Effects of Lonomia obliqua Venom on Vascular Smooth Muscle Cells: Contribution of NADPH Oxidase-Derived Reactive Oxygen Species
Abstract
:1. Introduction
2. Results
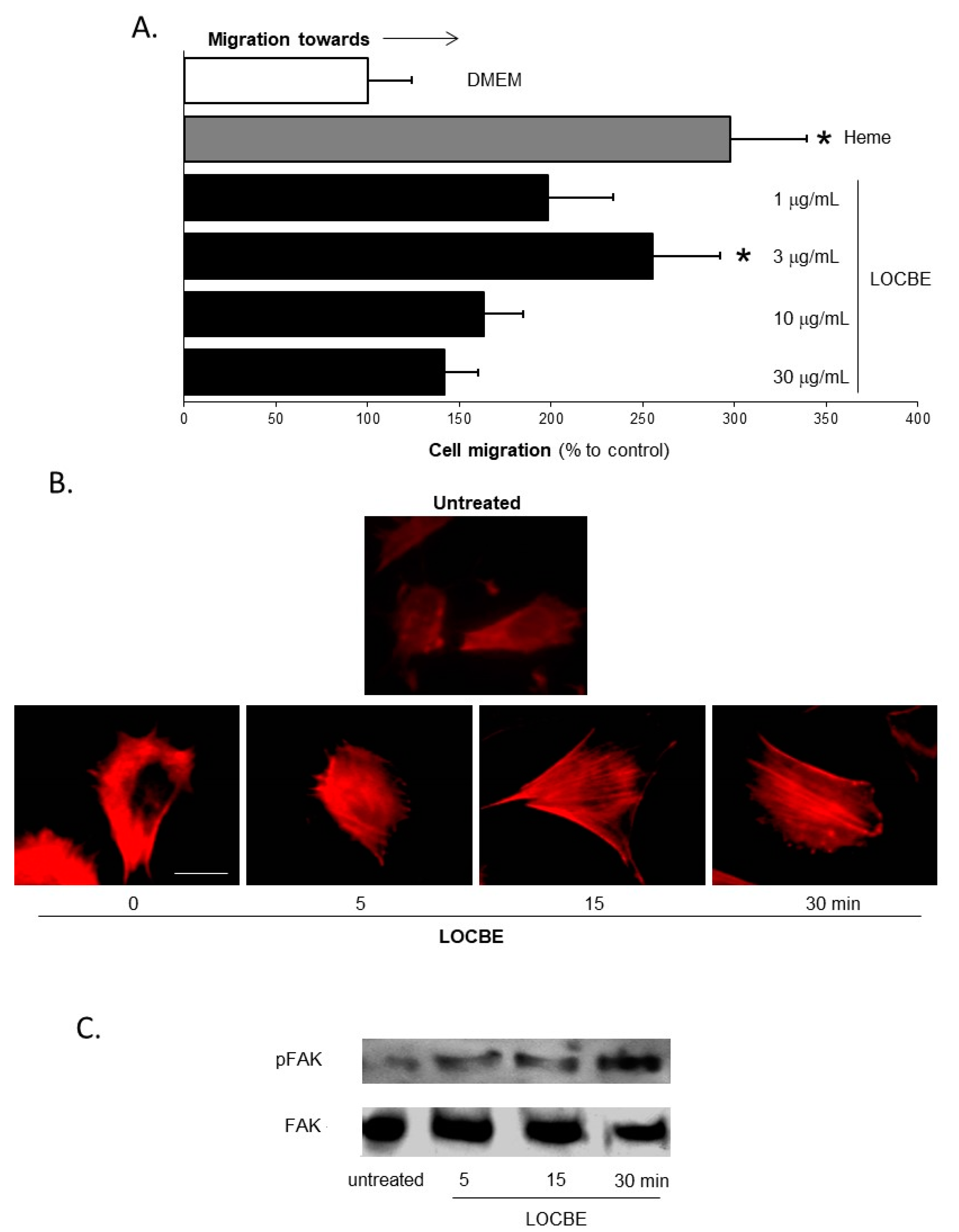
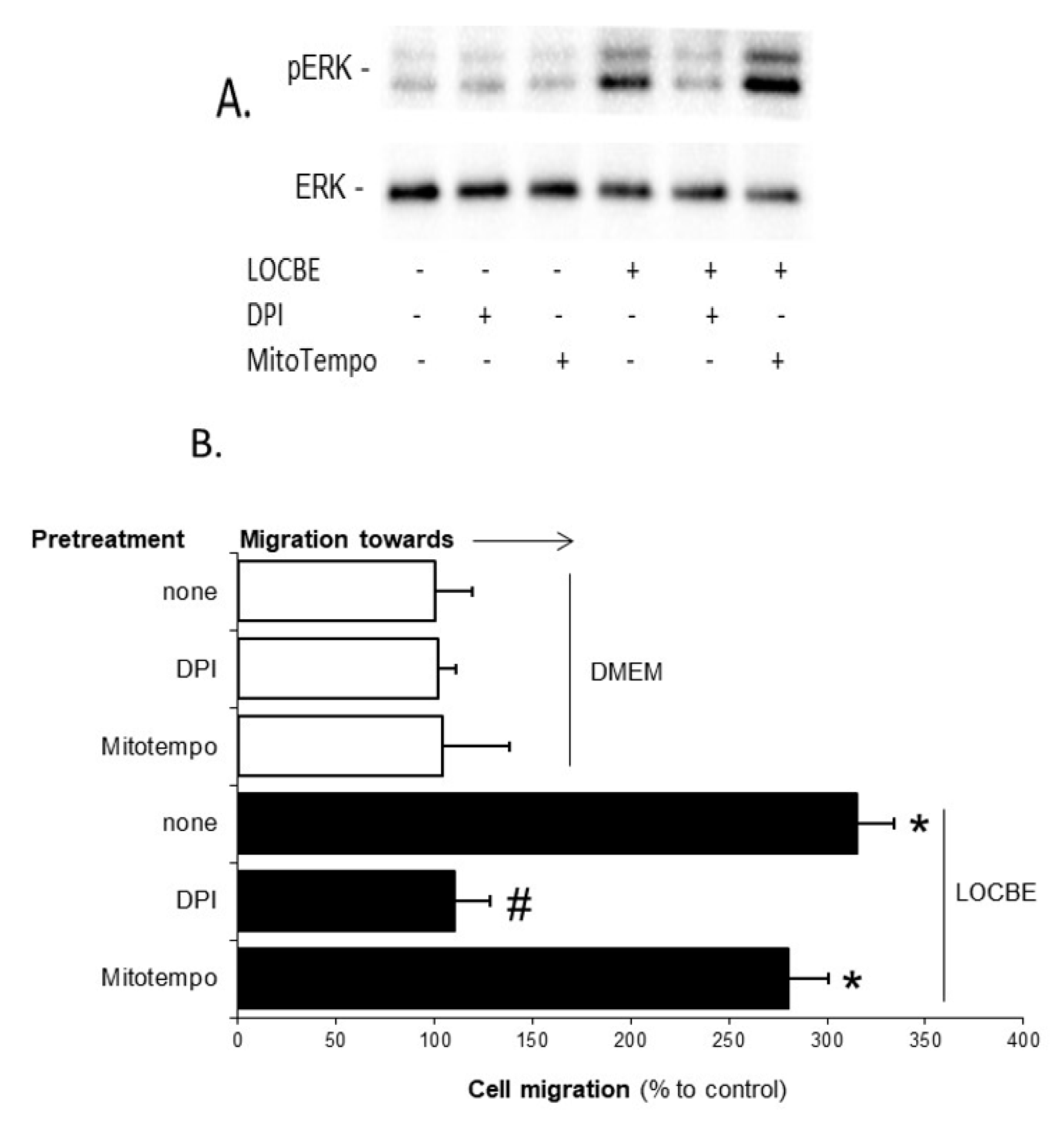
2.1. Effect of LOCBE on VSMC Migration
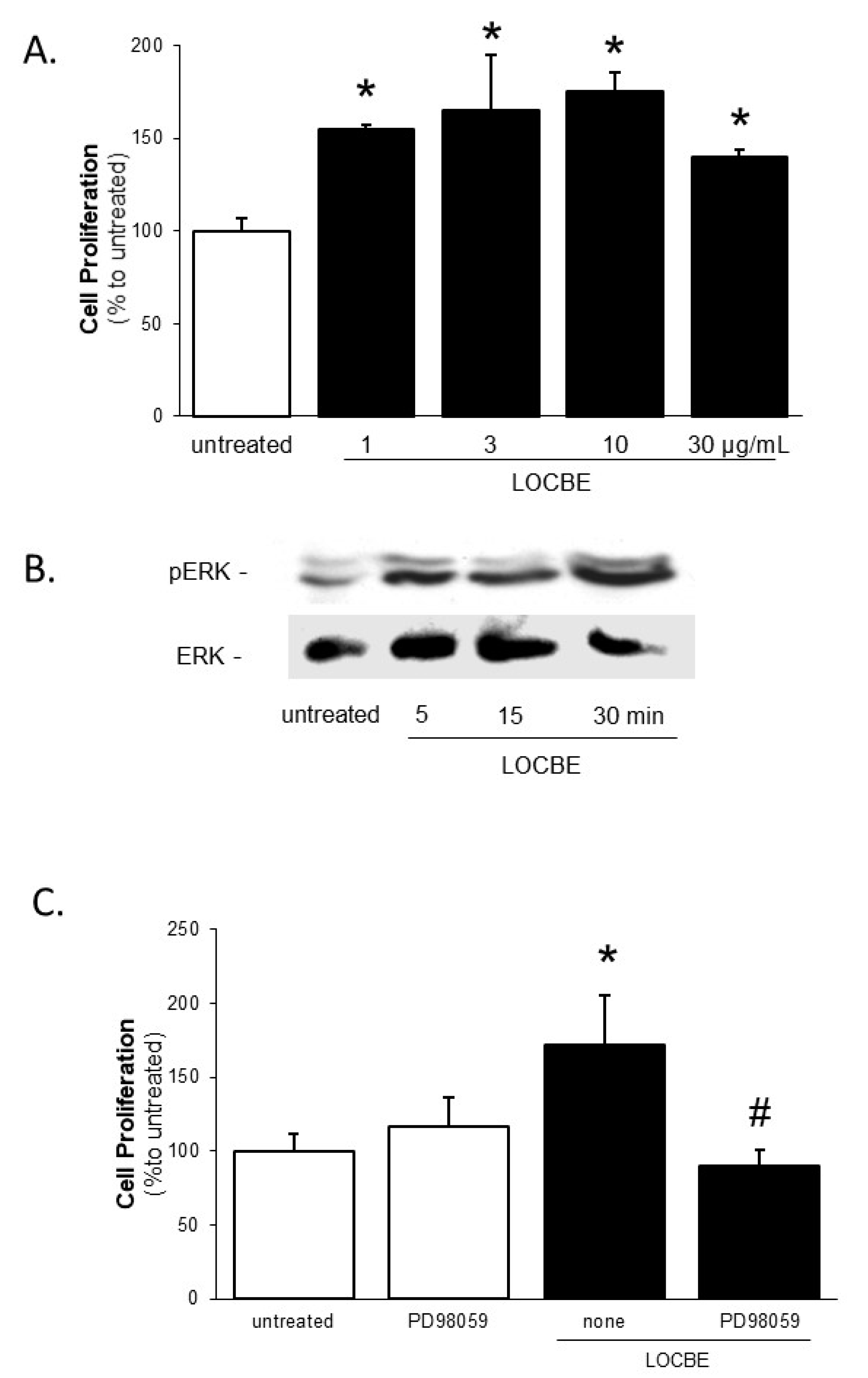
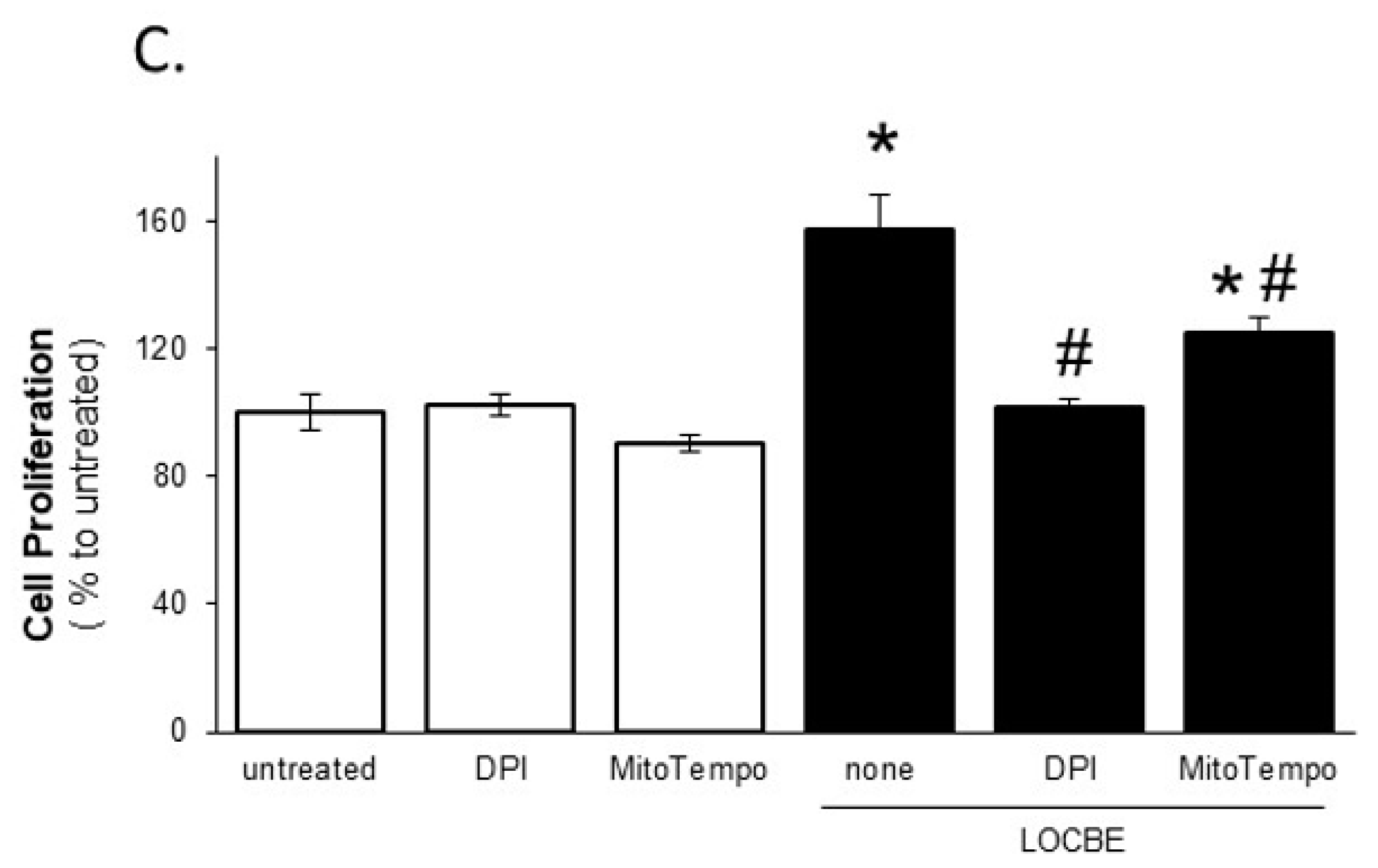
2.2. Effect of LOCBE on VSMC Proliferation
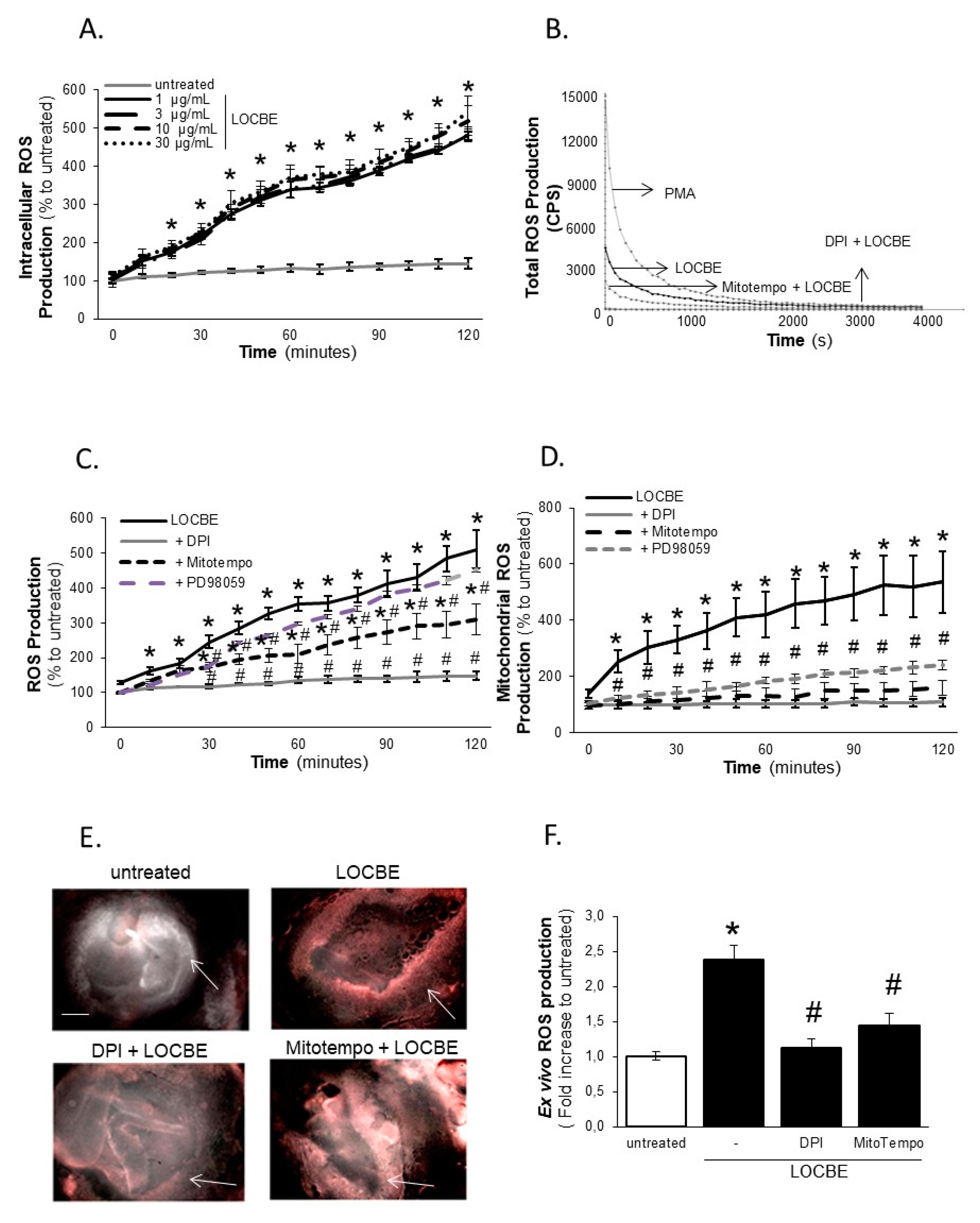
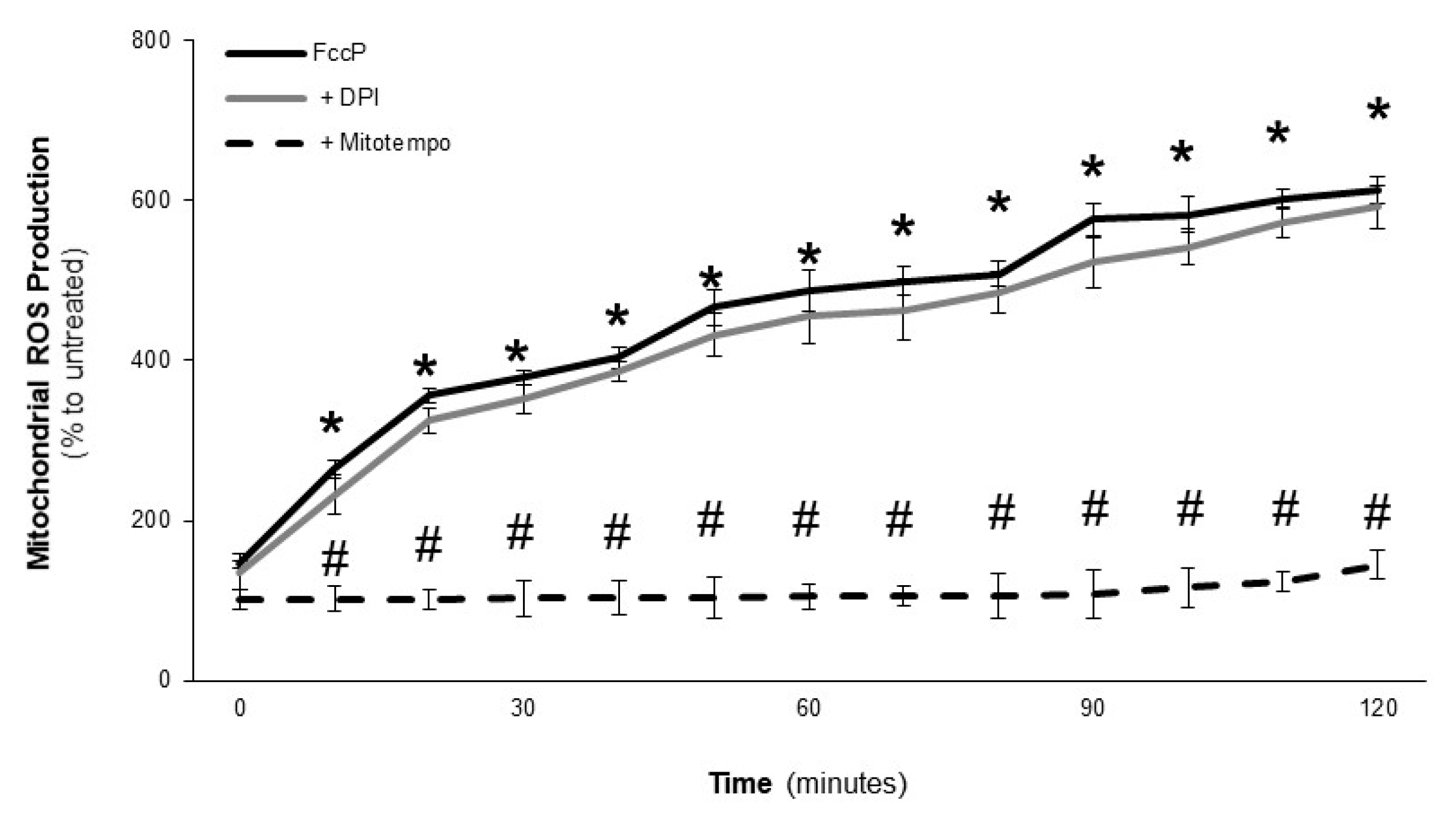
2.3. Effect of LOCBE on VSMC ROS Production
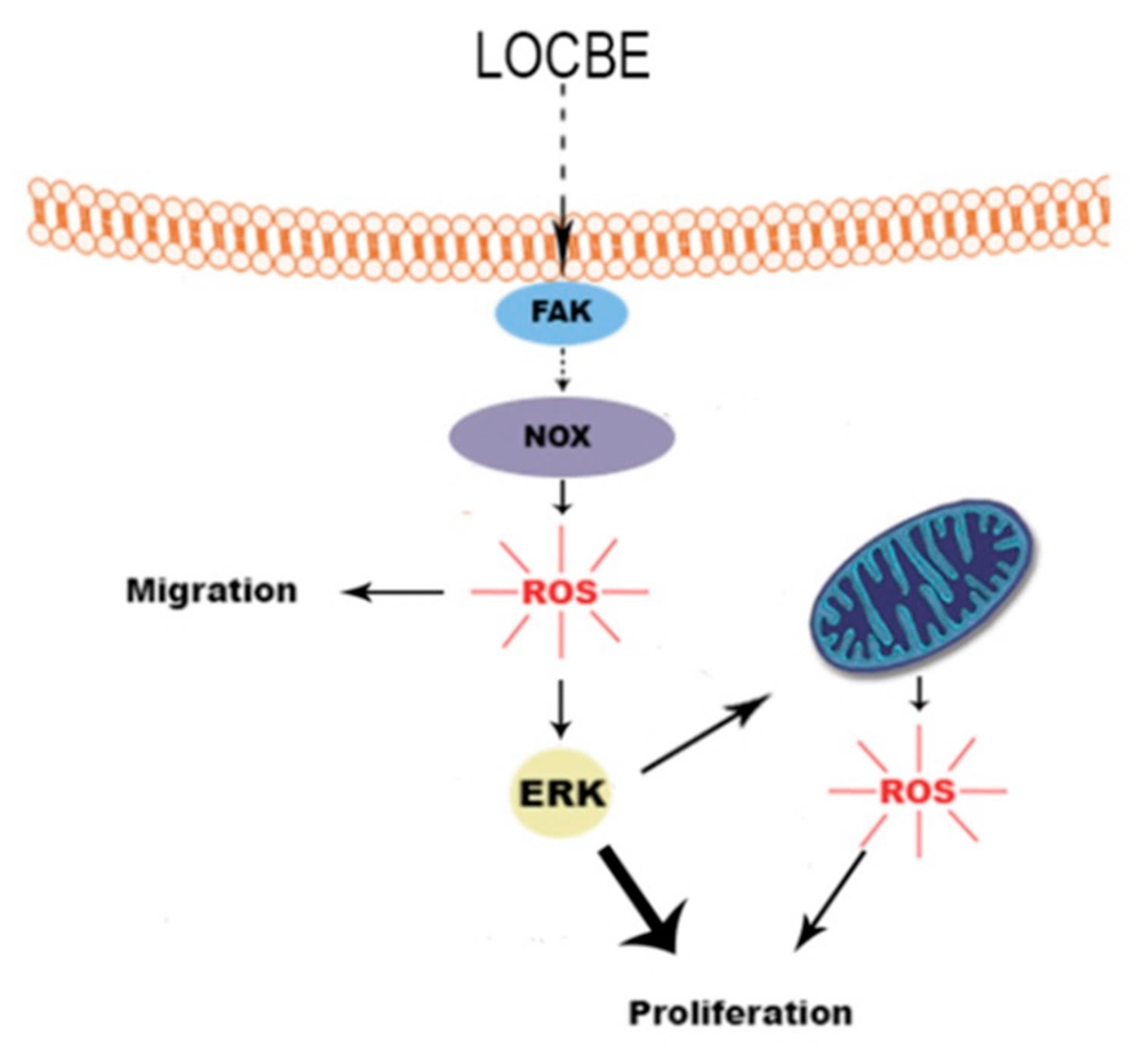
2.4. LOCBE-Induced ROS Production Modulates VSMC Functions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Venom
5.3. Cell Culture
5.4. Cell Migration
5.5. Cell Proliferation
5.6. Reactive Oxygen Species Production
5.7. Ex Vivo ROS Production Assay
5.8. Immunofluorescence Microscopy
5.9. Cellular Extract
5.10. Western Blot Analysis
5.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arocha-Pinango, C.L.; Guerrero, B. Lonomia genus caterpillar envenomation: Clinical and biological aspects. Haemostasis 2001, 31, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Arocha-Pinango, C.L.; Layrisse, M. Fibrinolysis produced by contact with a caterpillar. Lancet 1969, 1, 810–812. [Google Scholar] [CrossRef]
- Veiga, A.B.; Blochtein, B.; Guimaraes, J.A. Structures involved in production, secretion and injection of the venom produced by the caterpillar Lonomia obliqua (Lepidoptera, Saturniidae). Toxicon 2001, 39, 1343–1351. [Google Scholar] [CrossRef]
- Kelen, E.M.A.; Picarelli, Z.P.; Duarte, A.C. Hemorrhagic Syndrome Induced by Contact with Caterpillars of the Genus Lonomia (Saturniidae, Hemileucinae). J. Toxicol. Toxin Rev. 1995, 14, 283–308. [Google Scholar] [CrossRef]
- Arocha-Pinango, C.L.; Guerrero, B. Hemorrhagic syndrome induced by caterpillars. Clinical and experimental studies. Review. Investig. Clin. 2003, 44, 155–163. [Google Scholar]
- Carrijo-Carvalho, L.C.; Chudzinski-Tavassi, A.M. The venom of the Lonomia caterpillar: An overview. Toxicon 2007, 49, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Gamborgi, G.P.; Metcalf, E.B.; Barros, E.J. Acute renal failure provoked by toxin from caterpillars of the species Lonomia obliqua. Toxicon 2006, 47, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.V.; Andrade, S.A.; Ramos, O.H.; Ramos, C.R.; Ho, P.L.; Batista, I.F.; Chudzinski-Tavassi, A.M. Lopap, a prothrombin activator from Lonomia obliqua belonging to the lipocalin family: Recombinant production, biochemical characterization and structure-function insights. Biochem. J. 2006, 398, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.F.; Dragulev, B.; Guimaraes, J.A.; Fox, J.W. Novel perspectives on the pathogenesis of Lonomia obliqua caterpillar envenomation based on assessment of host response by gene expression analysis. Toxicon 2008, 51, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Reck, J., Jr.; Terra, R.M.; Beys da Silva, W.O.; Santi, L.; Pinto, A.F.; Vainstein, M.H.; Termignoni, C.; Guimaraes, J.A. Lonomia obliqua venomous secretion induces human platelet adhesion and aggregation. J. Thromb. Thrombolysis 2010, 30, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.H.; Panunto, P.C.; Hyslop, S.; Da Cruz-Hofling, M.A. Immunochemical detection of Lonomia obliqua caterpillar venom in rats. Microsc. Res. Tech. 2004, 65, 276–281. [Google Scholar] [CrossRef] [PubMed]
- De Castro Bastos, L.; Veiga, A.B.; Guimaraes, J.A.; Tonussi, C.R. Nociceptive and edematogenic responses elicited by a crude bristle extract of Lonomia obliqua caterpillars. Toxicon 2004, 43, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, C.B.; Reck Junior, J.; Fernandes, D.; Sordi, R.; Guimaraes, J.A.; Assreuy, J.; Termignoni, C. Kallikrein-kinin system activation by Lonomia obliqua caterpillar bristles: Involvement in edema and hypotension responses to envenomation. Toxicon 2007, 49, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Silva, V.; Rodrigues da Silva, G.; Moraes, J.A.; Cyrino, F.Z.; Seabra, S.H.; Bouskela, E.; Almeida Guimaraes, J.; Barja-Fidalgo, C. A pro-inflammatory profile of endothelial cell in Lonomia obliqua envenomation. Toxicon 2012, 60, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Santi, L.; Beys-da-Silva, W.O.; Oliveira, F.M.; Caliari, M.V.; Yates, J.R., III; Vieira, M.A.; Guimaraes, J.A. Mechanisms of acute kidney injury induced by experimental Lonomia obliqua envenomation. Arch. Toxicol. 2015, 89, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.A.; Frony, A.C.; Dias, A.M.; Renovato-Martins, M.; Rodrigues, G.; Marcinkiewicz, C.; Assreuy, J.; Barja-Fidalgo, C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis 2015, 243, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.A.; Frony, A.C.; Dias, A.M.; Renovato-Martins, M.; Rodrigues, G.; Marcinkiewicz, C.; Assreuy, J.; Barja-Fidalgo, C. Data in support of alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Data Brief 2016, 6, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.B.; Ribeiro, J.M.; Guimaraes, J.A.; Francischetti, I.M. A catalog for the transcripts from the venomous structures of the caterpillar Lonomia obliqua: Identification of the proteins potentially involved in the coagulation disorder and hemorrhagic syndrome. Gene 2005, 355, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Silva, M.E.; Valente, R.H.; Leon, I.R.; Tambourgi, D.V.; Ramos, O.H.; Perales, J.; Chudzinski-Tavassi, A.M. Immunochemical and proteomic technologies as tools for unravelling toxins involved in envenoming by accidental contact with Lonomia obliqua caterpillars. Toxicon 2008, 51, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.H.; Hyslop, S.; Alice da Cruz-Hofling, M. Lonomia obliqua caterpillar venom increases permeability of the blood-brain barrier in rats. Toxicon 2004, 44, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Hansson, G.K. Inflammation and immunity in diseases of the arterial tree: Players and layers. Circ. Res. 2015, 116, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Assreuy, J.; Canetti, C.; Barja-Fidalgo, C. Leukotriene B4 mediates vascular smooth muscle cell migration through alphavbeta3 integrin transactivation. Atherosclerosis 2010, 212, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.A.; Barcellos-de-Souza, P.; Rodrigues, G.; Nascimento-Silva, V.; Silva, S.V.; Assreuy, J.; Arruda, M.A.; Barja-Fidalgo, C. Heme modulates smooth muscle cell proliferation and migration via NADPH oxidase: A counter-regulatory role for heme oxygenase system. Atherosclerosis 2012, 224, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K. Focal adhesions: A personal perspective on a half century of progress. FEBS J. 2017. [CrossRef] [PubMed]
- Berger, M.; Beys-da-Silva, W.O.; Santi, L.; de Oliveira, I.M.; Jorge, P.M.; Henriques, J.A.; Driemeier, D.; Vieira, M.A.; Guimaraes, J.A. Acute Lonomia obliqua caterpillar envenomation-induced physiopathological alterations in rats: Evidence of new toxic venom activities and the efficacy of serum therapy to counteract systemic tissue damage. Toxicon 2013, 74, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.B.; Pinto, A.F.; Guimaraes, J.A. Fibrinogenolytic and procoagulant activities in the hemorrhagic syndrome caused by Lonomia obliqua caterpillars. Thromb. Res. 2003, 111, 95–101. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, J.A.; Rodrigues, G.; Nascimento-Silva, V.; Renovato-Martins, M.; Berger, M.; Guimarães, J.A.; Barja-Fidalgo, C. Effects of Lonomia obliqua Venom on Vascular Smooth Muscle Cells: Contribution of NADPH Oxidase-Derived Reactive Oxygen Species. Toxins 2017, 9, 360. https://doi.org/10.3390/toxins9110360
Moraes JA, Rodrigues G, Nascimento-Silva V, Renovato-Martins M, Berger M, Guimarães JA, Barja-Fidalgo C. Effects of Lonomia obliqua Venom on Vascular Smooth Muscle Cells: Contribution of NADPH Oxidase-Derived Reactive Oxygen Species. Toxins. 2017; 9(11):360. https://doi.org/10.3390/toxins9110360
Chicago/Turabian StyleMoraes, João Alfredo, Genilson Rodrigues, Vany Nascimento-Silva, Mariana Renovato-Martins, Markus Berger, Jorge Almeida Guimarães, and Christina Barja-Fidalgo. 2017. "Effects of Lonomia obliqua Venom on Vascular Smooth Muscle Cells: Contribution of NADPH Oxidase-Derived Reactive Oxygen Species" Toxins 9, no. 11: 360. https://doi.org/10.3390/toxins9110360





