Effects of Spider Venom Toxin PWTX-I (6-Hydroxytrypargine) on the Central Nervous System of Rats
Abstract
:1. Introduction

2. Materials and Methods
2.1. Animals
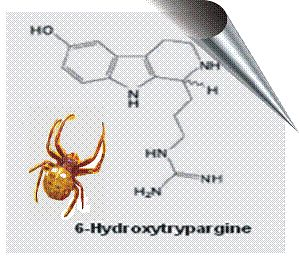
2.2. 6-Hydroxytrypargine (6-HT) and Trypargine
2.3. Monitoring the Expression of Fos-Protein
2.4. Double Labeling Immunohistochemistry
2.5. Venous Catheterization
2.6. Statistical Analysis
2.7. Electrophysiological Assay
2.8. Electrophysiological Recordings, Data Acquisition and Analysis
3. Results
3.1. Imunohistochemistry Assays


3.2. Electrophysiological Assay


| Control | After 6-HT Perfusion | |
|---|---|---|
| Vm (mV) | −71.63 ± 5.09 | −76,07 ± 10.31 |
| Rin (MΩ) | 42.7 ± 7.03 | 54,41 ± 8,17 |
| Tm (ms) | 11.43 ± 2.97 | 10.90 ± 3.60 |
| Spike Amplitude (mV) | 79.33 ± 10.97 | 84.33 ± 12.52 |


4. Discussion
5. Conclusions
Acknowledgements
References
- Hagiwara, K.; Sakai, T.; Miwa, A.; Kawai, N.; Nakajima, T. Agelenin. A new neurotoxin from the venom of the spider. Agelena opulenta. Pept. Chem. 1991, 1, 351–356. [Google Scholar]
- Glenn, F.; King, G.G. Modulation of insect Cav channels by peptidic spider toxins. Toxicon 2007, 49, 513–530. [Google Scholar]
- King, G.F.; Escoubas, P.; Nicholson, G.M. Peptide toxins that selectively target insect NaV and CaV channels. Channels 2008, 2, 100–116. [Google Scholar]
- Escoubas, P.; Diochot, S.; Corzo, G. Structure and pharmacology of spider venom neurotoxins. Biochimie 2000, 82, 893–907. [Google Scholar]
- Palma, M.S.; Nakajima, T. A Natural Combinatorial Chemistry Strategy in Acylpolyamine Toxins From Nephilinae Orb-Web Spiders. Toxin Rev. 2005, 24, 209–234. [Google Scholar]
- McCormick, K.D.; Meinwald, J. Neurotoxic acypoliamines from spider venoms. J. Chem. Ecol. 1993, 19, 2411–2451. [Google Scholar]
- Salamoni, S.D.; Costa, J.C.; Palma, M.S.; Konno, K.; Nihei, K.; Tavares, A.A.; Abreu, D.S.; Venturin, G.T.V.; Cunha, F.B.; Oliveira, R.M.; Breda, R.V. Antiepileptic effect of acylpolyaminetoxin JSTX-3 on rat hippocampal CA1 neurons in vitro. Brain Res. 2005, 1048, 170–176. [Google Scholar]
- Salamoni, S.D.; Da Costa, J.C.; Palma, M.S.; Konno, K.; Ken-Ichi, N.; Tavares, A.A.; Abreu, D.S.; Venturin, G.T.; Cunha, F.B.; Oliveira, R.M.; Breda, R.V. Antiepileptic effect of acypoliaminetoxin JSTX-3 on rat hippocampal neurons. Brain Res. 2005, 1048, 170–176. [Google Scholar]
- Quistad, G.B.; Lam, W.W.; Casida, J.E. Identification of bis(agmatine)oxalamide in venom from the primitive hunting spider. Plectreurys tristis (Simon). Toxicon 1993, 31, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Taggi, A.E.; Meinwald, J.; Schroeder, F.C. A new approach to natural products discovery exemplified by the identification of sulfated nucleosides in spider venom. J. Am. Chem. Soc. 2004, 126, 10364–10369. [Google Scholar]
- Cesar, L.M.M.; Tormena, C.F.; Marques, M.R.; Silva, G.V.J.; Mendes, M.A.; Palma, M.S. Structure Determination of Hydroxytrypargine: A Tetrahydro-β-carboline Toxin From the Venom of the Spider Parawixia bistriata. Helvetica Chim. Acta 2005, 88, 796–801. [Google Scholar]
- Marques, M.R.; Mendes, M.A.; Tormena, C.F.; Souza, B.M.; Cesar, L.M.; Rittner, R.; Palma, M.S. Structure determination of a tetrahydro-beta-carboline of arthropod origin: A novel alkaloid-toxin subclass from the web of spider Nephila clavipes. Chem. Biodivers. 2005, 2, 525–534. [Google Scholar]
- Saidemberg, D.M.; Ferreira, M.A.; Takahashi, T.N.; Gomes, P.C.; Cesar-Tognoli, L.M.; Da Silva-Filho, L.C.; Tormena, C.F.; Da Silva, G.V.; Palma, M.S. Monoamine oxidase inhibitory activities of indolylalkaloid toxins from the venom of the colonial spider Parawixia bistriata: Functional characterization of PwTX-I. Toxicon 2009, 54, 717–724. [Google Scholar]
- Martin, L.; Léon, A.; Martin, M.A.; Del Castilho, B.; Menédez, J.C. Detection and characterization of cyclodextrin complexes with β-carboline derivates by spectroscopic techniques. J. Pharm. Biomed. Anal. 2003, 32, 991–1001. [Google Scholar]
- Airaksinen, M.M.; Lecklin, A.; Saano, V.; Tuomisto, L.; Gynther, J. Tremorogenic effect and inhibition of tryptamine and serotonin receptor binding by β-carbolines. Pharmacol. Toxicol. 1987, 60, 5–8. [Google Scholar]
- Müller, W.E.; Fehske, K.J.; Borbe, H.O.; Wollert, U.; Nanz, C.; Rommelspacher, H. On the neuropharmacology of harmane and other β-carbolines. Pharmacol. Biochem. Behav. 1981, 14, 693–699. [Google Scholar]
- Husbands, S.M.; Glennon, R.A.; Gorgerat, S.; Gough, R.; Tyacke, R.; Corsby, J.; Nutt, D.J.; Lewis, J.W.; Hudson, A.L. Beta-carboline binding to imidazoline receptors. Drug Alchohol Depend. 2001, 64, 203–208. [Google Scholar]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.; Constantino, L.; Teitler, M.; Smith, C.; Ergan, C.; Davis, K.; Mattson, M.V. Binding of β-carbolines and related agents at serotonine (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug Alchohol Depend. 2000, 60, 121–132. [Google Scholar]
- Morgan, J.I.; Curran, T. Stimulus-transcription coupling in the nervous system: Involvement of inducible proto-oncogenes fos and jun. Ann. Rev. Neurosci. 1991, 14, 421–451. [Google Scholar]
- Lino de Oliveira, C.; Sales, A.J.; Del Bel, E.A.; Silveira, M.C.L.; Guimarães, F.S. Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res. Bull. 2001, 55, 747–754. [Google Scholar]
- Kobelt, P.; Tebbe, J.J.; Tjandra, I.; Bae, H.G.; Klapp, B.F.; Wiedenmann, B.; Monnikes, H. Two immunocytochemical protocols for immunofluorescent detection of c-Fos positive neurons in the rat brain. Brain Res. Protocol. 2004, 13, 45–52. [Google Scholar]
- McIlmwain, H.; Buddle, H.L. Techniques in tissue metabolism.1. A mechanical chopper. Biochem. J. 1953, 53, 412–420. [Google Scholar] [PubMed]
- National Academy of Sciences. Guide for the Care and Use of Laboratory Animals; Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; National Academy Press: Washington, DC, USA, 1996. Available online: http://www.nap.edu/readingroom/books/labrats/ (accessed on 1 February 2011).
- Shimizu, M.; Ishikawa, M.; Komoda, Y.; Nakajima, M. Total synthesis of (−)-Trypargine. Chem. Pharm. Bull. 1982, 30, 909–913. [Google Scholar]
- Gerfen, C.R.; Sawchenko, P.E. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localizations of an axonally transported plantlectin Phaseolus vulgaris leucoagglutinin. Brain Res. 1984, 290, 219–238. [Google Scholar]
- Shu, S.; Ju, G.; Fan, L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. 1988, 85, 169–171. [Google Scholar]
- Sita, L.V.; Elias, C.F.; Bittencourt, J.C. Dopamine and melanin-concentrating hormone neurons are distinct populations in the rat rostromedial zona incerta. Brain Res. 2003, 970, 232–237. [Google Scholar]
- Windle, H. A Nissl method using buffered solutions of thionin. Stain Tech. 1943, 18, 77–86. [Google Scholar]
- Bashir, Z.I. Neuroscience; Lynch, M.A., O’Mara, S.M., Eds.; LABFAX Series; Academic Press: London, UK, 1997; pp. 13–31. [Google Scholar]
- Jensen, M.S.; Azouz, R.; Yaari, Y. Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J. Physiol. 1996, 492, 199–210. [Google Scholar]
- Barker, S.A.; Harrison, R.E.W.; Brown, J.G.B.; Christian, S.T. Identification and quantification of 1,2,3,4-tetrahydro-b-carboline, 2-methyl-1,2,3,4-tetrahydro-b-carboline, and 6-methoxy-1,2,3,4-tetrahydro-b-carboline as in vivo constituents of rat brain and adrenal gland. Biochem. Pharmacol. 1981, 30, 9–15. [Google Scholar]
- Carmona, C.; Ghanem, R.; Balón, M.; Muñoz, M.A.; Guardado, P. Chemical and photochemical oxidation of tetrahydrobetacarboline. J. Photochem. Photobiol. A: Chem. 2000, 135, 171–177. [Google Scholar]
- Robertson, H.A. Harmaline-induced tremor: The benzodiazepine receptoras a site of action. Eur. J. Pharmacol. 1980, 67, 129–132. [Google Scholar]
- Solis-Maldonado, C.; Quintanilla-Licea, R.; Tamez-Guerra, R.; Rodriguez-Padilla, C.; Gomez-Flores, R. Differential effects of synthetic indoloquinolizines on in vitro rat lymphocytes and macrophage functions. Int. Immunopharmacol. 2003, 3, 1261–1271. [Google Scholar]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.; Costantino, L.; Teitler, M.; Smith, C.; Egan, C.; Davis, K.; Mattson, M.V. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend. 2000, 60, 121–132. [Google Scholar]
- Akizawa, T.; Yamazaki, K.; Yasuhara, T.; Nakajima, T.; Roseghini, M.; Esparmer, G.F.; Esparmer, M. Trypargine, a new tetrahydro-β-carboline of animal origin: Isolation and chemical characterization from the skin of the African rhacophorid frog. Kassina senegalensis Biomed. Res. 1982, 3, 232–234. [Google Scholar]
- Seyama, I.; Yakehiro, M.; Nakajima, T. Trypargine blocks tha sodium channels only from the inside of squid giant axon. JPN J. Physiol. 1985, 35, 367–373. [Google Scholar]
- Nistico, G.; de Sarro, G.B.; Langer, S.Z. Behavioural and electrocortical power spectrum effects of 5-methoxytryptoline and other analogs after intraventricular administration in rats. Eur. J. Pharmacol. 1987, 142, 121–128. [Google Scholar]
- Cesar, L.M.M.; Mendes, M.A.; Tormena, C.F.; Marques, M.R.; de Souza, B.M.; Saidemberg, D.M.; Bittencourt, J.C.; Palma, M.S. Isolation and chemical characterization of PwTx-II: A novel alkaloid toxin from the venom of the spider Parawixia bistriata (Araneidae, Araneae). Toxicon 2005, 46, 786–796. [Google Scholar]
- Yamada, M.; Yasuhara, H. Clinical pharmacology of MAO inhibitors: Safety and future. NeuroToxicology 2004, 25, 215–221. [Google Scholar]
- Herraiz, T.; Chaparro, C. Analysis of monoamine oxidase enzymatic activity by reversed-phase high performance liquid chromatography and inhibition by β-carboline alkaloids occurring in foods and plants. J. Chromatogr. A 2006, 1120, 237–243. [Google Scholar]
- Hoover, N.G.; Fortenberry, J.D. Use of antivenin to treat priapism after a black widow spider bite. Pediatrics 2004, 114, 128–129. [Google Scholar]
- Yildiz, A.; Biçeroglu, S.; Yakut, N.; Bilir, C.; Akdemir, R.; Akilli, A. Acute myocardial infarction in a young man caused by centipede Sting. Emerg. Med. J. 2006, 23, 30–33. [Google Scholar]
- Ucar, G.; Tasb, C.; Tümer, A. Monoamine oxidase inhibitory activities of the scorpion Mesobuthus gibbosus (Buthidae) venom peptides. Toxicon 2005, 45, 43–52. [Google Scholar]
- Singewald, N.; Salchner, P.; Sharp, T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psych. 2003, 15, 275–283. [Google Scholar]
- Adel, A.; Biggs, T.A.; Myers, R.D. Action of harman (1-methyl-β-carboline) on the brain: Body temperature and in vivo efflux of 5-HT from hippocampus of the rat. Neuropharmacology 1996, 35, 1101–1107. [Google Scholar]
- Myers, R.D. Serotonin and thermoregulation: Old and new views. Physiology (Paris) 1981, 77, 505–513. [Google Scholar]
- Harikai, N.; Tomogane, K.; Sugawara, T.; Tashiro, S. Differences in hypothalamic fos expression between two heat stress conditions in conscious mice. Brain Res. Bull. 2003, 61, 617–626. [Google Scholar]
- Ali, S.F.; Thiriet, N.; Zwiller, J. Acute ibogaine injection induces expression of the immediate early gene, erg-1 and c-fos, in mouse brain. Mol. Brain Res. 1999, 74, 237–241. [Google Scholar]
- Bac, G.; Seidel, G. Harmine action in rats with lymphostatic encephalopathy. Pharmacology 1977, 15, 127–133. [Google Scholar]
- Leino, M.; Airaksinen, M.M.; Antikainen, R.; Gynther, J.; Kari, E.; Kari, I.; Peura, P. Distribution of 1,2,3,4-tetrahydro-beta-carboline and 6-methoxy-1,2,3,4-tetrahydro-beta-carboline in mice. Acta Phamacol. Toxicol. 1984, 54, 361–371. [Google Scholar]
- Krause, W.; Mengel, H. Pharmacokinetics of the anxiolytic beta-carboline derivate abecarnil in the mouse, rat, rabbit, dog, cynomolgus monkey and baboon. Studies on species differences. Arzneimittelforschung 1990, 40, 522–529. [Google Scholar] [PubMed]
- Peyron, C.; Tighe, D.K.; van del Pol, A.N.; Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T. Neurons containing Hypocretin (Orexin) project to multiple neuronal systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar]
- Stone, T.W. Neurophamacology, 1st ed; W.H. Freeman/Spektrum: New York, NY, USA, 1995; p. 140. [Google Scholar]
- Prince, D.A.; Connors, B.W. Mechanisms of interictal epileptogenesis. Adv. Neurol. 1986, 44, 275–299. [Google Scholar]
- Köhr, G.; Mody, I. Kindling increases N-methyl-D-aspartate potency at single N-methyl-D-aspartate channels in dentate gyrus granule cells. Neuroscience 1994, 62, 975–981. [Google Scholar]
- Bernard, C.; Wheal, H.V. Plasticity of AMPA and NMDA receptor-mediated epileptiform activity in a chronic model of temporal lobe epilepsy. Epilepsy Res. 1995, 21, 95–107. [Google Scholar]
- Babb, T.L.; Matherm, G.W.; Leite, J.P.; Pretorius, J.K.; Yeoman, K.M.; Kuhlman, P.A. Glutamate AMPA receptors in the fascia dentata of human and kainate rat hippocampal epilepsy. Epilepsy Res. 1996, 26, 193–205. [Google Scholar]
- Traub, R.D.; Jefferys, J.G.; Whittington, M.A. Enhanced NMDA conductance can account for epileptoform activity induced by low Mg2+ in the rat hippocampal slice. J. Physiol. 1994, 478, 379–393. [Google Scholar]
- Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992, 5082, 597–603. [Google Scholar]
- Airaksinen, M.M.; Mikkonen, E. Affinity of β-carbolines on rat brain benzodiazepine and opiate receptors. Med. Biol. 1980, 58, 341–344. [Google Scholar]
- Saano, V.; Airaksinen, M.M. Binding of β-carbolines and cafeine on benzodiazepine receptors: Correlation to convulsions and tremor. Acta Pharmacol. Toxicol. 1982, 51, 300–308. [Google Scholar]
- Luttes, J.; Lorden, J.F.; Beales, M.; Lotmans, M.G.A. Tolerance to the tremorogenic effects of harmaline: Evidence for altered olivo-cerebellar function. Neuropharmacology 1988, 27, 849–855. [Google Scholar]
- Rommelspacher, H.; Brüning, G.; Susilo, R.; Nick, M.; Hill, R. Pharmacology of harmalan (1-methyl-3,4-dyhydro-β-carboline). Eur. J. Phamacol. 1985, 109, 363–371. [Google Scholar]
- Rommelspacher, H.; May, T.; Salewski, B. Harman (1-methyl-β-carboline) is a natural inhibitor of monoamine-oxidase type A in rats. Eur. J. Pharmacol. 1994, 252, 51–59. [Google Scholar]
- Pähkla, R.; Harro, J.; Rägo, L. Behavioural effects of the pinoline in the rat forced swimming open field avd elevated plus-maze tests. Pharm. Res. 1996, 34, 73–78. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cesar-Tognoli, L.M.M.; Salamoni, S.D.; Tavares, A.A.; Elias, C.F.; Da Costa, J.C.; Bittencourt, J.C.; Palma, M.S. Effects of Spider Venom Toxin PWTX-I (6-Hydroxytrypargine) on the Central Nervous System of Rats. Toxins 2011, 3, 142-162. https://doi.org/10.3390/toxins3020142
Cesar-Tognoli LMM, Salamoni SD, Tavares AA, Elias CF, Da Costa JC, Bittencourt JC, Palma MS. Effects of Spider Venom Toxin PWTX-I (6-Hydroxytrypargine) on the Central Nervous System of Rats. Toxins. 2011; 3(2):142-162. https://doi.org/10.3390/toxins3020142
Chicago/Turabian StyleCesar-Tognoli, Lilian M. M., Simone D. Salamoni, Andrea A. Tavares, Carol F. Elias, Jaderson C. Da Costa, Jackson C. Bittencourt, and Mario S. Palma. 2011. "Effects of Spider Venom Toxin PWTX-I (6-Hydroxytrypargine) on the Central Nervous System of Rats" Toxins 3, no. 2: 142-162. https://doi.org/10.3390/toxins3020142