Anticonvulsant Effects of Fractions Isolated from Dinoponera quadriceps (Kempt) Ant Venom (Formicidae: Ponerinae)
Abstract
:1. Introduction
2. Results
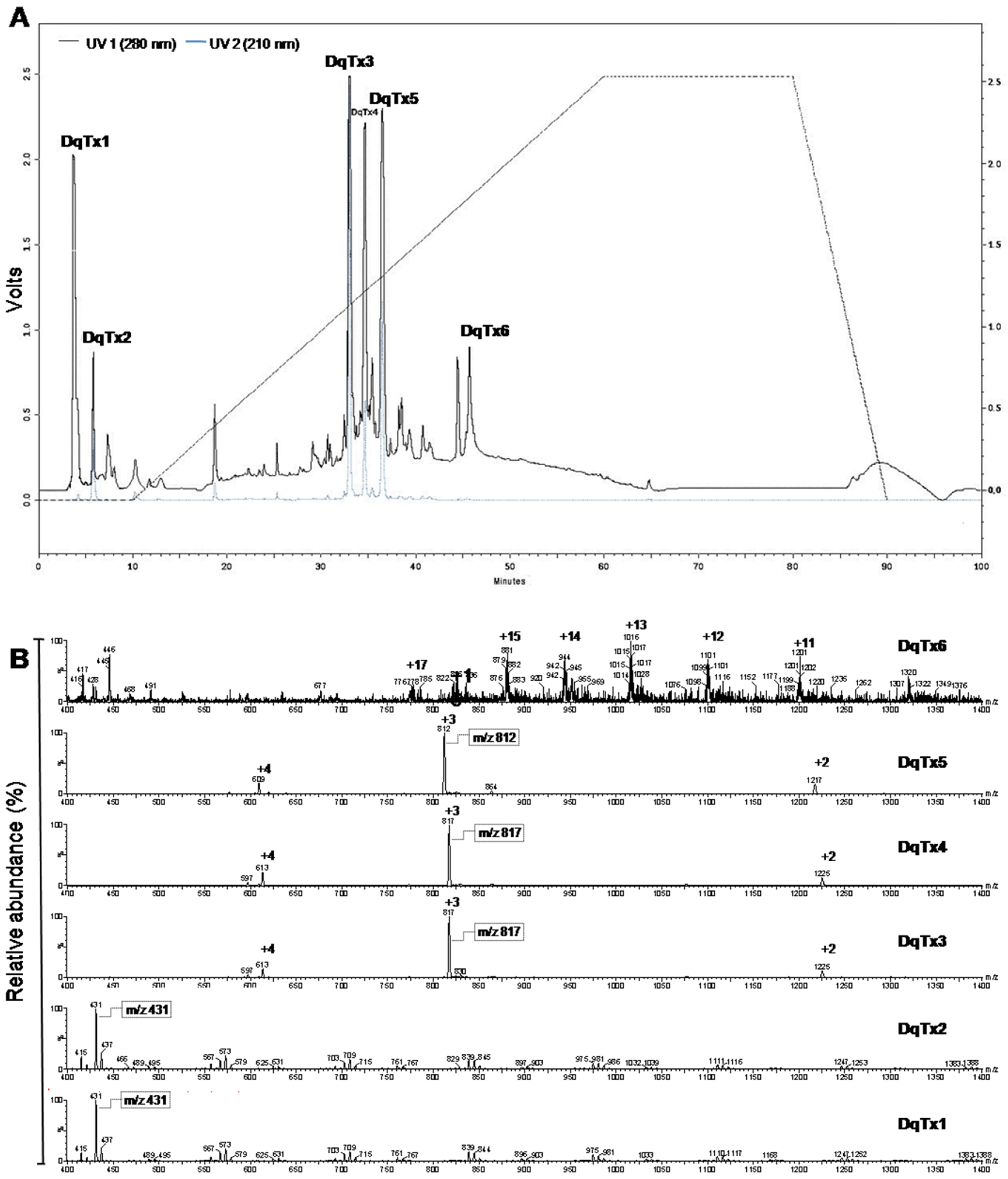
2.1. HPLC Fractionation and Mass Spectra
2.2. Primary Behavioral Screening
2.3. Anticonvulsant Assay
3. Discussion
4. Material and Methods
4.1. Ant Collection
4.2. Fractionation and Mass Spectrometry Analysis
4.3. Animals
4.4. Surgery
4.5. General Procedures
4.6. Behavioral Analysis
4.7. Verification of the Injection Site
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sitprija, V.; Suteparak, S. Animal toxins: An overview. Asian Biomed. 2008, 2, 451–457. [Google Scholar]
- Wong, E.S.W.; Belov, K. Venom evolution through gene duplications. Gene 2012, 496, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.-W.; Zhang, Y.; Wu, X.-F.; Peng, Y.; Cao, Z.; Ge, B.Y.; Wang, X.; Wu, Q.; Lin, J.T.; et al. Scorpion Venom Heat-Resistant Peptide (SVHRP) Enhances Neurogenesis and Neurite Outgrowth of Immature Neurons in Adult Mice by Up-Regulating Brain-Derived Neurotrophic Factor (BDNF). PLoS ONE 2014, 9, e109977. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-M.; Zhao, D.; Yu, D.-Q.; Li, S.-L.; An, D.; Peng, Y.; Xu, H.; Sun, Y.P.; Wang, D.M.; Zhao, J.; et al. Neuroprotection by scorpion venom heat resistant peptide in 6-hydroxydopamine rat model of early-stage Parkinson’s disease. Sheng Li Xue Bao 2014, 66, 658–666. [Google Scholar] [PubMed]
- Wang, C.-Z.; Chi, C.-W. Conus peptides—A rich pharmaceutical treasure. Acta Biochim. Biophys. Sin. 2004, 36, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Mellor, I.R.; Usherwood, P.N.R. Targeting ionotropic receptors with polyamine-containing toxins. Toxicon 2004, 43, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Mortari, M.R.; Cunha, A.O.S.; Ferreira, L.B.; dos Santos, W.F. Neurotoxins from invertebrates as anticonvulsants: From basic research to therapeutic application. Pharmacol. Ther. 2007, 114, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Beleboni, R.O.; Pizzo, A.B.; Fontana, A.C.K.; Carolino, R.O.G.; Coutinho-Netto, J.; dos Santos, W.F. Spider and wasp neurotoxins: Pharmacological and biochemical aspects. Eur. J. Pharmacol. 2004, 493, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Do Couto, L.L.; dos Anjos, L.C.; Araujo, M.A.F.; Mourão, C.A.; Schwartz, C.A.; Ferreira, L.B.; Mortari, M.R. Anticonvulsant and anxiolytic activity of the peptide fraction isolated from the venom of the social wasp Polybia paulista. Pharmacogn. Mag. 2012, 8, 292–299. [Google Scholar] [PubMed]
- Gelfuso, E.A.; Cunha, A.O.S.; Mortari, M.R.; Liberato, J.L.; Paraventi, K.H.; Beleboni, R.O.; Coutinho-Netto, J.; Lopes, N.P.; dos Santos, W.F. Neuropharmacological profile of FrPbAII, purified from the venom of the social spider Parawixia bistriata (Araneae, Araneidae), in Wistar rats. Life Sci. 2007, 80, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Gelfuso, E.A.; Liberato, J.L.; Cunha, A.O.S.; Mortari, M.R.; Beleboni, R.O.; Lopes, N.P.; dos Santos, W.F. Parawixin2, a novel non-selective GABA uptake inhibitor from Parawixia bistriata spider venom, inhibits pentylenetetrazole-induced chemical kindling in rats. Neurosci. Lett. 2013, 543, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Thurman, D.J.; Beghi, E.; Begley, C.E.; Berg, A.T.; Buchhalter, J.R.; Ding, D.; Hesdorffer, D.C.; Hauser, W.A.; Kazis, L.; Kobau, R.; et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011, 52, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Rosillo-de la Torre, A. Pharmacoresistant epilepsy and nanotechnology. Front. Biosci. 2014, 6, 329–340. [Google Scholar] [CrossRef]
- Löscher, W. Animal models of intractable epilepsy. Prog. Neurobiol. 1997, 53, 239–258. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Gomes, F.M.M.; Campos, G.A.A.; de Castro Silva, J.; Biolchi, A.M.; dos Anjos, L.C.; Gonçalves, J.C.; Lopes, K.S.; Mortari, M.R. Neuroactive compounds obtained from arthropod venoms as new therapeutic platforms for the treatment of neurological disorders. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.S.; Rios, E.R.; Lima, C.N.; Linhares, M.I.; Torres, A.F.; Havt, A.; Quinet, Y.P.; Fonteles, M.M.; Martins, A.M. The effects of the Brazilian ant Dinoponera quadriceps venom on chemically induced seizure models. Neurochem. Int. 2013, 63, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Nôga, D.A.M.F.; Cagni, F.C.; Santos, J.R.; Silva, D.; Azevedo, D.L.O.; Araújo, A.; Silva, R.H.; Ribeiro, A.M. Pro- and Anticonvulsant Effects of the Ant Dinoponera quadriceps (Kempf) Venom in Mice. Neotrop. Entomol. 2015, 44, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Aili, S.R.; Touchard, A.; Escoubas, P.; Padula, M.P.; Orivel, J.; Dejean, A.; Nicholson, G.M. Diversity of peptide toxins from stinging ant venoms. Toxicon 2014, 92C, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Orivel, J.; Redeker, V.; le Caer, J.P.; Krier, F.; Revol-Junelles, A.M.; Longeon, A.; Chafotte, A.; Dejean, A.; Rossier, J. Ponericins, new antibacterial and insecticidal peptides from the venom of the ant Pachycondyla goeldii. J. Biol. Chem. 2001, 276, 17823–17829. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Akagi, M.; Imai, H.T.; Taylor, R.W.; Kubo, T. Molecular cloning and biological characterization of novel antimicrobial peptides, pilosulin 3 and pilosulin 4, from a species of the Australian ant genus Myrmecia. Arch. Biochem. Biophys. 2004, 428, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Cologna, C.T.; Cardoso, J.D.S.; Jourdan, E.; Degueldre, M.; Upert, G.; Gilles, N.; Uetanabaro, A.P.T.; Costa Neto, E.M.; Thonart, P.; Pauw, E.; et al. Peptidomic comparison and characterization of the major components of the venom of the giant ant Dinoponera quadriceps collected in four different areas of Brazil. J. Proteom. 2013, 94, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Malécot, C.O.; Pelhate, M.; Piek, T. Poneratoxin, a new toxin from an ant venom, reveals an interconversion between two gating modes of the Na channels in frog skeletal muscle fibres. Pflugers Arch. 1992, 420, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Piek, T.; Duval, A.; Hue, B.; Karst, H.; Lapied, B.; Mantel, P.; Nakajima, T.; Pelhate, M.; Schmidt, J.O. Poneratoxin, a novel peptide neurotoxin from the venom of the ant, Paraponera clavata. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1991, 99, 487–495. [Google Scholar] [CrossRef]
- Szolajska, E.; Poznanski, J.; Ferber, M.L.; Michalik, J.; Gout, E.; Fender, P.; Bailly, I.; Dublet, B.; Chroboczeck, J. Poneratoxin, a neurotoxin from ant venom. Structure and expression in insect cells and construction of a bio-insecticide. Eur. J. Biochem. 2004, 271, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.; Mozrzymas, J. The effect of poneratoxin on neuromuscular transmission in the rat diaphragm. Cell. Mol. Biol. Lett. 2002, 7, 195–202. [Google Scholar] [PubMed]
- Touchard, A.; Brust, A.; Cardoso, F.C.; Chin, Y.K.-Y.; Herzig, V.; Jin, A.-H.; Dejean, A.; Alewood, G.F.; King, J.; Orivel, P. Isolation and characterization of a structurally unique β-hairpin venom peptide from the predatory ant Anochetus emarginatus. Biochim. Biophys. Acta. 2016, 1860, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, X.-Y.; Yang, J.; Weng, C.-C.; Jiang, L.-L.; Zhang, J.-W.; Shu, X.-Q.; Ji, Y.-H. Anticonvulsant effect of BmK IT2, a sodium channel-specific neurotoxin, in rat models of epilepsy. Br. J. Pharmacol. 2008, 154, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Weng, C.-C.; Feng, Q.; Chen, L.; Zhang, X.-Y.; Zhu, H.-Y.; Wang, Y.; Ji, Y.-H. Anticonvulsant activity of BmK AS, a sodium channel site 4-specific modulator. Epilepsy Behav. 2011, 20, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.; Zhou, L.; Layer, R.; Nielson, J.; McCabe, R.T.; White, H.S. Anticonvulsant profile of Conantokin-G: A Novel, broad spectrum NMDA antagonist. Epilepsia 1998, 39, 39–40. [Google Scholar]
- Barton, M.E.; White, H.S. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on kindling acquisition and expression. Epilepsy Res. 2004, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.C.; Scheideler, M.A. Behavioural and anticonvulsant effects of Ca2+ channel toxins in DBA/2 mice. Psychopharmacology 1996, 126, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Capasso, A.; Gallo, C. Anticonvulsant activity of new GABA prodrugs. Med. Chem. 2009, 5, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Faggion, S.A.; Cunha, A.O.S.; Fachim, H.A.; Gavin, A.S.; dos Santos, W.F.; Pereira, A.M.S.; Beleboni, R.O. Anticonvulsant profile of the alkaloids (+)-erythravine and (+)-11-α-hydroxy-erythravine isolated from the flowers of Erythrina mulungu Mart ex Benth (Leguminosae-Papilionaceae). Epilepsy Behav. 2011, 20, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.O.S.; Mortari, M.R.; Oliveira, L.; Carolino, R.O.G.; Coutinho-Netto, J.; dos Santos, W.F. Anticonvulsant effects of the wasp Polybia ignobilis venom on chemically induced seizures and action on GABA and glutamate receptors. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2005, 141, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mussi-Ribeiro, A.; Miranda, A.; Gobbo-Netto, L.; Peporine Lopes, N.; dos Santos, W.F. A anticonvulsive fraction from Scaptocosa raptoria (Araneae: Lycosidae) spider venom. Neurosci. Lett. 2004, 371, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Thompson, K.S.; Sargent, B.J.; Heal, D.J. A novel CNS drug with potential anticonvulsant, neuroprotective, and antimigraine properties. CNS Drug Rev. 2001, 7, 146–171. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.E.; Baldwin, R.A.; Niquet, J.; Stafford, G.I.; van Staden, J.; Wasterlain, C.G.; Jäger, K.A. Anticonvulsant effects of Searsia dentata (Anacardiaceae) leaf extract in rats. Phytother. Res. 2009, 24, 924–927. [Google Scholar]
- White, H.S.; McCabe, R.T.; Armstrong, H.; Donevan, S.D.; Cruz, L.J.; Abogadie, F.C.; Torres, J.; Rivier, J.E.; Paarmann, I.; Hollmann, M.; et al. In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiates. J. Pharmacol. Exp. Ther. 2000, 292, 425–432. [Google Scholar] [PubMed]
- Liberato, J.L.; Cunha, A.O.S.; Mortari, M.R.; Gelfuso, E.A.; Beleboni, R.O.; Coutinho-Netto, J.; dos Santos, W.F. Anticonvulsant and anxiolytic activity of FrPbAII, a novel GABA uptake inhibitor isolated from the venom of the social spider Parawixia bistriata (Araneidae: Araneae). Brain Res. 2006, 1124, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fachim, H.A.; Cunha, A.O.S.; Pereira, A.C.; Beleboni, R.O.; Gobbo-Neto, L.; Lopes, N.P.; Coutinho-Netto, J.; dos Santos, W.F. Neurobiological activity of Parawixin 10, a novel anticonvulsant compound isolated from Parawixia bistriata spider venom (Araneidae: Araneae). Epilepsy Behav. 2011, 22, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-Z.; Guo, C.; Tian, Y.; Chen, D.; Greenaway, F.T.; Liu, S. Biochemical, functional and structural characterization of Akbu-LAAO: A novel snake venom l-amino acid oxidase from Agkistrodon blomhoffii ussurensis. Biochimie 2010, 92, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.C.; Chacón, A.M.; Vargas, L.; Segura, C.; Gutiérrez, J.M.; Alarcón, J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012, 124, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rocha-e-Silva, T.A.A.; Rostelato-Ferreira, S.; Leite, G.B.; da Silva, P.I.; Hyslop, S.; Rodrigues-Simioni, L. VdTX-1, a reversible nicotinic receptor antagonist isolated from venom of the spider Vitalius dubius (Theraphosidae). Toxicon 2013, 70, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.N.; Vo, H.D.M.; Vo, N.P.; Kudryashova, K.S.; Nekrasova, O.V.; Feofanov, A.V.; Kispichnikov, M.P.; Andreeva, T.V.; Serebryakova, M.V.; Tsetlin, V.I.; et al. Vietnamese Heterometrus laoticus scorpion venom: Evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 2014, 77, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Stiles, B.G.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Franco, O.L.; Rowan, E.G.; Kumar, A.P.; Lim, L.H.K.; Sethi, G. Viperatoxin-II: A novel viper venom protein as an effective bactericidal agent. FEBS Open Bio 2015, 5, 928–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olamendi-Portugal, T.; Bartok, A.; Zamudio-Zuñiga, F.; Balajthy, A.; Becerril, B.; Panyi, G.; Possani, L.D. Isolation, chemical and functional characterization of several new K+-channel blocking peptides from the venom of the scorpion Centruroides tecomanus. Toxicon 2016, 115, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, L.C.; Gomes, F.M.M.; do Couto, L.L.; Mourão, C.A.; Moreira, K.G.; Silva, L.P.; Mortari, M.R. Anxiolytic activity and evaluation of potentially adverse effects of a bradykinin-related peptide isolated from a social wasp venom. Life Sci. 2016, 149, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hink, W.F. Isolation and characterization of myrmexins, six isoforms of venom proteins with anti-inflammatory activity from the tropical ant, Pseudomyrmex triplarinus. Toxicon 2000, 38, 1403–1413. [Google Scholar] [CrossRef]
- Mendes, M.A.; Palma, M.S. Two new bradykinin-related peptides from the venom of the social wasp Protopolybia exigua (Saussure). Peptides 2006, 27, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Turillazzi, S.; Mastrobuoni, G.; Dani, F.R.; Moneti, G.; Pieraccini, G.; Marca, G.; Bartolucci, G.; Perito, B.; Lombardi, D.; Cavallini, V.; et al. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. J. Am. Soc. Mass Spectrom. 2006, 17, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Kong, Y.; Zhai, L.; Wu, X.; Jia, P.; Liu, J.; Yu, H. Two novel antimicrobial peptides from centipede venoms. Toxicon 2010, 55, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Picolo, G.; Hisada, M.; Moura, A.B.; Machado, M.F.M.; Sciani, J.M.; Conceição, I.M.; Melo, R.L.; Oliveira, V.; Lima-Landman, M.T.R.; Cury, Y.; et al. Bradykinin-related peptides in the venom of the solitary wasp Cyphononyx fulvognathus. Biochem. Pharmacol. 2010, 79, 478–486. [Google Scholar] [PubMed]
- Ferreira, M.J.; Lima, C.; Lopes-Ferreira, M. Anti-inflammatory effect of Natterins, the major toxins from the Thalassophryne nattereri fish venom is dependent on TLR4/MyD88/PI3K signaling pathway. Toxicon 2014, 87, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Vulfius, C.A.; Spirova, E.N.; Serebryakova, M.V.; Shelukhina, I.V.; Kudryavtsev, D.S.; Kryukova, U.V.; Starkov, V.G.; Kopylova, N.V.; Zhmak, M.N.; Ivanov, I.A.; et al. Peptides from puff adder Bitis arietans venom, novel inhibitors of nicotinic acetylcholine receptors. Toxicon 2016, 121, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Sills, G.J.; Brodie, M.J. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol. Ther. 2001, 90, 21–34. [Google Scholar] [CrossRef]
- Gasior, M.; White, N.A.; Rogawski, M.A. Prolonged attenuation of amygdala-kindled seizure measures in rats by convection-enhanced delivery of the N-type calcium channel antagonists omega-conotoxin GVIA and omega-conotoxin MVIIA. J. Pharmacol. Exp. Ther. 2007, 323, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Kelly, K.M. Antiepileptic drug mechanisms of action. Epilepsia 1995, 36, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Dalby, N.O. GABA-level increasing and anticonvulsant effects of three different GABA uptake inhibitors. Neuropharmacology 2000, 39, 2399–2407. [Google Scholar] [CrossRef]
- Duncan, J.S.; Sander, J.W.; Sisodiya, S.M.; Walker, M.C. Adult epilepsy. Lance 2006, 367, 1087–1100. [Google Scholar] [CrossRef]
- Rogawski, M.A. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006, 69, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Beleboni, R.O.; Guizzo, R.; Fontana, A.C.K.; Pizzo, A.B.; Carolino, R.O.G.; Gobbo-Neto, L.; Lopes, N.P.; Coutinho-Netto, J.; dos Santos, W.F. Neurochemical characterization of a neuroprotective compound from Parawixia bistriata spider venom that inhibits synaptosomal uptake of GABA and glycine. Mol. Pharmacol. 2006, 69, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.L.; Quinet, Y.; Ponte, E.L.; do Vale, J.F.; Torres, A.F.C.; Pereira, M.G.; Assreuy, A.M.S. Venom’s antinociceptive property in the primitive ant Dinoponera quadriceps. J. Ethnopharmacol. 2012, 144, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; Torres, A.F.C.; Mello, C.P.; de Menezes, R.R.; Sampaio, T.L.; Canuto, J.A.; da Silva, J.A.A.; Freire, V.N.; Quinet, Y.P.; Havt, A.; et al. Antimicrobial effect of Dinoponera quadriceps (Hymenoptera: Formicidae) venom against Staphylococcus aureus strains. J. Appl. Microbiol. 2014, 117, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; Sousa, P.L.; Torres, A.F.C.; Rodrigues, K.A.F.; Mello, C.P.; de Menezes, R.R.P.P.B.; Tessarolo, L.D.; Quinet, Y.P.; de Oliveira, M.R.; Martins, A.M.C. Antiparasitic effect of Dinoponera quadriceps giant ant venom. Toxicon 2016, 120, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Madeira, J.C.; Quinet, Y.P.; Nonato, D.T.T.; Sousa, P.L.; Chaves, E.M.C.; Honório Júnior, J.E.R.; Pereira, M.G.; Assreuy, A.M.S. Novel Pharmacological Properties of Dinoponera quadriceps Giant Ant Venom. Nat. Prod. Commun. 2015, 10, 1607–1609. [Google Scholar]
- Sousa, P.L.; Quinet, Y.P.; Brizeno, L.A.C.; Sampaio, T.L.; Torres, A.F.C.; Martins, A.M.C.; Assreuy, A.M.S. The acute inflammatory response induced in mice by the venom of the giant ant Dinoponera quadriceps involves macrophage and interleukin-1β. Toxicon 2016, 117, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.F.C.; Huang, C.; Chong, C.-M.; Leung, S.W.; Prieto-da-Silva, A.R.B.; Leung, S.W.; Prieto-da-Silva, A.R.B.; Havt, A.; Quinet, Y.P.; Martins, A.M.C.; et al. Transcriptome analysis in venom gland of the predatory giant ant Dinoponera quadriceps: Insights into the polypeptide toxin arsenal of hymenopterans. PLoS ONE 2014, 9, e87556. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K. The Mouse Brain in Stereotaxic Coordenates, 3th ed.; Elsevier Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]

| Treatment | Behavioral Cluster (s) | ||
|---|---|---|---|
| Exploration | Grooming | Immobility | |
| Control (n = 9) | 1395.48 ± 56.38 | 293.14 ± 55.36 | 111.36 ± 36.47 |
| DqTx1 (n = 7) | 1281.37 ± 110.25 | 352.47 ± 104.24 | 166.15 ± 93.05 |
| DqTx2 (n = 7) | 1250.36 ± 128.08 | 267.05 ± 58.79 | 282.58 ± 119.69 |
| DqTx3 (n = 8) | 1367.91 ± 58.9 | 294.31 ± 37.89 | 137.77 ± 53.78 |
| DqTx4 (n = 8) | 1193.58 ± 49.32 | 493.80 ± 50.81 | 112.61 ± 44.11 |
| DqTx5 (n = 8) | 1250.40 ± 87.55 | 406.00 ± 38.47 | 143.59 ± 62.27 |
| DqTx6 (n = 8) | 1378.41 ± 84.41 | 280.44 ± 43.68 | 141.14 ± 64.03 |
| Parameters | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Control | DqTx1 | DqTx2 | DqTx3 | DqTx4 | DqTx5 | DqTx6 | |
| Median seizure score | 5 | 5 | 5 | 5 | 5 | 5 | 4 |
| Incidence of seizures | |||||||
| Stage 1 | 0/8 | 6/7 | 4/7 | 3/7 | 5/7 | 3/7 | 5/8 |
| Stage 2 | 0/8 | 4/7 | 4/7 | 3/7 | 5/7 | 3/7 | 5/8 |
| Stage 3 | 0/8 | 2/7 | 5/7 | 2/7 | 3/7 | 2/7 | 4/8 |
| Stage 4 | 0/8 | 1/7 | 2/7 | 2/7 | 0/7 | 1/7 | 1/8 |
| Stage 5 | 8/8 | 4/7 | 7/7 | 5/7 | 4/7 | 4/7 | 3/8 |
| Percentage of protection | 0 | 42.8 | 0 | 28.6 | 42.8 | 42.8 | 62.5 # |
| Percentage of survival | 0 | 71.4 | 42.8 | 71.4 | 57.1 | 57.1 | 100 # |
| Latency for the onset of seizures (s) | 16 ± 6 | 1191 ± 306 * | 710 ± 216 | 879 ± 310 + | 923 ± 318 * | 812 ± 350 | 1225 ±273 * |
| Latency for death (s) | 509 ± 253 | 1528 ± 180 * | 1471 ± 169 * | 1453 ± 199 * | 1342 ± 257 * | 1087 ± 337 | 1800 * |
| Incidence of death | 8/8 | 2/7 | 4/7 | 2/7 | 3/7 | 3/7 | 0/8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nôga, D.A.M.F.; Brandão, L.E.M.; Cagni, F.C.; Silva, D.; De Azevedo, D.L.O.; Araújo, A.; Dos Santos, W.F.; Miranda, A.; Da Silva, R.H.; Ribeiro, A.M. Anticonvulsant Effects of Fractions Isolated from Dinoponera quadriceps (Kempt) Ant Venom (Formicidae: Ponerinae). Toxins 2017, 9, 5. https://doi.org/10.3390/toxins9010005
Nôga DAMF, Brandão LEM, Cagni FC, Silva D, De Azevedo DLO, Araújo A, Dos Santos WF, Miranda A, Da Silva RH, Ribeiro AM. Anticonvulsant Effects of Fractions Isolated from Dinoponera quadriceps (Kempt) Ant Venom (Formicidae: Ponerinae). Toxins. 2017; 9(1):5. https://doi.org/10.3390/toxins9010005
Chicago/Turabian StyleNôga, Diana Aline Morais Ferreira, Luiz Eduardo Mateus Brandão, Fernanda Carvalho Cagni, Delano Silva, Dina Lilia Oliveira De Azevedo, Arrilton Araújo, Wagner Ferreira Dos Santos, Antonio Miranda, Regina Helena Da Silva, and Alessandra Mussi Ribeiro. 2017. "Anticonvulsant Effects of Fractions Isolated from Dinoponera quadriceps (Kempt) Ant Venom (Formicidae: Ponerinae)" Toxins 9, no. 1: 5. https://doi.org/10.3390/toxins9010005





