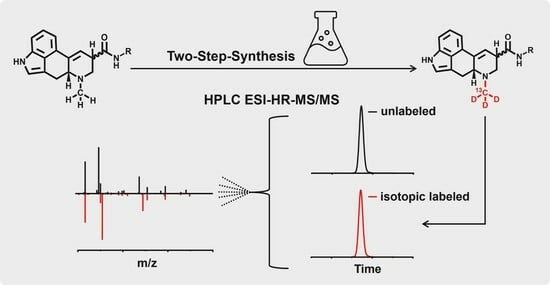
First Synthesis of Ergotamine-13CD3 and Ergotaminine-13CD3 from Unlabeled Ergotamine
Abstract
:1. Introduction
2. Results
2.1. Electrochemical N-Demethylation
2.2. Synthesis of Norergotamine/-inine via an Iron-Catalyzed N-Demethylation Reaction
2.3. Synthesis of Ergotamine-13CD3 and Ergotaminine-13CD3
3. Discussion
4. Materials and Methods
4.1. Chemicals and Equipment
4.2. HPLC-HR-MS/MS
4.3. Electrochemical Experiments
4.4. Synthesis of Norergotamine and Norergotaminine
4.5. Synthesis of Ergotamine-13CD3 and Ergotaminine-13CD3
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky, P.; Kos, G.; Nahrer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-Occurrence of Regulated, Masked and Emerging Mycotoxins and Secondary Metabolites in Finished Feed and Maize-An Extensive Survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Debegnach, F.; Patriarca, S.; Brera, C.; Gregori, E.; Sonego, E.; Moracci, G.; De Santis, B. Ergot Alkaloids in Wheat and Rye Derived Products in Italy. Foods 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798. [Google Scholar] [CrossRef]
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. Ergot alkaloids--biology and molecular biology. Alkaloids Chem. Biol. 2006, 63, 48. [Google Scholar] [CrossRef]
- Tudzynski, P.; Scheffer, J. Claviceps purpurea: Molecular aspects of a unique pathogenic lifestyle. Mol. Plant Pathol. 2004, 5, 377–388. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Crews, C. Analysis of Ergot Alkaloids. Toxins 2015, 7, 2024–2050. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2021/1399 of 24 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ergot Sclerotia and Ergot Alkaloids in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2021/1399/oj (accessed on 17 January 2024).
- EN 17425:2021; Foodstuffs—Determination of Ergot Alkaloids in Cereals and Cereal Products by dSPE Clean-Up and HPLC-MS/MS. European Committee for Standardization: Bruxelles, Belgium. Available online: https://standards.iteh.ai/catalog/standards/cen/4861d773-3ff6-4d7f-90ca-0a48397e9080/en-17425-2020 (accessed on 16 April 2024).
- Panuwet, P.; Hunter, R.E., Jr.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef]
- Van Eeckhaut, A.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC-MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, S.V.; Diana Di Mavungu, J.; Goryacheva, I.Y.; De Saeger, S. A systematic assessment of the variability of matrix effects in LC-MS/MS analysis of ergot alkaloids in cereals and evaluation of method robustness. Anal. Bioanal. Chem. 2013, 405, 5595–5604. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Jestoi, M. Determination of ergot alkaloids from grains with UPLC-MS/MS. J. Sep. Sci. 2010, 33, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Chakraborty, S.; Bera, R.; Karmakar, S.; Pal, T.K. Internal Standard an Important Analyte Use in Drug Analysis by Liquid Chromatography Mass Spectrometry- An Article. Int. J. Pharm. Bio-Med. Sci. 2022, 2, 10–17. [Google Scholar] [CrossRef]
- Chung, S.W.C. A critical review of analytical methods for ergot alkaloids in cereals and feed and in particular suitability of method performance for regulatory monitoring and epimer-specific quantification. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- European Commission. COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Braun, B.; Köppen, R.; Wedler, C.; Theil, F. Synthesis of M + 4 Stable Isotopomers of Ergometrine and Ergometrinine. Nat. Prod. Commun. 2017, 12, 373–376. [Google Scholar] [PubMed]
- Moldvai, I.; Temesvari-Major, E.; Incze, M.; Szentirmay, E.; Gacs-Baitz, E.; Szantay, C. Enantioefficient synthesis of alpha-ergocryptine: First direct synthesis of (+)-lysergic acid. J. Org. Chem. 2004, 69, 5993–6000. [Google Scholar] [CrossRef]
- Stadler, P.A.; Frey, A.J.; Ott, H.; Hofamann, A. Die Synthese des Ergosins und des Valin-Analogen der Ergotamin-Gruppe. 61. Mitteilung über Mutterkornalkaloide. Helv. Chim. Acta 2004, 47, 1911–1921. [Google Scholar] [CrossRef]
- Alipour Najmi, A.; Xiao, Z.; Bischoff, R.; Dekker, F.J.; Permentier, H.P. Electrochemical N-demethylation of tropane alkaloids. Green Chem. 2020, 22, 6455–6463. [Google Scholar] [CrossRef]
- Schotten, C.; Nicholls, T.P.; Bourne, R.A.; Kapur, N.; Nguyen, B.N.; Willans, C.E. Making electrochemistry easily accessible to the synthetic chemist. Green Chem. 2020, 22, 3358–3375. [Google Scholar] [CrossRef]
- Glotz, G.; Kappe, C.O.; Cantillo, D. Electrochemical N-Demethylation of 14-Hydroxy Morphinans: Sustainable Access to Opioid Antagonists. Org. Lett. 2020, 22, 6891–6896. [Google Scholar] [CrossRef]
- Reddy, P.; Hemsworth, J.; Guthridge, K.M.; Vinh, A.; Vassiliadis, S.; Ezernieks, V.; Spangenberg, G.C.; Rochfort, S.J. Ergot alkaloid mycotoxins: Physiological effects, metabolism and distribution of the residual toxin in mice. Sci. Rep. 2020, 10, 9714. [Google Scholar] [CrossRef]
- Uhlig, S.; Rangel-Huerta, O.D.; Divon, H.H.; Rolen, E.; Pauchon, K.; Sumarah, M.W.; Vralstad, T.; Renaud, J.B. Unraveling the Ergot Alkaloid and Indole Diterpenoid Metabolome in the Claviceps purpurea Species Complex Using LC-HRMS/MS Diagnostic Fragmentation Filtering. J. Agric. Food Chem. 2021, 69, 7137–7148. [Google Scholar] [CrossRef]
- Csutoras, C.; Zhang, A.; Bidlack, J.M.; Neumeyer, J.L. An investigation of the N-demethylation of 3-deoxymorphine and the affinity of the alkylation products to mu, delta, and kappa receptors. Bioorg. Med. Chem. 2004, 12, 2687–2690. [Google Scholar] [CrossRef]
- Machara, A.; Endoma-Arias, M.A.A.; Císařová, I.; Cox, D.P.; Hudlicky, T. Direct Synthesis of Noroxymorph-one from Thebaine: Unusual CeIV Oxidation of a Methoxydiene-Iron Complex to an Enone-γ-Nitrate. Eur. J. Org. Chem. 2016, 2016, 1500–1503. [Google Scholar] [CrossRef]
- Thavaneswaran, S.; Scammells, P.J. Further investigation of the N-demethylation of tertiary amine alkaloids using the non-classical Polonovski reaction. Bioorg. Med. Chem. Lett. 2006, 16, 2868–2871. [Google Scholar] [CrossRef]
- Schummer, C.; Zandonella, I.; van Nieuwenhuyse, A.; Moris, G. Epimerization of ergot alkaloids in feed. Heliyon 2020, 6, e04336. [Google Scholar] [CrossRef]
- Jahn, S.; Baumann, A.; Roscher, J.; Hense, K.; Zazzeroni, R.; Karst, U. Investigation of the biotransformation pathway of verapamil using electrochemistry/liquid chromatography/mass spectrometry—A comparative study with liver cell microsomes. J. Chromatogr. A 2011, 1218, 9210–9220. [Google Scholar] [CrossRef]
- Torres, S.; Brown, R.; Szucs, R.; Hawkins, J.M.; Scrivens, G.; Pettman, A.; Kraus, D.; Taylor, M.R. Rapid Synthesis of Pharmaceutical Oxidation Products Using Electrochemistry: A Systematic Study of N-Dealkylation Reactions of Fesoterodine Using a Commercially Available Synthesis Cell. Org. Process Res. Dev. 2014, 19, 1596–1603. [Google Scholar] [CrossRef]
- Najmi, A.A.; Bhat, M.F.; Bischoff, R.; Poelarends, G.J.; Permentier, H.P. TEMPO-Mediated Electrochemical N-demethylation of Opiate Alkaloids. ChemElectroChem 2021, 8, 2590–2596. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]





| Working/Auxiliary Electrode | Electrolyte | 10 µM Ergotamine in Water/Acetonitrile or Water/Methanol (v%/v%) | N-Demethylated Product Detected |
|---|---|---|---|
| GC, BDD, and Pt/ PEEK | 10 mM NH4OOCH | 0/100, 20/80, and 50/50 | Yes (only GC) |
| GC, BDD, and Pt/ PEEK | 10 mM FA | 0/100, 20/80, and 50/50 | No |
| GC, BDD, and Pt/ PEEK | 10 mM (NH4)2CO3 | 20/80 and 50/50 | Yes (only GC) |
| Parameter—ESI Source | Parameter—Mass Spectrometer | ||
|---|---|---|---|
| Temperature | 300 °C | MS1 | |
| Ion Source Gas 1 | 60 psi | Collision energy | 5 V |
| Ion Source Gas 2 | 70 psi | Declustering Potential | 80 V |
| Curtain Gas | 25 psi | Mass range | 400–800 |
| Ionspray Voltage | 5500 V | MS 2 | |
| Collision Energy | Rolling collision energy | ||
| Collision Energy Spread | 5 V | ||
| Declustering Potential | 80 V | ||
| Mass range | 100–800 | ||
| Time [min] | H2O + 20 mM NH3 [%] | Acetonitrile [%] |
|---|---|---|
| 0 | 80 | 20 |
| 8.0 | 20 | 80 |
| 11.9 | 20 | 80 |
| 12 | 80 | 20 |
| 15 | 80 | 20 |
| Time [min] | H2O + 20 mM NH3 [%] | Acetonitrile [%] |
|---|---|---|
| 0 | 66 | 34 |
| 20 | 66 | 34 |
| 20.1 | 0 | 100 |
| 25 | 0 | 100 |
| 25.1 | 66 | 34 |
| 31 | 66 | 34 |
| Time [min] | H2O + 20 mM NH3 [%] | Acetonitrile [%] |
|---|---|---|
| 0 | 62 | 38 |
| 14 | 62 | 38 |
| 16 | 35 | 65 |
| 22 | 35 | 65 |
| 22.1 | 0 | 100 |
| 27 | 0 | 100 |
| 27.1 | 62 | 38 |
| 33 | 62 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herter, S.-O.; Haase, H.; Koch, M. First Synthesis of Ergotamine-13CD3 and Ergotaminine-13CD3 from Unlabeled Ergotamine. Toxins 2024, 16, 199. https://doi.org/10.3390/toxins16040199
Herter S-O, Haase H, Koch M. First Synthesis of Ergotamine-13CD3 and Ergotaminine-13CD3 from Unlabeled Ergotamine. Toxins. 2024; 16(4):199. https://doi.org/10.3390/toxins16040199
Chicago/Turabian StyleHerter, Sven-Oliver, Hajo Haase, and Matthias Koch. 2024. "First Synthesis of Ergotamine-13CD3 and Ergotaminine-13CD3 from Unlabeled Ergotamine" Toxins 16, no. 4: 199. https://doi.org/10.3390/toxins16040199