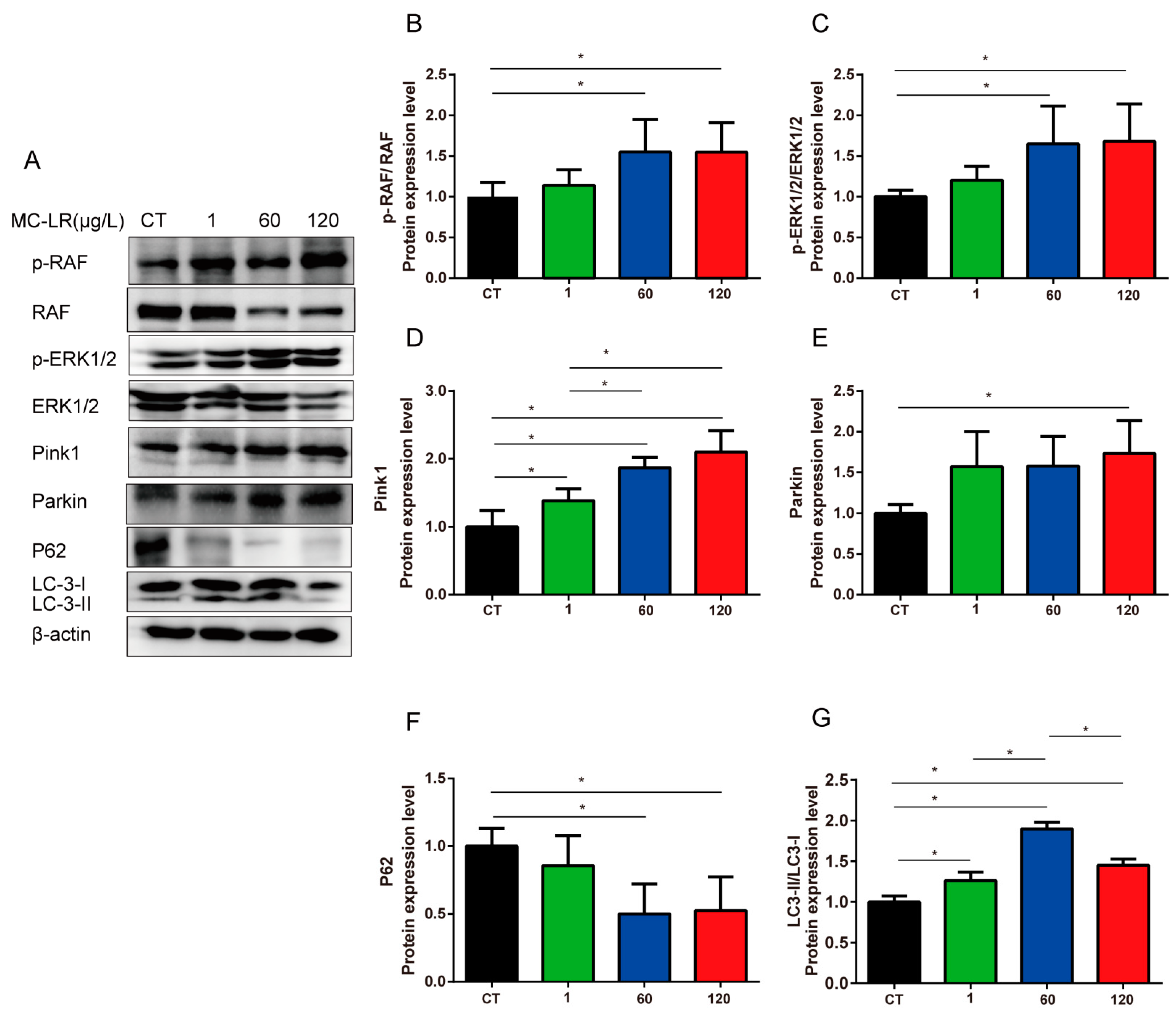
2.6. MAPK Signaling Pathway and Mitophagy-Related Proteins Expression
3. Discussion
4. Conclusions
5. Methods and Materials
5.1. Animals and Treatments
5.2. Histology Staining
5.3. qRT-PCR
5.4. Western Blot
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Fulton, R.S., 3rd; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, F.; Feng, H.; Wei, J.; Massey, I.Y.; Liang, G.; Zhang, F.; Yin, L.; Kacew, S.; Zhang, X.; et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water. Res. 2020, 174, 115638. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Tan, Y.; Wang, L.; Xiang, M.; Zhou, Z.; Chen, J.A.; Wang, J.; Zhang, R.; Tian, Y.; Luo, J.; et al. Association of serum microcystin levels with neurobehavior of school-age children in rural area of Southwest China: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 212, 111990. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Li, Y.W.; Liu, B.L.; Zhao, H.M.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H.; Li, Q.X. High ecological and human health risks from microcystins in vegetable fields in southern China. Environ. Int. 2019, 133 Pt A, 105142. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svircev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Bouaicha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Guo, J.; Wei, J.; Huang, F.; Massey, I.Y.; Luo, J.; Yang, F. Optimization of microcystin biodegradation by bacterial community YFMCD4 using response surface method. Chemosphere 2021, 274, 129897. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef] [PubMed]
- AlKahtane, A.A.; Abushouk, A.I.; Mohammed, E.T.; ALNasser, M.; Alarifi, S.; Ali, D.; Alessia, M.S.; Almeer, R.S.; AlBasher, G.; Alkahtani, S.; et al. Fucoidan alleviates microcystin-LR-induced hepatic, renal, and cardiac oxidative stress and inflammatory injuries in mice. Environ. Sci. Pollut. Res. Int. 2020, 27, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, Y.; Wen, C.; Liu, W.; Cao, L.; Feng, X.; Chen, J.; Wang, H.; Tang, Y.; Tian, L.; et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ. Int. 2021, 154, 106555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Y.; Wang, F.; Xu, D.; Xie, P. N-acetylcysteine protects against microcystin-LR-induced endoplasmic reticulum stress and germ cell apoptosis in zebrafish testes. Chemosphere 2018, 204, 463–473. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Zeng, H.; Pu, C.; Zhang, R.; Qiu, Z.; Chen, J.A.; Wang, L.; Tan, Y.; Zheng, C.; et al. Determination of Environmental Exposure to Microcystin and Aflatoxin as a Risk for Renal Function Based on 5493 Rural People in Southwest China. Environ. Sci. Technol. 2016, 50, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Massey, I.Y.; Feng, H.; Yang, F. A Review of Cardiovascular Toxicity of Microcystins. Toxins 2019, 11, 507. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine Enhances the Antioxidant and Anti-Inflammatory Activities of Thymoquinone against Microcystin-LR-Induced Hepatotoxicity and Neurotoxicity in Mice. Oxid. Med. Cell. Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef]
- Cao, L.; Huang, F.; Massey, I.Y.; Wen, C.; Zheng, S.; Xu, S.; Yang, F. Effects of Microcystin-LR on the Microstructure and Inflammation-Related Factors of Jejunum in Mice. Toxins 2019, 11, 482. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC. Monogr. Eval. Carcinog. Risks. Hum. 2010, 94, 1–412. [Google Scholar]
- Fawell, J.K.; Mitchell, R.E.; Everett, D.J.; Hill, R.E. The toxicity of cyanobacterial toxins in the mouse: I microcystin-LR. Hum. Exp. Toxicol. 1999, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Xie, P. The dose makes the poison. Sci. Total Environ. 2018, 621, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Huang, H.; Yang, L.; Zhang, X.F.; Zhang, S.S.; Liu, H.H.; Wang, Y.Q.; Yuan, L.; Cheng, X.M.; Zhuang, D.G.; et al. Gastrointestinal toxicity induced by microcystins. World J. Clin. Cases 2018, 6, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Furukawa, T.; Ikeda, R.; Takumi, S.; Nong, Q.; Aoyama, K.; Akiyama, S.; Keppler, D.; Takeuchi, T. Involvement of mitogen-activated protein kinase signaling pathways in microcystin-LR-induced apoptosis after its selective uptake mediated by OATP1B1 and OATP1B3. Toxicol. Sci. 2007, 97, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mandery, K.; Bujok, K.; Schmidt, I.; Wex, T.; Treiber, G.; Malfertheiner, P.; Rau, T.T.; Amann, K.U.; Brune, K.; Fromm, M.F.; et al. Influence of cyclooxygenase inhibitors on the function of the prostaglandin transporter organic anion-transporting polypeptide 2A1 expressed in human gastroduodenal mucosa. J. Pharm. Exp. Ther. 2010, 332, 345–351. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, P.; Chen, J.; Liang, G. Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection. Toxicon 2008, 52, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Delerme, C.; Edery, M.; Huet, H.; Puiseux-Dao, S.; Bernard, C.; Fontaine, J.J.; Crespeau, F.; de Luze, A. Microcystin-LR and embryo-larval development of medaka fish, Oryzias latipes. I. Effects on the digestive tract and associated systems. Toxicon 2005, 46, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quijada, L.; Medrano-Padial, C.; Llana-Ruiz-Cabello, M.; Catunescu, G.M.; Moyano, R.; Risalde, M.A.; Camean, A.M.; Jos, A. Cylindrospermopsin-Microcystin-LR Combinations May Induce Genotoxic and Histopathological Damage in Rats. Toxins 2020, 12, 348. [Google Scholar] [CrossRef]
- Vidal, F.; Sedan, D.; D’Agostino, D.; Cavalieri, M.L.; Mullen, E.; Parot Varela, M.M.; Flores, C.; Caixach, J.; Andrinolo, D. Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report. Toxins 2017, 9, 267. [Google Scholar] [CrossRef]
- Svircev, Z.; Drobac, D.; Tokodi, N.; Luzanin, Z.; Munjas, A.M.; Nikolin, B.; Vuleta, D.; Meriluoto, J. Epidemiology of cancers in Serbia and possible connection with cyanobacterial blooms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 319–337. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Wang, Y.; Losiewicz, M.D.; Chen, X.; Du, X.; Wang, Y.; Zhang, B.; Guo, X.; Yuan, S.; et al. Chronic Exposure to Environmentally Relevant Concentrations of Microcystin-Leucine Arginine Causes Lung Barrier Damage through PP2A Activity Inhibition and Claudin1 Ubiquitination. J. Agric. Food Chem. 2022, 70, 10907–10918. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Huang, P.; Wang, H.; Xu, K.; Wang, X.; Xu, L.; Guo, Z. Microcystin-LR promotes cell proliferation in the mice liver by activating Akt and p38/ERK/JNK cascades. Chemosphere 2016, 163, 14–21. [Google Scholar] [CrossRef]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced Cytotoxicity and Apoptosis. Mini Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef]
- Peters, C.; Andrews, P.D.; Stark, M.J.R.; Tadic, S.C.; Glatz, A.; Podtelejnikov, A.; Mann, M.; Mayer, A. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 1999, 285, 1084–1087. [Google Scholar] [CrossRef]
- Aggarwal, S.; Suzuki, T.; Taylor, W.L.; Bhargava, A.; Rao, R.K. Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem. J. 2011, 433, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Gomez, N.; Moorhead, G.; Lewis, T.; Keyse, S.M.; Cohen, P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 1995, 5, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Geng, Z.Z.; Wang, Y.; Tong, Z.H.; Yu, H.Q. Essential roles of p53 and MAPK cascades in microcystin-LR-induced germline apoptosis in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Mei, F.; Ren, G.; Long, L.; Chen, M.; Fang, X.; Li, J.; Li, K.; Tang, Y.; Huang, T.; et al. Synergistic Effect of MC-LR and C-Terminal Truncated HBx on HepG2 Cells and Their Effects on PP2A Mediated Downstream Target of MAPK Signaling Pathway. Front. Genet. 2020, 11, 537785. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bian, R.; Li, J.; Qiu, L.; Lu, B.; Ouyang, X. Chronic exposure to microcystin-LR reduces thyroid hormone levels by activating p38/MAPK and MEK/ERK signal pathway. Ecotoxicol. Environ. Saf. 2019, 173, 142–148. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ho, P.W.; Leung, C.T.; Pang, S.Y.; Chang, E.E.S.; Choi, Z.Y.; Kung, M.H.; Ramsden, D.B.; Ho, S.L. Aberrant mitochondrial morphology and function associated with impaired mitophagy and DNM1L-MAPK/ERK signaling are found in aged mutant Parkinsonian LRRK2(R1441G) mice. Autophagy 2021, 17, 3196–3220. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Yamashita, S.; Kurihara, Y.; Jin, X.; Aihara, M.; Saigusa, T.; Kang, D.; Kanki, T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy 2015, 11, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, M.; Li, J.; Zhou, L.; Tamm, M.; Roth, M. Airway Smooth Muscle Cell Mitochondria Damage and Mitophagy in COPD via ERK1/2 MAPK. Int. J. Mol. Sci. 2022, 23, 13987. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef]
- Wan, X.; Steinman, A.D.; Gu, Y.; Zhu, G.; Shu, X.; Xue, Q.; Zou, W.; Xie, L. Occurrence and risk assessment of microcystin and its relationship with environmental factors in lakes of the eastern plain ecoregion, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 45095–45107. [Google Scholar] [CrossRef]
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Geng, X.; Chen, Y.; Shi, H.; Yang, Y.; Zhu, C.; Yu, G.; Tang, Z. Essential roles of Akt/Snail pathway in microcystin-LR-induced tight junction toxicity in Sertoli cell. Food Chem. Toxicol. 2018, 112, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Van Itallie, C.M.; McCrea, H.J.; Rahner, C.; Anderson, J.M. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell. Physiol. 2002, 283, C142–C147. [Google Scholar] [CrossRef] [PubMed]
- Hirase, T.; Kawashima, S.; Wong, E.Y.; Ueyama, T.; Rikitake, Y.; Tsukita, S.; Yokoyama, M.; Staddon, J.M. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem. 2001, 276, 10423–10431. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Occludin and claudins in tight-junction strands: Leading or supporting players? Trends Cell. Biol. 1999, 9, 268–273. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Shen, L.; Ogden, S.; Romero-Gallo, J.; Lapierre, L.A.; Israel, D.A.; Turner, J.R.; Peek, R.M., Jr. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 2009, 136, 236–246. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A.; Kawasaki, H.; Yoshida, K.; Ishii, K.; Furuse, M.; Amagai, M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J. Derm. Sci. 2015, 77, 28–36. [Google Scholar] [CrossRef]
- Brautigan, D.L. Protein Ser/Thr phosphatases--the ugly ducklings of cell signalling. FEBS J. 2013, 280, 324–345. [Google Scholar] [CrossRef]
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Goncalves, F.J.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 2006, 24, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.W. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell. Biol. 2008, 40, 2707–2719. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xu, C.B.; Zhang, Y.; Cao, Y.X.; Edvinsson, L. Secondhand smoke exposure induces Raf/ERK/MAPK-mediated upregulation of cerebrovascular endothelin ETA receptors. BMC Neurosci. 2011, 12, 109. [Google Scholar] [CrossRef]
- Chin, P.C.; Liu, L.; Morrison, B.E.; Siddiq, A.; Ratan, R.R.; Bottiglieri, T.; D’Mello, S.R. The c-Raf inhibitor GW5074 provides neuroprotection in vitro and in an animal model of neurodegeneration through a MEK-ERK and Akt-independent mechanism. J. Neurochem. 2004, 90, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Breton, S. The MAPK/ERK-Signaling Pathway Regulates the Expression and Distribution of Tight Junction Proteins in the Mouse Proximal Epididymis. Biol. Reprod. 2016, 94, 22. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Wang, J.; Wang, L.; Xiang, Z.; Li, D.; Han, X. Microcystin-Leucine Arginine Causes Cytotoxic Effects in Sertoli Cells Resulting in Reproductive Dysfunction in Male Mice. Sci. Rep. 2016, 6, 39238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, X.; Yu, B.; Yu, G. Characterization of in vitro effects of microcystin-LR on intestinal epithelial cells. Environ. Toxicol. 2017, 32, 1539–1547. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Padman, B.S.; Usher, J.; Oorschot, V.; Ramm, G.; Lazarou, M. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 2016, 215, 857–874. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, M. Autophagosome Trafficking. Adv. Exp. Med. Biol. 2021, 1208, 67–77. [Google Scholar]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, K.F.; Chen, F.J.; Chen, Y.H.; Yang, X.; Cai, Z.H.; Jiang, Y.B.; Wang, X.B.; Zhang, G.P.; Wang, F.Y. Deoxynivalenol triggers porcine intestinal tight junction disorder: Insights from mitochondrial dynamics and mitophagy. Ecotoxicol. Environ. Saf. 2022, 248, 114291. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | TCAAGATCATTGCTCCTCCTGAG | ACATCTGCTGGAAGGTGGACA |
| TNF-α | GTGCCTATGTCTCAGCCTCT | AGGCTTGTCACTCGAATTTTGA |
| IL-6 | CCACGGCCTTCCCTACTTC | TTGGGAGTGGTATCCTCTGTGA |
| IL-10 | ATAACTGCACCCACTTCCCA | GGGCATCACTTCTACCAGGT |
| ZO-1 | GCGATTCAGCAGCAACAGAACC | AGGACCGTGTAATGGCAGACTC |
| Occludin | GCGGCTATGGAGGCTATGGCTA | AGGAAGCGATGAAGCAGAAGGC |
| Claudin-1 | GGACAACATCGTGACCGCTCAG | TCCAGGCACCTCATGCACTTCA |
| Antibody Name | Source | Dilution Ratios |
|---|---|---|
| MC-LR | Alexis Corporation (Lausen, Switzerland) | 1:3000 |
| RAF | Proteintech, Wuhan, China | 1:1000 |
| p-RAF | Cell Signaling Technology Company, USA | 1:1000 |
| ERK | Proteintech, Wuhan, China | 1:1000 |
| p-ERK | Cell Signaling Technology Company, USA | 1:1000 |
| Pink-1 | Proteintech, Wuhan, China | 1:1000 |
| Parkin | Abcam, The United Kingdom | 1:1000 |
| P62 | Proteintech, Wuhan, China | 1:1000 |
| LC-3 | Proteintech, Wuhan, China | 1:500 |
| β-actin | Proteintech, Wuhan, China | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Y.; Tan, Q.; Lv, Y.; Tang, Y.; Yang, Y.; Yao, X.; Yang, F. Long-Term Exposure to Microcystin-LR Induces Gastric Toxicity by Activating the Mitogen-Activated Protein Kinase Signaling Pathway. Toxins 2023, 15, 574. https://doi.org/10.3390/toxins15090574
Liu Y, Li Y, Tan Q, Lv Y, Tang Y, Yang Y, Yao X, Yang F. Long-Term Exposure to Microcystin-LR Induces Gastric Toxicity by Activating the Mitogen-Activated Protein Kinase Signaling Pathway. Toxins. 2023; 15(9):574. https://doi.org/10.3390/toxins15090574
Chicago/Turabian StyleLiu, Ying, Yafang Li, Qinmei Tan, Yilin Lv, Yan Tang, Yue Yang, Xueqiong Yao, and Fei Yang. 2023. "Long-Term Exposure to Microcystin-LR Induces Gastric Toxicity by Activating the Mitogen-Activated Protein Kinase Signaling Pathway" Toxins 15, no. 9: 574. https://doi.org/10.3390/toxins15090574





