Synthesis of Mono- and Di-Glucosides of Zearalenone and ?-/?-Zearalenol by Recombinant Barley Glucosyltransferase HvUGT14077
Abstract
:1. Introduction
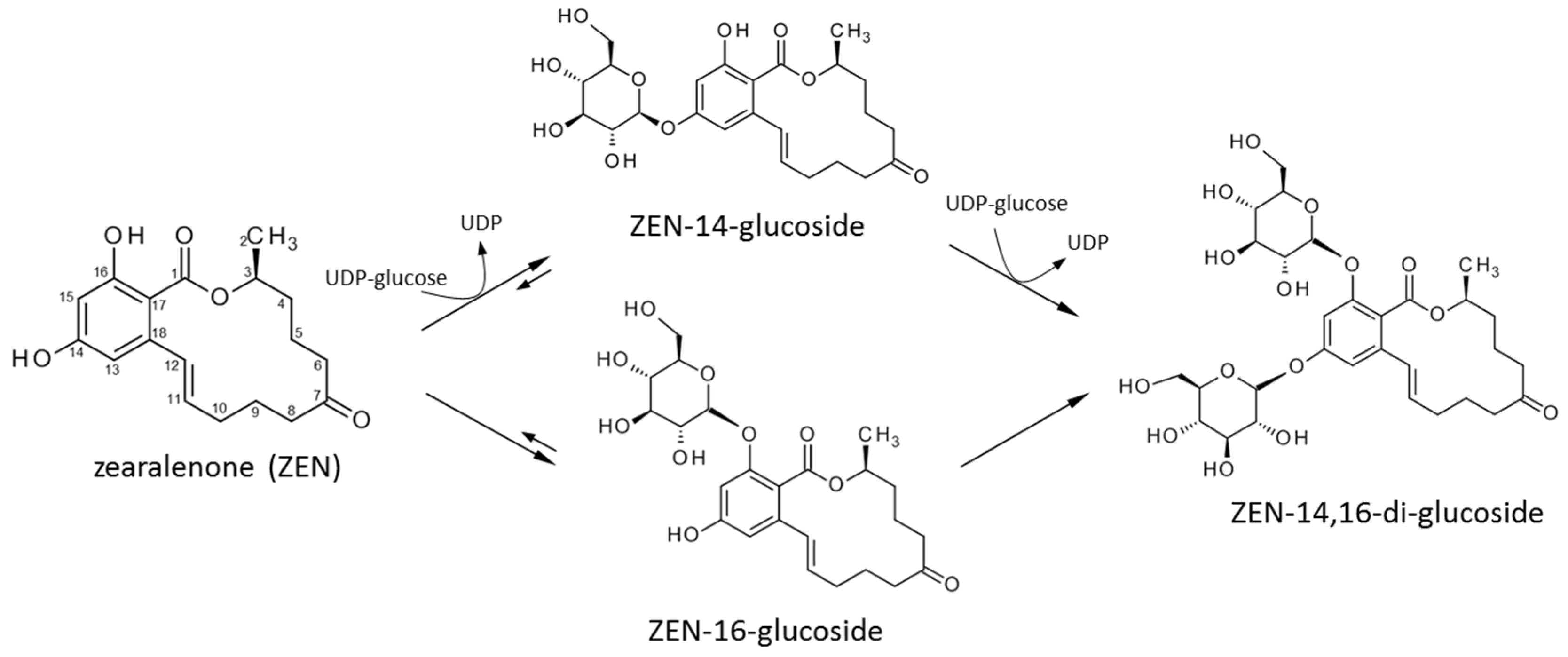
2. Results
2.1. Biochemical Characteristics of HvUGT14077
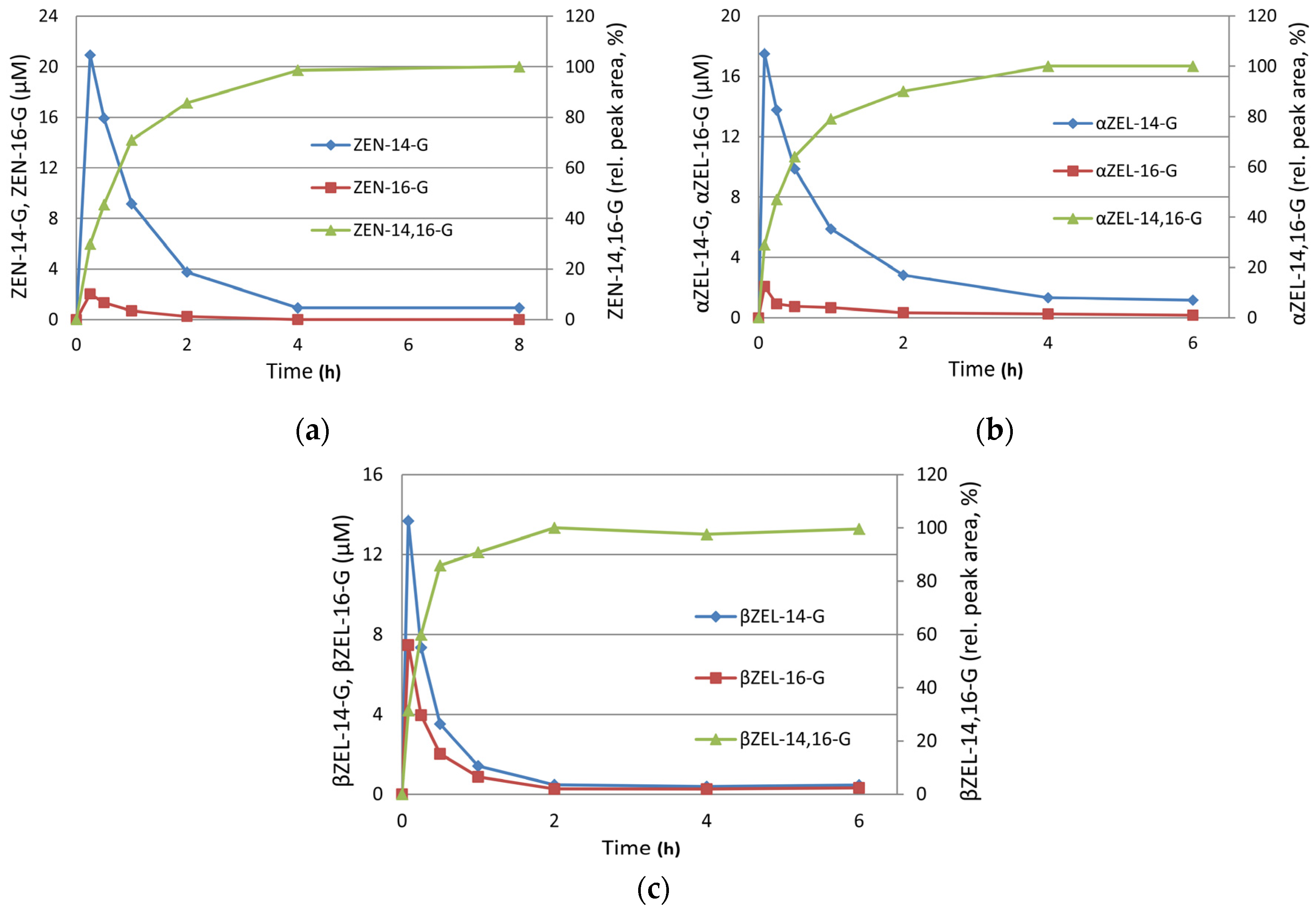
2.2. Preparative Synthesis of ZEN-16-Glucoside
2.3. Structure Elucidation of ZEN-14,16-diG and α/βZEL-14,16-diG
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Cloning
5.3. Protein Expression and Purification
5.4. Glycosylation Assays
5.5. Batch Glycosylation and ZEN-16-G Production
5.6. LC-MS/MS Analysis
5.7. ZEN-16-G Preparative Purification
5.8. αZEL/βZEL-14/16-G Production and Purification
5.9. Characterization of ZEN-14,16-diG, αZEL-14,16-diG, and βZEL-14,16-diG
5.10. Confirmation of ZEN-14,16-diG Structure by NMR
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl sulfoxide |
| DON | Deoxynivalenol |
| GT | Glycosyltransferase |
| HPLC | High performance liquid chromatography |
| IMAC | Immobilized metal ion chromatography |
| IPTG | Isopropyl-β-d-1-thiogalactopyranoside |
| LC-MS | Liquid chromatography coupled to mass spectrometry |
| LC-MS/MS | Liquid chromatography coupled to tandem mass spectrometry |
| MS/HRMS | High resolution tandem mass spectrometry |
| NMR | Nuclear magnetic resonance spectroscopy |
| UDP | Uridine diphosphate |
| UGT | Uridine diphosphate (UDP) glycosyltransferase |
| UHPLC | Ultra high performance liquid chromatography |
| PSPG | Plant secondary product glycosyltransferase |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide electrophoresis |
| SRM | Selected reaction monitoring |
| TEV | Tobacco etch virus |
| QTL | Quantitative trait locus |
| αZEL | α-Zearalenol |
| βZEL | β-Zearalenol |
| ZEN | Zearalenone |
| ZEN-14-G | Zearalenone-14-O-glucoside |
| ZEN-14,16-diG | Zearalenone-14,16-diglucoside |
| ZEN-16-G | Zearalenone-16-O-glucoside |
References
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the Toxicity, Occurrence, Metabolism, Detoxification, Regulations and Intake of Zearalenone: An Oestrogenic Mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S. Zearalenone risk in European wheat. World Mycotoxin J. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Adam, G.; Wiesenberger, G.; Güldener, U. Fusarium Mycotoxins and Their Role in Plant–Pathogen Interactions. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Zeilinger, S., Martín, J.-F., García-Estrada, C., Eds.; Springer: New York, NY, USA, 2015; Volume 2, pp. 199–233. [Google Scholar]
- Gaffoor, I.; Brown, D.W.; Plattner, R.; Proctor, R.H.; Qi, W.; Trail, F. Functional Analysis of the Polyketide Synthase Genes in the Filamentous Fungus Gibberella zeae (Anamorph Fusarium graminearum). Eukaryot. Cell 2005, 4, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, Y.R.; Jin, J.; Han, K.H.; Kim, H.; Kim, J.C.; Lee, T.; Yun, S.H.; Lee, Y.W. Two Different Polyketide Synthase Genes are Required for Synthesis of Zearalenone in Gibberella zeae. Mol. Microbiol. 2005, 58, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Lysøe, E.; Klemsdal, S.S.; Bone, K.R.; Frandsen, R.J.; Johansen, T.; Thrane, U.; Giese, H. The PKS4 Gene of Fusarium graminearum Is Essential for Zearalenone Production. Appl. Environ. Microbiol. 2006, 72, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Werner, U. Characterisation of the Effect of the Fusarium Mycotoxin Zearalenone in Arabidopsis thaliana. Ph.D. Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 2005. [Google Scholar]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of Xenobiotics by Plants: Chemical Modification and Vacuolar Compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Berthiller, F.; Häubl, G.; Jaunecker, G.; Adam, G.; Krska, R.; Schuhmacher, R. Stable Isotopic Labelling-Assisted Untargeted Metabolic Profiling Reveals Novel Conjugates of the Mycotoxin Deoxynivalenol in Wheat. Anal. Bioanal. Chem. 2013, 405, 5031–5036. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Fröhlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and Quantification of Novel Conjugated (Masked) Mycotoxins in Wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the Mycotoxin Deoxynivalenol in Fusarium Resistant and Susceptible Near Isogenic Wheat Lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-Glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant Secondary Metabolism Glycosyltransferases: The Emerging Functional Analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lairson, L.; Henrissat, B.; Davies, G.; Withers, S. Glycosyltransferases: Structures, Functions, and Mechanisms. Biochemistry 2008, 77, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.M.; Deleury, E.; Davies, G.J.; Henrissat, B. An Evolving Hierarchical Family Classification for Glycosyltransferases. J. Mol. Biol. 2003, 328, 307–317. [Google Scholar] [CrossRef]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A Genome-wide Phylogenetic Reconstruction of Family 1 UDP-Glycosyltransferases Revealed the Expansion of the Family during the Adaptation of Plants to Life on Land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Desmond, O.J.; Manners, J.M.; Schenk, P.M.; Maclean, D.J.; Kazan, K. Gene Expression Analysis of the Wheat Response to Infection by Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 2008, 73, 40–47. [Google Scholar] [CrossRef]
- Hill-Ambroz, K.; Webb, C.A.; Matthews, A.R.; Li, W.; Gill, B.S.; Fellers, J.P. Expression Analysis and Physical Mapping of a cDNA Library of Fusarium Head Blight Infected Wheat Spikes. Crop Sci. 2006, 46. [Google Scholar] [CrossRef]
- Lulin, M.; Yi, S.; Aizhong, C.; Zengjun, Q.; Liping, X.; Peidu, C.; Dajun, L.; Xiu-E, W. Molecular Cloning and Characterization of an Up-regulated UDP-Glucosyltransferase Gene Induced by DON from Triticum aestivum L. cv. Wangshuibai. Mol. Biol. Rep. 2010, 37, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Kurz, H.; Lemmens, M.; Buerstmayr, H. Differential Gene Expression of Related Wheat Lines with Contrasting Levels of Head Blight Resistance after Fusarium graminearum Inoculation. Theor. Appl. Genet. 2009, 118, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, Á.; Krska, R.; et al. The Ability to Detoxify the Mycotoxin Deoxynivalenol Colocalizes With a Major Quantitative Trait Locus for Fusarium Head Blight Resistance in Wheat. Mol. Plant-Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Gunnaiah, R.; Kushalappa, A.C.; Duggavathi, R.; Fox, S.; Somers, D.J. Integrated Metabolo-Proteomic Approach to Decipher the Mechanisms by Which Wheat QTL (Fhb1) Contributes to Resistance against Fusarium graminearum. PLoS ONE 2012, 7, e40695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shin, S.; Heinen, S.; Dill-Macky, R.; Berthiller, F.; Nersesian, N.; Clemente, T.; McCormick, S.; Muehlbauer, G.J. Transgenic Wheat Expressing a Barley UDP-Glucosyltransferase Detoxifies Deoxynivalenol and Provides High Levels of Resistance to Fusarium graminearum. Mol. Plant-Microbe Interact. 2015, 28, 1237–1246. [Google Scholar] [PubMed]
- Engelhardt, G.; Zill, G.; Wohner, B.; Wallnöfer, P. Transformation of the Fusarium Mycotoxin Zearalenone in Maize Cell Suspension Cultures. Naturwissenschaften 1988, 75, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Zill, G.; Engelhardt, G.; Wohner, B.; Wallnoefer, P. The Fate of the Fusarium Mycotoxin Zearalenone in Maize Cell Suspension Cultures. Mycotoxin Res. 1990, 6, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M. Proposal for A Uniform Designation of Zearalenone and Its Metabolites. Mycotoxin Res. 2011, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Berthiller, F.; Bachmann, H.; Lucyshyn, D.; Peterbauer, C.; Mitterbauer, R.; Schuhmacher, R.; Krska, R.; Glössl, J.; Adam, G. Heterologous Expression of Arabidopsis UDP-Glucosyltransferases in Saccharomyces cerevisiae for Production of Zearalenone-4-O-Glucoside. Appl. Environ. Microbiol. 2006, 72, 4404–4410. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Werner, U.; Sulyok, M.; Krska, R.; Hauser, M.-T.; Schuhmacher, R. Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS) Determination of Phase II Metabolites of the Mycotoxin Zearalenone in the Model Plant Arabidopsis thaliana. Food Addit. Contam. 2006, 23, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Pasquet, J.-C.; Nussbaumer, T.; Paris, M.P.K.; Wiesenberger, G.; Macadré, C.; Ametz, C.; Berthiller, F.; Lemmens, M.; Saindrenan, P.; et al. Functional Characterization of Two Clusters of Brachypodium distachyon UDP-Glycosyltransferases Encoding Putative Deoxynivalenol Detoxification Genes. Mol. Plant-Microbe Interact. 2013, 26, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Boddu, J.; Cho, S.; Kruger, W.M.; Muehlbauer, G.J. Transcriptome Analysis of the Barley-Fusarium graminearum Interaction. Mol. Plant-Microbe Interact. 2006, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.A.; Boddu, J.; Berthiller, F.; Hametner, C.; Stupar, R.M.; Adam, G.; Muehlbauer, G.J. Transcriptome Analysis of the Barley-Deoxynivalenol Interaction: Evidence for a Role of Glutathione in Deoxynivalenol Detoxification. Mol. Plant-Microbe Interact. 2010, 23, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Boddu, J.; Shin, S.; Poppenberger, B.; Berthiller, F.; Lemmens, M.; Muehlbauer, G.J.; Adam, G. Validation of a Candidate Deoxynivalenol-Inactivating UDP-Glucosyltransferase from Barley by Heterologous Expression in Yeast. Mol. Plant-Microbe Interact. 2010, 23, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky Paris, M.P.; Schweiger, W.; Hametner, C.; Stückler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A New Masked Mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mikula, H.; Fruhmann, P.; Hametner, C.; Varga, E.; Berthiller, F.; Krska, R.; Fröhlich, J. Gentiobiosylation of β-Resorcylic Acid Esters and Lactones: First Synthesis and Characterization of Zearalenone-14-β, D-Gentiobioside. Synlett 2013, 24, 1830–1834. [Google Scholar] [CrossRef]
- Michlmayr, H.; Malachová, A.; Varga, E.; Kleinová, J.; Lemmens, M.; Newmister, S.; Rayment, I.; Berthiller, F.; Adam, G. Biochemical Characterization of a Recombinant UDP-Glucosyltransferase from Rice and Enzymatic Production of Deoxynivalenol-3-O-β-D-glucoside. Toxins 2015, 7, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Barilli, A.; Galaverna, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Study on the Uptake and Deglycosylation of the Masked Forms of Zearalenone in Human Intestinal Caco-2 Cells. Food Chem. Toxicol. 2016, 98, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Gandia-Herrero, F.; Lorenz, A.; Larson, T.; Graham, I.A.; Bowles, D.J.; Rylott, E.L.; Bruce, N.C. Detoxification of the Explosive 2, 4, 6-Trinitrotoluene in Arabidopsis: Discovery of Bifunctional O- and C-Glucosyltransferases. Plant J. 2008, 56, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Messner, B.; Nakajima, J.-I.; Schäffner, A.R.; Saito, K. UGT73C6 and UGT78D1, Glycosyltransferases Involved in Flavonol Glycoside Biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 43910–43918. [Google Scholar] [CrossRef] [PubMed]
- Eng-Kiat, L.; Higgins, G.S.; Yi, L.; Bowles, D.J. Regioselectivity of Glucosylation of Caffeic Acid by a UDP-Glucose: Glucosyltransferase is Maintained in Planta. Biochem. J. 2003, 373, 987–992. [Google Scholar]
- Husar, S.; Berthiller, F.; Fujioka, S.; Rozhon, W.; Khan, M.; Kalaivanan, F.; Elias, L.; Higgins, G.S.; Li, Y.; Schuhmacher, R. Overexpression of the UGT73C6 Alters Brassinosteroid Glucoside Formation in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, Y.; Tinker, N.; Colville, A.; Miki, B. CSR1, the Sole Target of Imidazolinone Herbicide in Arabidopsis thaliana. Plant Cell Physiol. 2007, 48, 1340–1358. [Google Scholar] [CrossRef] [PubMed]
- Baerson, S.R.; Sánchez-Moreiras, A.; Pedrol-Bonjoch, N.; Schulz, M.; Kagan, I.A.; Agarwal, A.K.; Reigosa, M.J.; Duke, S.O. Detoxification and Transcriptome Response in Arabidopsis Seedlings Exposed to the Allelochemical Benzoxazolin-2 (3H)-one. J. Biol. Chem. 2005, 280, 21867–21881. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Galletti, R.; Pontiggia, D.; Manfredini, C.; Lionetti, V.; Bellincampi, D.; Cervone, F.; de Lorenzo, G. Transgenic Expression of a Fungal endo-Polygalacturonase Increases Plant Resistance to Pathogens and Reduces Auxin Sensitivity. Plant Physiol. 2008, 146, 669–681. [Google Scholar] [CrossRef] [PubMed]
- He, J.X.; Gendron, J.M.; Sun, Y.; Gampala, S.S.; Gendron, N.; Sun, C.Q.; Wang, Z.Y. BZR1 Is a Transcriptional Repressor with Dual Roles in Brassinosteroid Homeostasis and Growth Responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, S.S.; Srivastava, S.; Mohammadi, M.; Rahman, M.H.; Deyholos, M.K.; Kav, N.N. Transcriptional profiling of pea ABR17 mediated changes in gene expression in Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Li, Y.; Lim, E.-K.; Bowles, D.J. Higher plant glycosyltransferases. Genome Biol. 2001, 2, REVIEWS3004. [Google Scholar] [CrossRef] [PubMed]
- El Sharkawy, S.H. Microbial Transformation of Zearalenone. III. Formation of 2,4-O-β-Diglucoside. Acta Pharm. Jugosl. 1989, 39, 303–310. [Google Scholar]
- Brodehl, A.; Moller, A.; Kunte, H.J.; Koch, M.; Maul, R. Biotransformation of the Mycotoxin Zearalenone by Fungi of the Genera Rhizopus and Aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mikula, H.; Weber, J.; Svatunek, D.; Skrinjar, P.; Adam, G.; Krska, R.; Hametner, C.; Frohlich, J. Synthesis of Zearalenone-16-β,d-Glucoside and Zearalenone-16-Sulfate: A Tale of Protecting Resorcylic Acid Lactones for Regiocontrolled Conjugation. Beilstein J. Org. Chem. 2014, 10, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.; Summerell, B. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Rocco, C.; Dennison, K.; Klenchin, V.A.; Rayment, I.; Escalante-Semerena, J. Construction and Use of New Cloning Vectors for the Rapid Isolation of Recombinant Proteins from Escherichia coli. Plasmid 2008, 59, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Lemke, S.L.; Mayura, K.; Reeves, W.R.; Wang, N.; Fickey, C.; Phillips, T.D. Investigation of Organophilic Montmorillonite Clay Inclusion in Zearalenone-Contaminated Diets Using the Mouse Uterine Weight Bioassay. J. Toxicol. Environ. Health A 2001, 62, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Varga, E.; Malachova, A.; Nguyen, N.T.; Lorenz, C.; Haltrich, D.; Berthiller, F.; Adam, G. A Versatile Family 3 Glycoside Hydrolase from Bifidobacterium adolescentis Hydrolyzes β-Glucosides of the Fusarium Mycotoxins Deoxynivalenol, Nivalenol, and HT-2 Toxin in Cereal Matrices. Appl. Environ. Microbiol. 2015, 81, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Hametner, C.; Krenn, P.; Schweiger, W.; Ludwig, R.; Adam, G.; Krska, R.; Schuhmacher, R. Preparation and Characterization of the Conjugated Fusarium Mycotoxins Zearalenone-4O-β-d-Glucopyranoside, α-Zearalenol-4O-β-d-Glucopyranoside and β-Zearalenol-4O-β-d-Glucopyranoside by MS/MS and Two-Dimensional NMR. Food Addit. Contam. 2009, 26, 207–213. [Google Scholar] [CrossRef] [PubMed]


| Reaction | Reaction Rate (nmol·min−1·mg−1) | ||
|---|---|---|---|
| ZEN | αZEL | βZEL | |
| Z → Z-14-G 1 | 213 ± 14 | 176 ± 7 | 351 ± 20 |
| Z → Z-16-G 1 | 45 ± 1 | 61 ± 7 | 227 ± 19 |
| Z-14-G → Z-14,16-diG 2 | 0.64 ± 0.13 | 0.47 ± 0.13 | 0.82 ± 0.14 |
| Z-16-G → Z-14,16-diG 2 | 1.6 ± 0.1 | 2.6 ± 0.1 | 1.6 ± 0.1 |
| Z-14-G → Z 3 | 0.55 ± 0.01 | 0.32 ± < 0.01 | 0.75 ± 0.01 |
| Z-16-G → Z 3 | 1.1 ± < 0.1 | 0.59 ± 0.01 | 0.93 ± 0.02 |
| Substrate | Kinetic Constant | |||
|---|---|---|---|---|
| Km (µM) | Vmax (µmol·min−1·mg−1) | kcat (s−1) | kcat/Km (s−1·mM−1) | |
| Zearalenone | 3 ± 1 | 0.34 ± 0.06 | 0.54 ± 0.10 | 190 |
| α-Zearalenol | 13 ± 2 | 0.32 ± 0.03 | 0.52 ± 0.05 | 40 |
| β-Zearalenol | 27 ± 3 | 1.2 ± 0.1 | 2.0 ± < 0.1 | 74 |
| Position | 1H | 13C |
|---|---|---|
| 1 | - | 169.4 |
| 2 | 1.37 (d, 6.3) | 20.3 |
| 3 | 5.29 (m) | 73.2 |
| 4 | 1.74 (m); 1.58 (m) | 36.0 |
| 5 | 1.78 (m); 1.60 (m) | 22.3 |
| 6 | 2.48 (m); 2.28 (m) | 44.7 |
| 7 | - | 214.3 |
| 8 | 2.66 (m); 2.24 (m) | 38.3 |
| 9 | 2.02 (m); 1.60 (m) | 22.7 |
| 10 | 2.33 (m); 2.05 (m) | 32.5 |
| 11 | 6.10 (ddd, 15.6, 9.8, 4.5) | 135.1 |
| 12 | 6.32 (d, 15.5) | 129.6 |
| 13 | 6.97 (d, 2.1) | 108.6 |
| 14 | - | 160.7 |
| 15 | 6.90 (d, 2.1) | 104.2 |
| 16 | - | 156.6 |
| 17 | - | 119.6 |
| 18 | - | 138.1 |
| 1′ | 4.97 (d, 7.4) | 102.1 |
| 1″ | 4.98 (d, 7.3) | 102.1 |
| 2′-5′; 2″-5″ | 3.62–3.28 (m) | 78.5; 78.4; 78.4; 78.0; 75.0; 74.9; 71.8; 71.6 |
| 6′; 6″ | 3.95–3.88 (m); 3.70–3.62 (m) | 62.9; 62.9 |
| 6′; 6″ | 3.95–3.88 (m); 3.70–3.62 (m) | 62.9; 62.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michlmayr, H.; Varga, E.; Lupi, F.; Malachová, A.; Hametner, C.; Berthiller, F.; Adam, G. Synthesis of Mono- and Di-Glucosides of Zearalenone and ?-/?-Zearalenol by Recombinant Barley Glucosyltransferase HvUGT14077. Toxins 2017, 9, 58. https://doi.org/10.3390/toxins9020058
Michlmayr H, Varga E, Lupi F, Malachová A, Hametner C, Berthiller F, Adam G. Synthesis of Mono- and Di-Glucosides of Zearalenone and ?-/?-Zearalenol by Recombinant Barley Glucosyltransferase HvUGT14077. Toxins. 2017; 9(2):58. https://doi.org/10.3390/toxins9020058
Chicago/Turabian StyleMichlmayr, Herbert, Elisabeth Varga, Francesca Lupi, Alexandra Malachová, Christian Hametner, Franz Berthiller, and Gerhard Adam. 2017. "Synthesis of Mono- and Di-Glucosides of Zearalenone and ?-/?-Zearalenol by Recombinant Barley Glucosyltransferase HvUGT14077" Toxins 9, no. 2: 58. https://doi.org/10.3390/toxins9020058
APA StyleMichlmayr, H., Varga, E., Lupi, F., Malachová, A., Hametner, C., Berthiller, F., & Adam, G. (2017). Synthesis of Mono- and Di-Glucosides of Zearalenone and ?-/?-Zearalenol by Recombinant Barley Glucosyltransferase HvUGT14077. Toxins, 9(2), 58. https://doi.org/10.3390/toxins9020058








