2. Results and Discussion
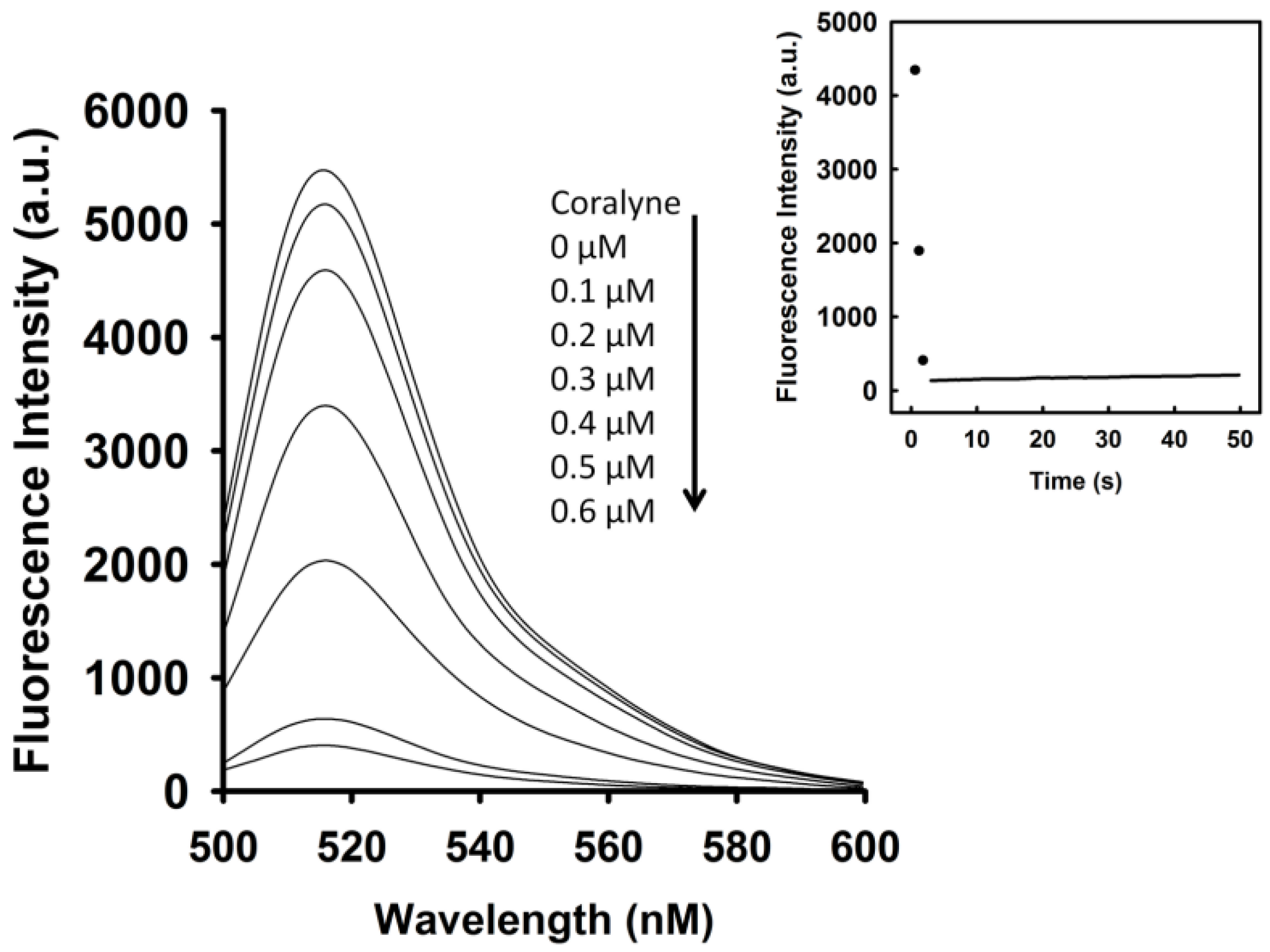
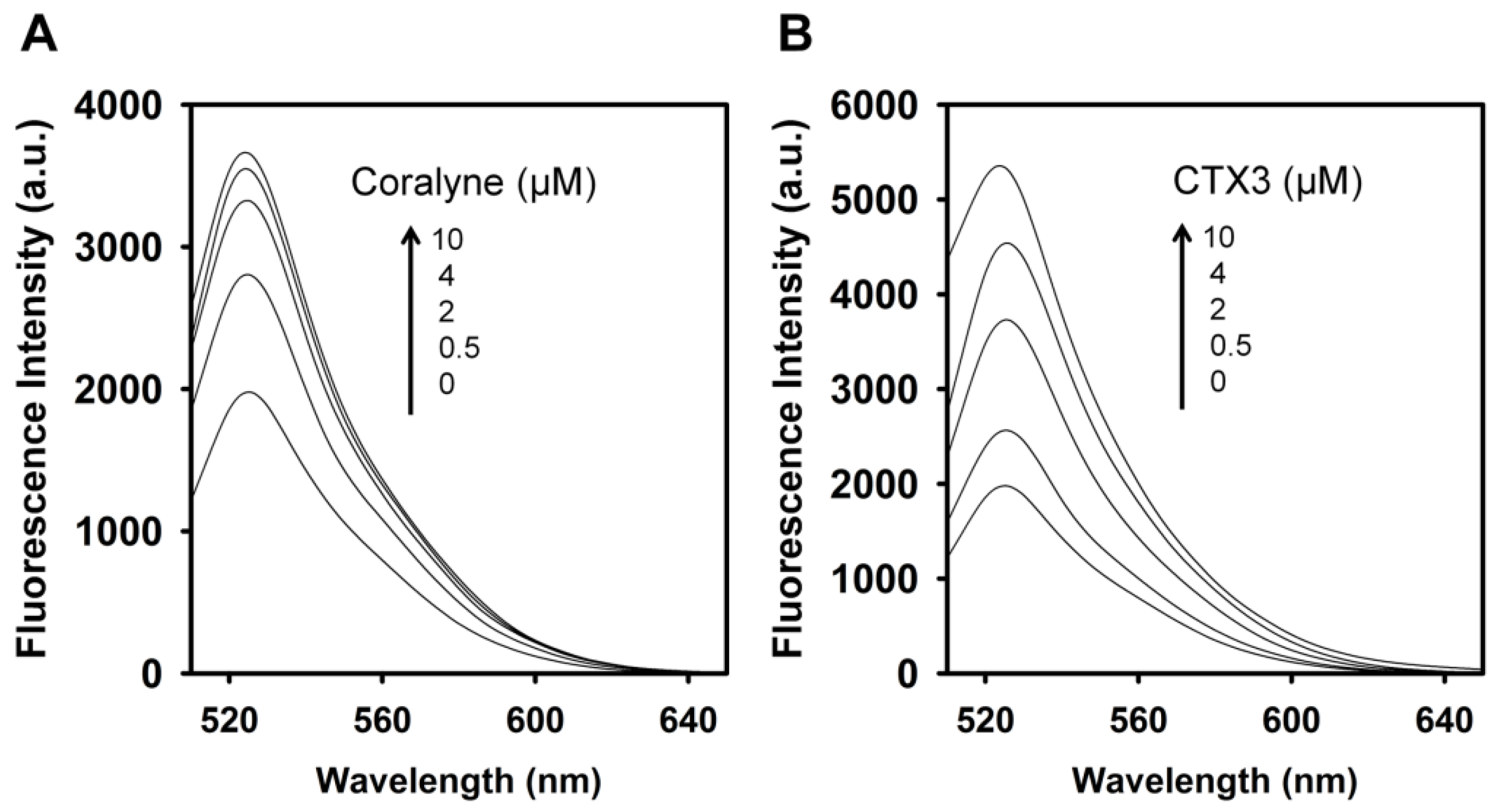
To identify the optimal concentration of coralyne for maximal quenching of the fluorescence between FAM and DABCYL, a 10 nM A
12-MB-A
12 DNA probe was titrated with coralyne. As shown in
Figure 1, the fluorescence intensity of FAM was reduced by addition of coralyne, and it reduced sharply within 10 s at 520 nm. The fluorescence quenching reached a saturation level after addition of 0.6 μM coralyne, indicating that the hairpin structure of A
12-MB-A
12 was more stable at coralyne concentrations exceeding 0.6 μM. According to the change in the FAM fluorescence intensity, the binding affinity of coralyne towards A
12-MB-A
12 was calculated. The dissociation constant (K
d) of coralyne for A
12-MB-A
12 was 0.5 μM. This is in agreement with the finding that coralyne binds to poly(A) with an association constant of 1.8 × 10
6 M
−1 at pH 7.0 [
7,
8].
To assess the concentration-dependent effect of CTX3 on the FAM fluorescence of hairpin-shaped MB, a solution containing 10 nM MB and 0.6 μM coralyne was titrated with CTX3. As shown in
Figure 2A, maximum restoration of FAM fluorescence was noted with the addition of 80 nM CTX3. The fluorescence turn-on assay required 10 s to achieve maximum recovery of FAM fluorescence (
Figure S1, Supplementary Materials). The fitted curve was used for quantification of CTX3 with a correlation coefficient of 0.995, and the detection limit of 0.03 nM could be reached based on the definition of three times the deviation of the blank signal (3σ). Noticeably, CTX3 caused a recovery of FAM fluorescence to approximately 50% of that noted with coralyne-free MB. Restoration of FAM fluorescence increased with increasing the CTX3 concentration up to 80 nM (
Figure 2B). Likewise, the addition of 80 nM CTX1, CTX2, CTX4, CTX5, and CTXn restored the FAM fluorescence of hairpin-shaped MB (Inset of
Figure 2A). To test the specificity of hairpin-shaped MB in detecting CTXs, the effect of cobrotoxin (a short-chain α-neurotoxin from
N. atra venom) and α-bungarotoxin (a long-chain α-neurotoxin from
Bungarus multicinctus venom) on FAM fluorescence was analyzed. CTXs, cobrotoxin, and α-bungarotoxin structurally adopt a three-loop folding topology [
9,
10,
11,
12], but CTXs and α-neurotoxins show distinct pharmacological activities [
13]. Moreover, genetic analyses have proven that CTXs, cobrotoxin, and α-bungarotoxin share a common gene structure and originate from the same ancestor [
13,
14,
15,
16,
17]. As shown in
Figure 2B, cobrotoxin and α-bungarotoxin did not notably restore the FAM fluorescence of hairpin-shaped MB at test concentrations up to 1000 nM. These results indicated that hairpin-shaped MB was more selective for detecting CTXs over α-neurotoxin. Nevertheless, a notable reduction in the FAM fluorescence of A
12-MB-A
12 was noted when the concentration of added CTX3 was >100 nM, and CTX3 quenched the fluorescence signal of A
12-MB-A
12 in a concentration-dependent manner. Likewise, titration of coralyne-A
12-MB-A
12 complexes with other CTX isotoxins also yielded bell-shaped curves of FAM fluorescence signal (
Figure S2, Supplementary Materials).
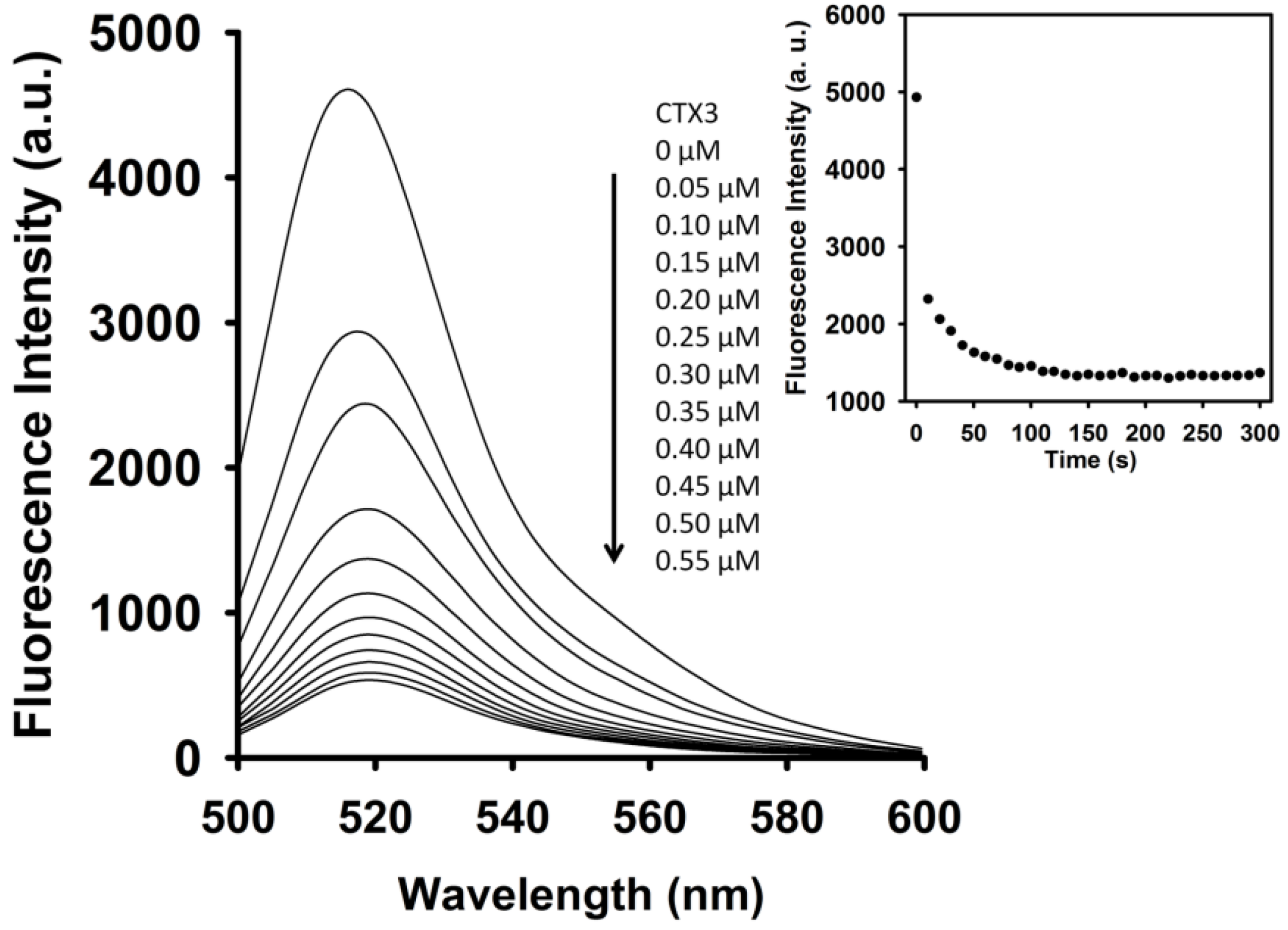
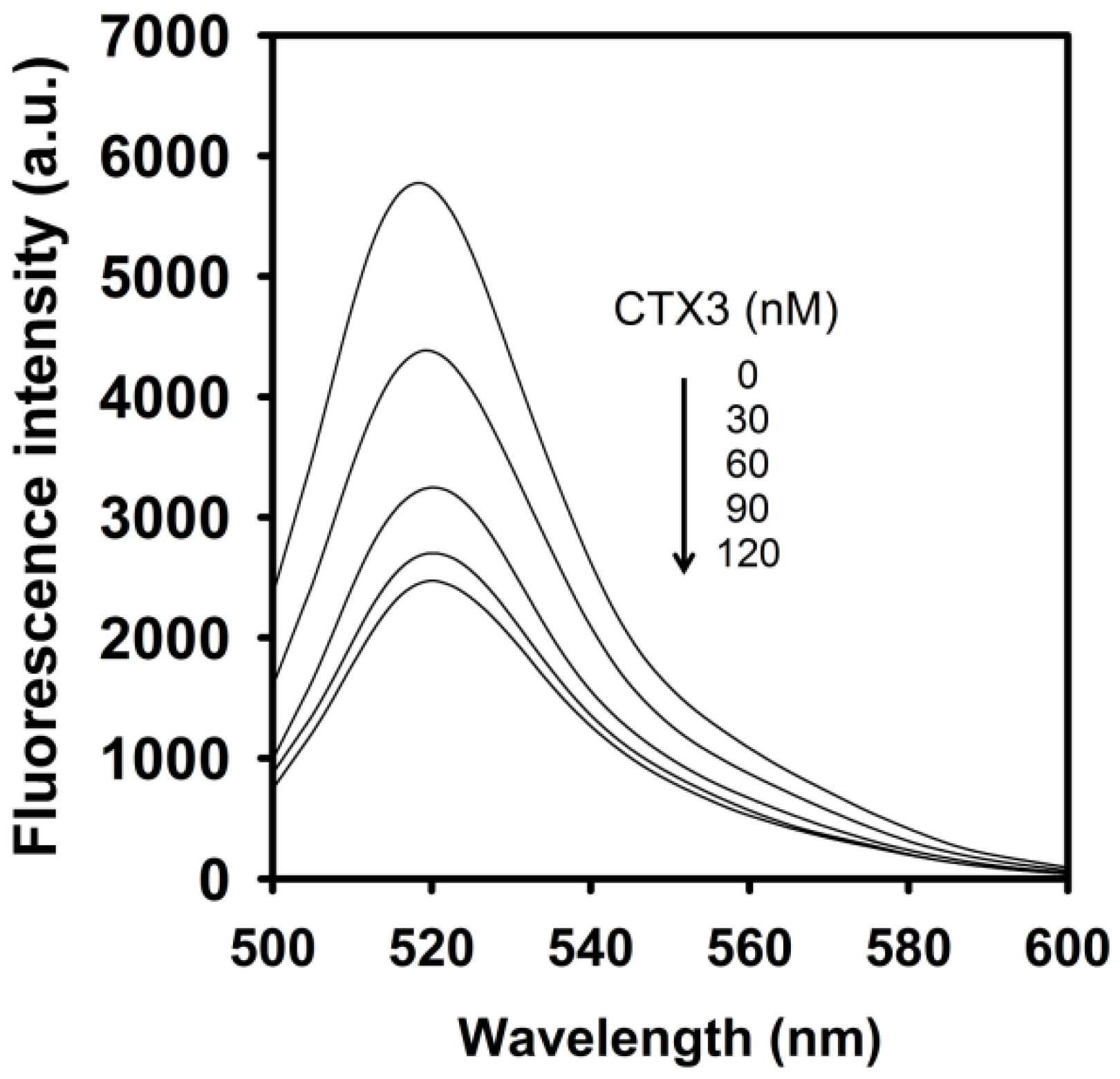
As shown in
Figure 3, CTX3 induced a decrease in the FAM fluorescence of A
12-MB-A
12 in the absence of coralyne in a concentration-dependent manner, and maximal reduction in FAM fluorescence was noted when CTX3 was ≥550 nM. As shown in inset of
Figure 3, CTX3-induced fluorescence turn-off required 120 s to achieve maximum reduction in FAM fluorescence. Likewise, titration of A
12-MB-A
12 with other CTX isotoxins also resulted in the quenching of FAM fluorescence (
Figure S3, Supplementary Materials). To prove that the reduction in fluorescence occurred only upon binding of CTX3 to the A
12-MB-A
12, a control experiment was conducted by titrating FAM solution with CTX3. CTX3 did not significantly affect the fluorescence intensity of the FAM solution. Thus, any change in the fluorescence intensity of A
12-MB-A
12 was caused by the binding of CTX3. These observations indicated that the binding of CTXs with A
12-MB-A
12 might keep DABCYL and FAM in close proximity. The binding affinity of CTX isotoxins for A
12-MB-A
12 was calculated using the titration data derived from the change in FAM fluorescence intensity induced by toxin molecules. The dissociation constants (K
d) of CTX1, CTX2, CTX3, CTX4, CTX5, and CTXn for A
12-MB-A
12 were 0.2 μM, 0.5 μM, 0.1 μM, 0.6 μM, 0.1 μM, and 0.1 μM, respectively.
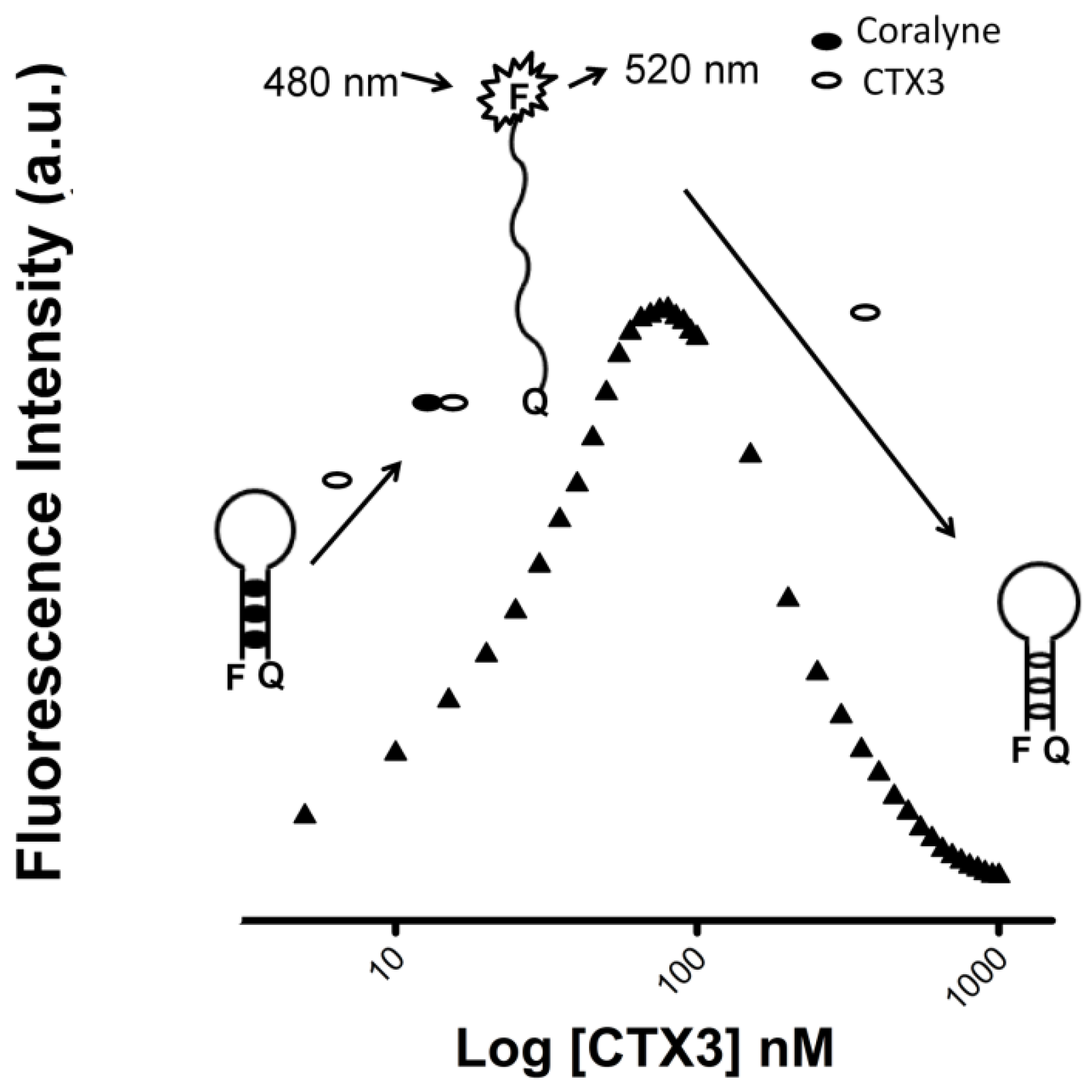
As per the above findings, CTX3 disrupted the coralyne-induced stem-loop MB structure and quenched the FAM fluorescence of A
12-MB-A
12 irrespective of coralyne, hence two possibilities could be considered: (1) CTX3 bound to coralyne; and (2) CTX3 induced formation of the stem-loop MB structure. As shown in
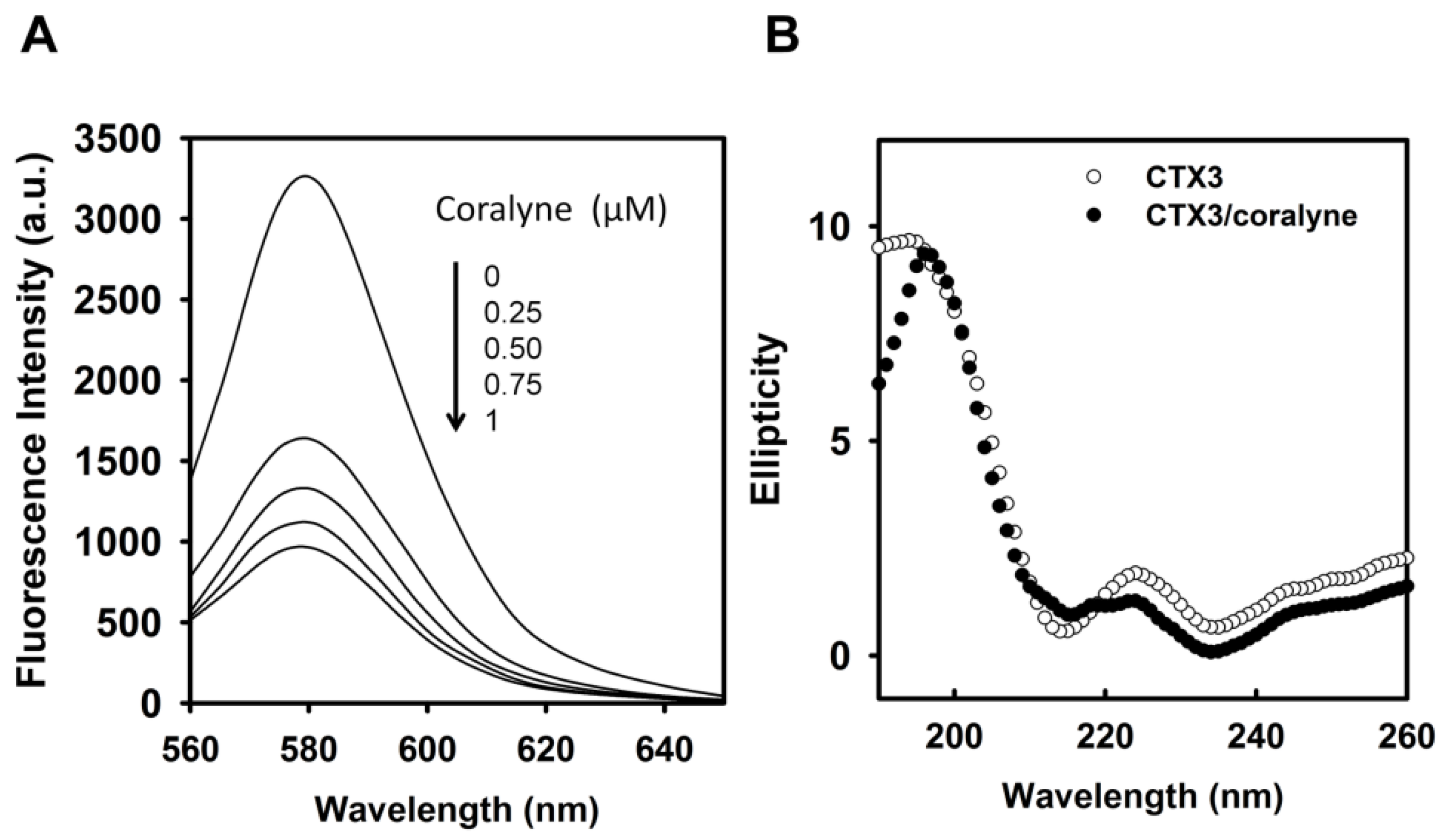
Figure 4A, coralyne induced a reduction in the fluorescence intensity of rhodamine-labeled CTX3 in a concentration-dependent manner. The affinity of CTX3 with coralyne was calculated from the change in fluorescence intensity. The dissociation constant (K
d) of CTX3 for coralyne was 0.2 μM. CD spectra also showed that coralyne induced a change in the gross conformation of CTX3 (
Figure 4B). These observations indicated the binding of CTX3 with coralyne. Molecular docking analyses suggest the involvement of Lys2, Tyr 11, Thr13, Asp57, and Arg58 of CTX3 in binding with coralyne (
Figure S4, Supplementary Materials). To test whether CTX3 induced a stem-loop conformation of MB, SG fluorescence enhancement was used to detect folded MB structure. SG is an organic dye that has weak fluorescence but exhibits a fluorescence enhancement upon binding to double-stranded DNA. Compared with the fluorescence intensity of SG in the presence of coralyne-free A
12-MB-A
12, the fluorescence intensity in the presence of coralyne-A
12-MB-A
12 complexes was markedly enhanced (
Figure 5A). It was consistent with the notion that coralyne induced the hairpin-shaped structure of A
12-MB-A
12. Likewise, following the addition of CTX3, an obvious increase in the fluorescence intensity at 525 nm of SG was noted (
Figure 5B). Apparently, CTX3 rendered MB to form a folded structure.
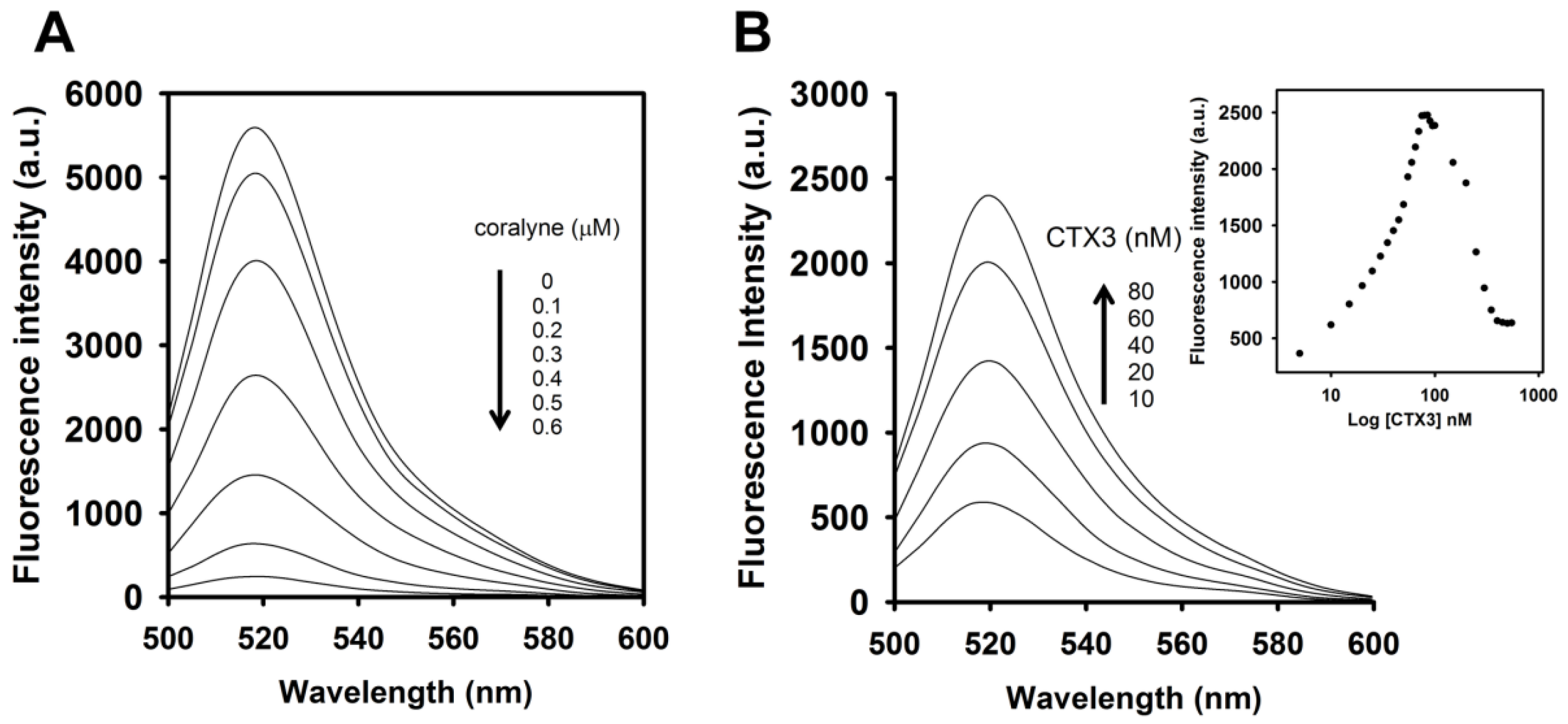
Previous studies showed that coralyne could induce hairpin structure of poly A
40 [
18]. Thus, the effect of coralyne on fluorescence of FAM-A
40-DABCYL was analyzed. As shown in
Figure 6A, the fluorescence intensity of FAM was reduced by the addition of coralyne. The fluorescence quenching reached a saturation level after addition of 0.6 μM coralyne, indicating that the hairpin structure of A
40 was more stable at coralyne concentration exceeding 0.6 μM. To assess the concentration-dependent effect of CTX3 on the FAM fluorescence of hairpin-shaped A
40, a solution containing 10 nM FAM-A
40-DABCYL and 0.6 μM coralyne was titrated with CTX3. As shown in
Figure 6B, maximum restoration of FAM fluorescence was noted with the addition of 80 nM CTX3. Moreover, a notable reduction in the FAM fluorescence of FAM-A
40-DABCYL was noted when the concentration of added CTX3 was >100 nM (Inset of
Figure 6B), and thus titration of coralyne- FAM-A
40-DABCYL complexes with CTX3 also yielded a bell-shaped curve of FAM fluorescence signal. As shown in
Figure 7, CTX3 induced a decrease in the FAM fluorescence of FAM/DABCYL-labeled A
40 in a concentration-dependent manner, and maximal reduction in FAM fluorescence was noted when CTX3 was ≥120 nM. The binding affinity of CTX3 for A
40 was calculated using the titration data derived from the change in FAM fluorescence intensity induced by toxin molecules. The dissociation constants (K
d) of CTX3 for A
40 was 0.17 μM. These findings supported that CTX3 binds with the poly dA part of MB.
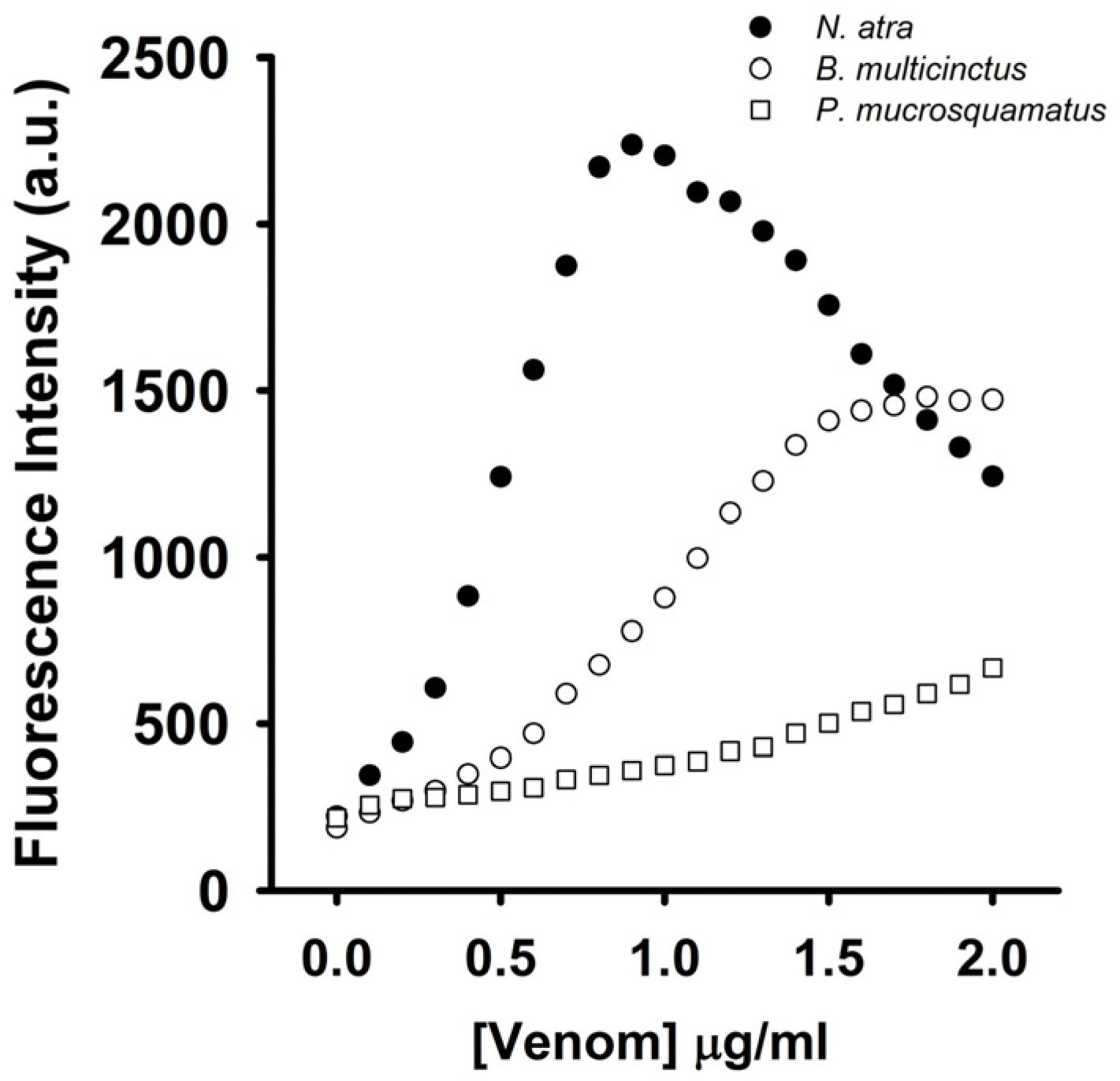
To test whether the coralyne-A
12-MB-A
12 complexes could specifically detect CTX-containing venom, the effect of
N. atra,
B. multicinctus, and
P. mucrosquamatus venoms on the FAM fluorescence of hairpin-shaped MB was analyzed. To date, no studies indicate that
B. multicinctus and
P. mucrosquamatus venoms contain CTXs. As shown in
Figure 8,
N. atra venom maximally restored MB fluorescence signal at concentration of 0.8 μg/mL, and further increase in the concentration of
N. atra venom caused a reduction in MB fluorescence intensity. Additionally,
P. mucrosquamatus venom slightly increased the FAM fluorescence of hairpin-shaped MB at the test concentrations. Although
B. multicinctus venom could notably restore MB fluorescence intensity,
B. multicinctus venom did not function as
N. atra venom to yield a bell-shaped concentration-dependent curve of MB fluorescence signal. Moreover, at concentration ≤0.8 μg/mL,
N. atra venom showed a superior ability to restore the FAM fluorescence of hairpin-shaped MB compared to
B. multicinctus and
P. mucrosquamatus venoms. Taken together, these results show that the hairpin-shaped MB can discriminate
N. atra venom from
P. mucrosquamatus and
B. multicinctus venoms. A linear relationship (
R2 = 0.976) between fluorescent quenching and crude
N. atra venom was obtained over the range of 0–0.8 μg/mL. The limit of detection for
N. atra venom was determined to be 0.028 μg/mL at a signal-to-noise ratio of 3 (3σ).
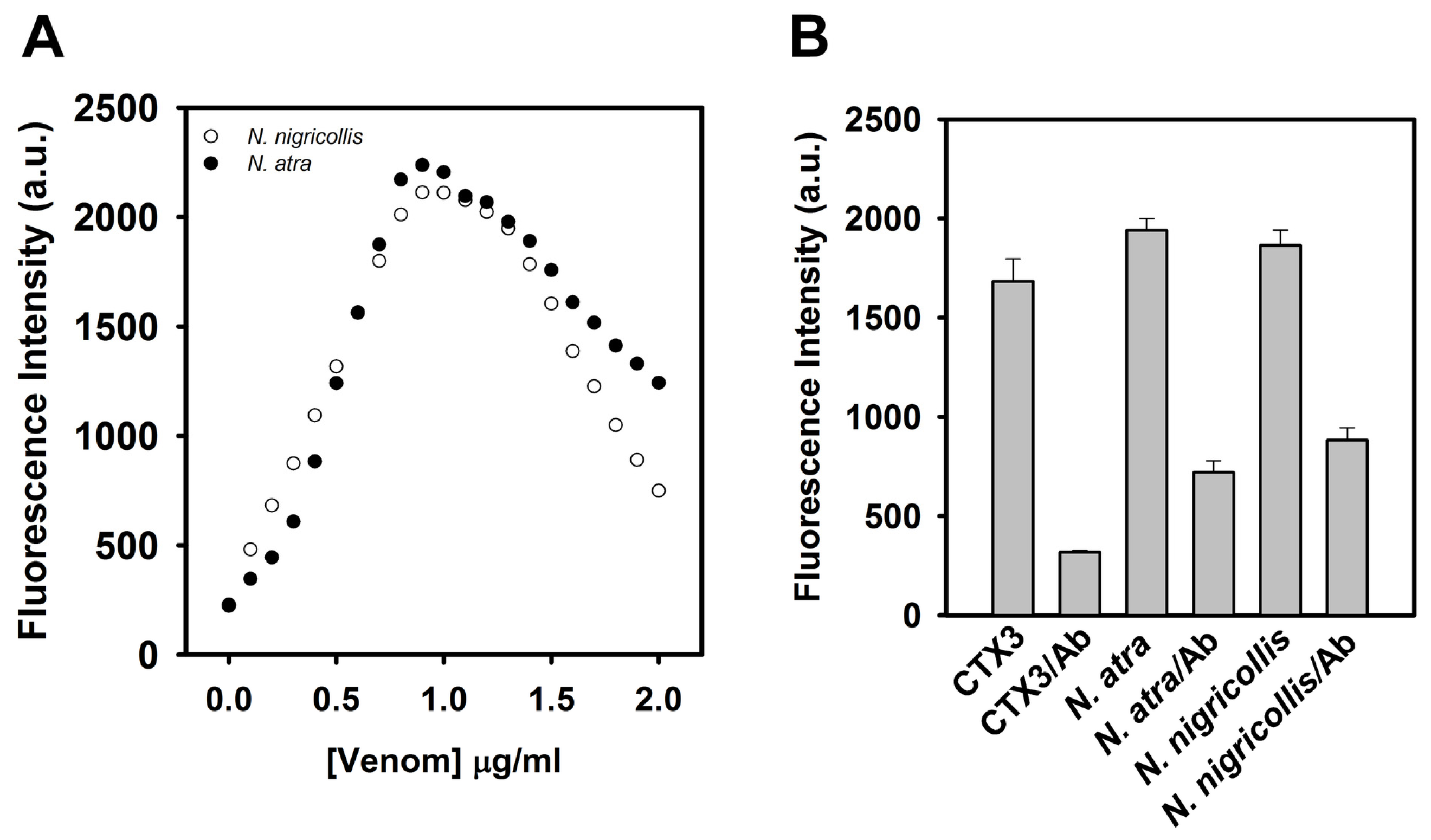
Given that the tested CTX isotoxins caused a recovery of FAM fluorescence of hairpin-shaped MB, it was likely that snake venom containing CTXs might restore FAM fluorescence. Previous studies showed that
N. nigricollis venom contained CTX isotoxins [
19]. As shown in
Figure 9A,
N. nigricollis venom functioned as
N. atra venom to yield a bell-shaped concentration-dependent curve of MB fluorescence signal.
Figure 9B shows that preincubation with anti-
N. atra CTX3 antibodies reduced the ability of CTX3,
N. atra venom and
N. nigricollis venom to restore FAM fluorescence of hairpin-shaped MB. These findings indicated that the hairpin-shaped MB could detect crude venoms containing CTXs, and suggested that the change of MB fluorescence signal could not differentiate the venom of
N. atra from the venoms of other
Naja species.
Previous studies revealed that CTXs could bind with aptamers against α-bungarotoxin [
20]. Aptamers are synthetic oligonucleotides, such as RNA and single-stranded DNA, that can bind to their targets due to their specific secondary or tertiary structures [
21,
22]. Therefore, aptamer-binding by proteins is largely determined by how well the molecules fit into the cavities of the target proteins [
23,
24]. Chen et al. [
20] propose that the binding of CTXs to aptamers against α-bungarotoxin is due to the structural complementarity. Noticeably, CTXs and α-bungarotoxin show similar binding affinity for aptamers, and thus the aptamers are unable to discriminate CTXs from α-bungarotoxin. Obviously, compared to aptamers against α-bungarotoxin, the coralyne-MB complexes platform specifically detects CTXs. In the present study, our data revealed that CTX3 could bind with coralyne and A
12-MB-A
12. At concentrations ≤80 nM, CTX3 converts MB from a stem-loop structure to a random-coil structure via binding with coralyne in a concentration-dependent manner; when toxin concentration further increased, CTX3 causes the random-coil MB structure to become a folded structure irrespective of coralyne (
Figure 10). Thus, the FAM fluorescent signal of A
12-MB-A
12-coralyne complexes yielded a bell-shaped curve as a function of CTX3 concentration. Likewise, the FAM fluorescence signal of A
12-MB-A
12-coralyne complexes also yielded bell-shaped curves in response to other CTX isotoxins (
Figure S3, Supplementary Materials). Given that the binding affinity of CTX3 for coralyne (K
d, 0.2 μM) was greater than that of A
12-MB-A
12 for coralyne (K
d, 0.5 μM), it is conceivable that CTX3 can competitively remove coralyne from the MB. Noticeably,
Figure 3 shows that 550 nM CTX3 maximally induces fluorescence quenching of MB, while 1000 nM CTX3 is required for maximally quenching MB fluorescence in the presence of 0.6 μM coralyne (
Figure 2B). These observations suggest that the interaction between coralyne and CTX3 attenuates the binding capability of CTX3 with A
12-MB-A
12. However, the binding affinity of CTX3 for coralyne (K
d, 0.2 μM) was lower than that of CTX3 for A
12-MB-A
12 (K
d, 0.1 μM). Thus, increasing CTX3 concentration results in the folded MB structure in the presence of coralyne. Our data show that CTX3 causes a ‘turn-on’ fluorescence signal of hairpin-shaped MB at a concentration ≤80 nM in a concentration-dependent manner, while cobrotoxin and α-bungarotoxin marginally affects the FAM fluorescence of hairpin-shaped MB at the test concentrations (
Figure 2B). These observations suggest that the coralyne-induced hairpin conformation of A
12-MB-A
12 can discriminate CTX isotoxins from α-neurotoxin. When CTX3 concentration was ≤80 nM, CTX3 concentration-dependently disrupted stem-loop structure of MB via removal of coralyne from MB. Thus, CTX3 concentration-dependently restores FAM fluorescence of MB. Increase in CTX3 concentration further caused the formation of folded MB structure, leading to quenched FAM fluorescence by DABCYL. Thus, a bell-shaped curve was noted with FAM fluorescence intensity of coralyne-A
12-MB-A
12 complexes in response to titration with CTX3. Hung et al. [
25] reported that the serum venom levels of
N. atra snakebite patients are 228–1270 ng/mL after 2 h snakebite. Our data show that the detection limit for crude
N. naja atra venom is 28 ng/mL. Thus, human serum samples spiked with
N. atra,
B. multicinctus, and
P. mucrosquamatus venoms were used to test the utility of the MB for detecting CTXs in the presence of serum proteins. Unfortunately, serum proteins alone caused a notable recovery of hairpin-shaped MB fluorescence signal, and hence the effect
N. atra,
B. multicinctus, and
P. mucrosquamatus venoms on hairpin-shaped MB fluorescence signal were unable to be detected (data not shown). Evidently, the MB probe could not be free from matrix effect of the plasma sample. Thus, the potential applicability of the method for detecting serum snake venom from snakebite patients needs some alterations. Previous studies revealed that human serum albumin and bovine serum albumin could bind with coralyne [
26]. Removal of the serum proteins might be the required step before the use of MB probe for detecting CTXs in human serum samples. On the other hand, the detection sensitivity of ELISA for crude
N. atra venom has been reported to be 1 ng/mL [
25]. It is obvious that the sensitivity of MB should be further improved.
In summary, the present findings suggest the utility of an adenosine-based MB for rapidly sensing CTXs. The detected sensitivity can be achieved at nanomolar level, suggesting that this is a sensitive method for detecting CTXs. Moreover, the fact that the FAM fluorescence of A12-MB-A12-coralyne complexes yielded bell-shaped curves in response to CTXs indicates that this specific character could also be used for detecting CTXs.
3. Materials and Methods
Crude venoms of
Naja atra (Taiwan cobra)
, Bungarus multicinctus (Taiwan banded krait), and
Protobothrops mucrosquamatus (Taiwan habu) were milked from the venom glands of at least 40 adult snakes in Taiwan. The crude venoms were pooled together and stored at −20 °C after lyophilization. Cobrotoxin and CTX isotoxins (CTX1, CTX2, CTX3, CTX4, CTX5, and CTXn) were isolated from the venom of
N. atra according to the procedure described in Lin et al. [
27]. Purification of α-bungarotoxin from
B. multicinctus venom was carried out according to the procedure described in Chang et al. [
13]. Moreover, the pooled crude venoms were used for studying their effect on the fluorescent signal of coralyne-MB complexes. The chromatographic profiles for separation of CTX3, cobrotoxin, and α-bungarotoxin were shown in
Figure S5A (Supplementary Materials), and the purity of CTXs, cobrotoxin, and α-bungarotoxin was verified using MALDI-TOF analyses (
Figure S5B, Supplementary Materials). Rabbit anti-
N. atra CTX3 sera were prepared in our laboratory [
28], and anti-CTX3 antibodies were further purified using a CTX3-Sepharose column that was prepared by covalent coupling of CTX3 to CNBr-activated Sepharose 4B column. SYBR Green I (SG) was purchased from Molecular Probe Inc. (Eugene, OR, USA). Calcein, carboxyfluorescein (FAM), coralyne sulfoacetate, HEPES, crude venom of
N. nigricollis, and rhodamine B isothiocyanate were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA); and 5′-FAM/3′-DABCYL-labeled A
12-CATCATAGTCGAGTGTCCAGGG-A
12 DNA MB, 5′-FAM/3′-DABCYL-labeled A
40, and label-free A
12-CATCATAGTCGAGTGTCCAGGG-A
12 were synthesized from Neogene Biomedicals Corporation (Taipei, Taiwan). CNBr-activated Sepharose 4B was obtained from GH Healthcare Bio-Sciences Corp. (Piscataway, NJ, USA). Unless otherwise specified, all other reagents were analytical grade.
3.1. Circular Dichroism (CD) Measurement
CD spectra were obtained on a Jasco J-810 spectropolarimeter (JASCO corporation, Tokyo, Japan) with a cell path-length of 0.5 mm. The CD spectra were measured from 260 nm to 190 nm, and CD spectra were obtained by averaging the signals of five scans.
3.2. Competitive Binding of CTXs and Coralyne to MB
All samples were prepared in solution containing 10 mM HEPES (pH 7.3). The FAM/DABCYL-labeled A12-MB-A12 (10 nM) were titrated with small aliquots of CTXs in the presence of 0.6 μM coralyne. Total dilution never exceeded 10% and the relative fluorescence values were uniformly corrected for dilution. Titration of CTXs was stopped when fluorescence intensity of MB no longer changing. The fluorescence spectra were measured on a Hitachi F-4500 Fluorescence spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) with excitation wavelength at 480 nm. To measurement the dissociation constant of CTXs with MB, FAM/DABCYL-labeled A12-MB-A12 (10 nM) were titrated with small aliquots of CTXs in the absence of coralyne. A plot of the 1/(Fo-F) versus 1/[Toxin] gives lines with a slope corresponding to the dissociation constant of MB-toxin complexes. Fo and F are fluorescence intensity in the absence or presence of toxins. To study the effect of anti-CTX3 antibodies on the binding of CTX3 and crude venom to hairpin-shaped MB, CTX3, and crude venom were preincubated with anti-CTX3 antibodies for 10 min. The FAM fluorescence of MB was measured after addition of the reaction mixtures.
3.3. Binding of Rhodamine-Labeled CTX3 with Coralyne
Rhodamine-labeled CTX3 was prepared according to the procedure described in Chen et al. [
29]. Rhodamine-labeled CTX3 (0.2 μM) was titrated with increasing concentrations of coralyne until maximal changes in fluorescence intensity of rhodamine-labeled CTX3 was achieved. The binding was monitored at excitation wavelength and emission wavelength at 550 and 580 nm, respectively. A plot of the 1/(Fo-F) versus 1/[coralyne] gives lines with a slope corresponding to the dissociation constant of CTX3-coralyne complexes. Fo and F are fluorescence intensity in the absence or presence of coralyne.
3.4. Measurement of Coralyne- and CTX3-Induced Folded Structure of Label-Free MB Using SG Fluorescence Enhancement
A stock solution of SG (12.3 μM) was prepared according to the procedure described in Lin and Tseng [
18]. SG (160 nM) and label-free MB (0.5 μM) were incubated in 10 mM HEPES (pH 7.3) for 40 min until the SG fluorescence intensity was no more changed. Then the solution containing SG and label-free MB was titrated with coralyne and CTX, respectively. The fluorescence spectra were measured on a Hitachi F-4500 Fluorescence spectrophotometer with the excitation wavelength at 494 nm.
3.5. Molecular Docking
Molecular docking of the binding of coralyne with CTX3 was conducted using iGEMDOCK v2.1 (BioXGEM Lab., Hsinchu, Taiwan). We used its molecular docking platform to dock the coralyne to CTX3 with a population size of 300, a number of generations of 80 and the number of solutions set to 100. The 100 docking scores were then used for statistical analysis to evaluate the binding model of CTX3 with coralyne.