Tetracycline Reduces Kidney Damage Induced by Loxosceles Spider Venom
Abstract
:1. Introduction
2. Results
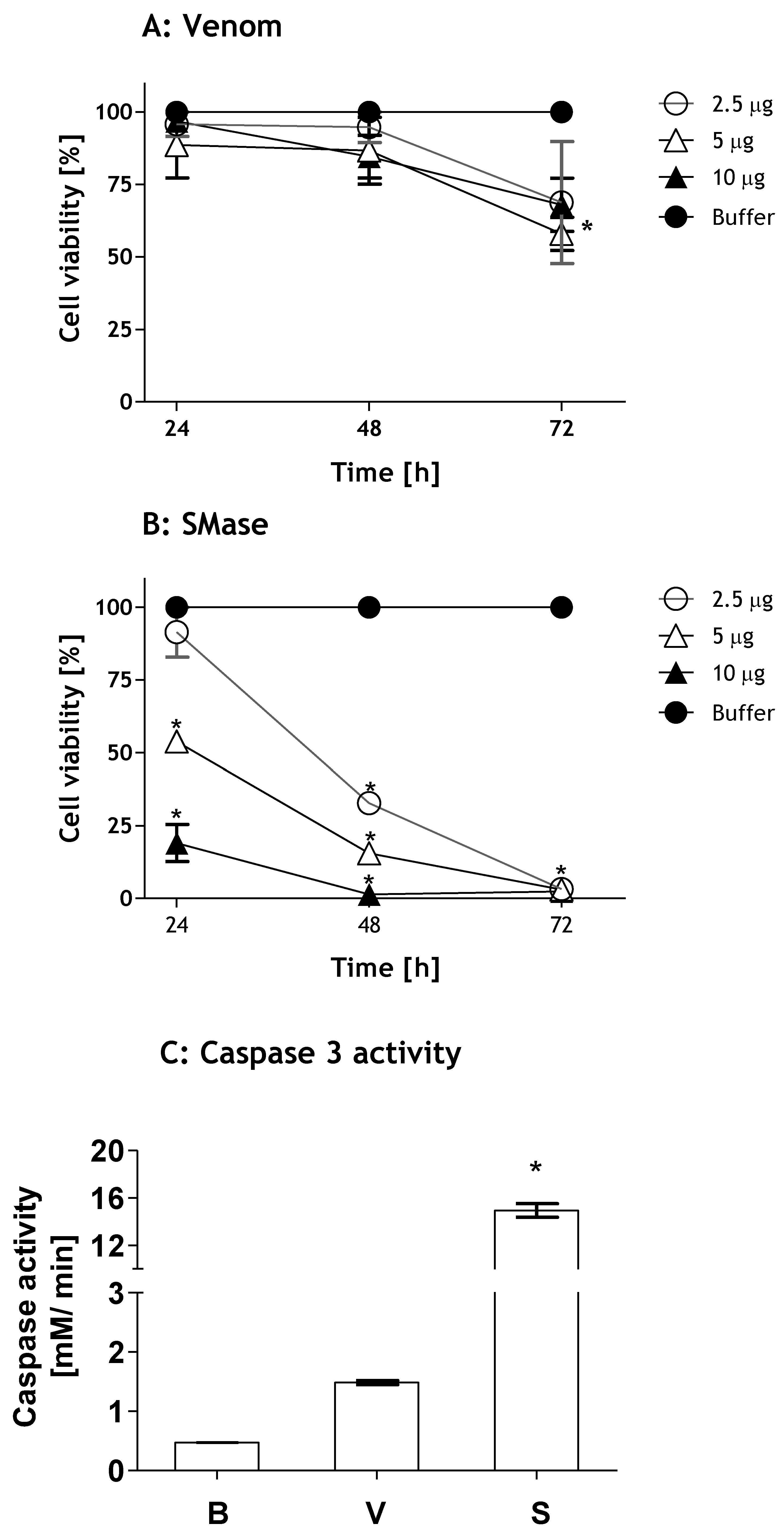
2.1. Loxosceles Venom and SMase D Induce Renal Cell Death by Apoptosis
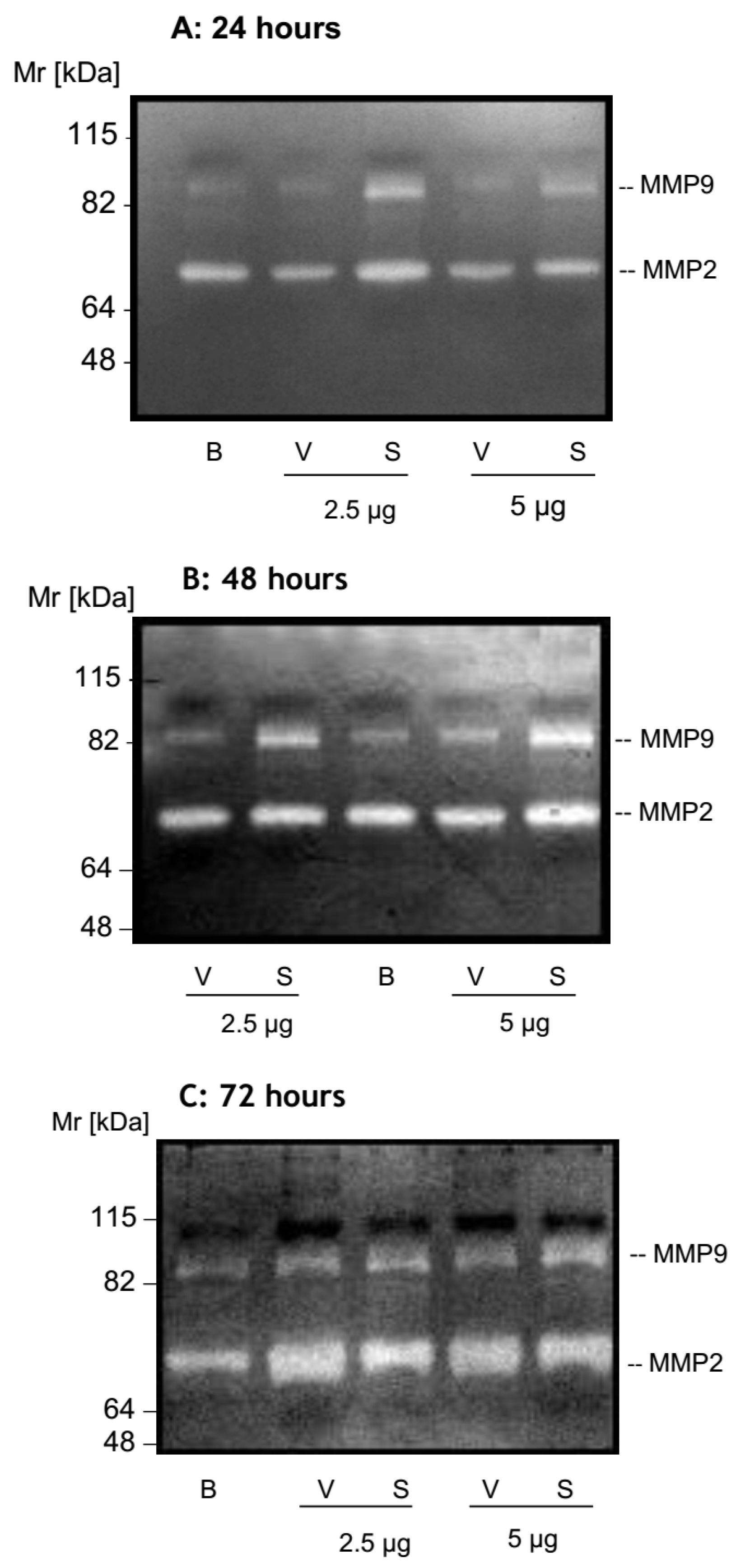
2.2. MMP Secretion by HK-2 Cells
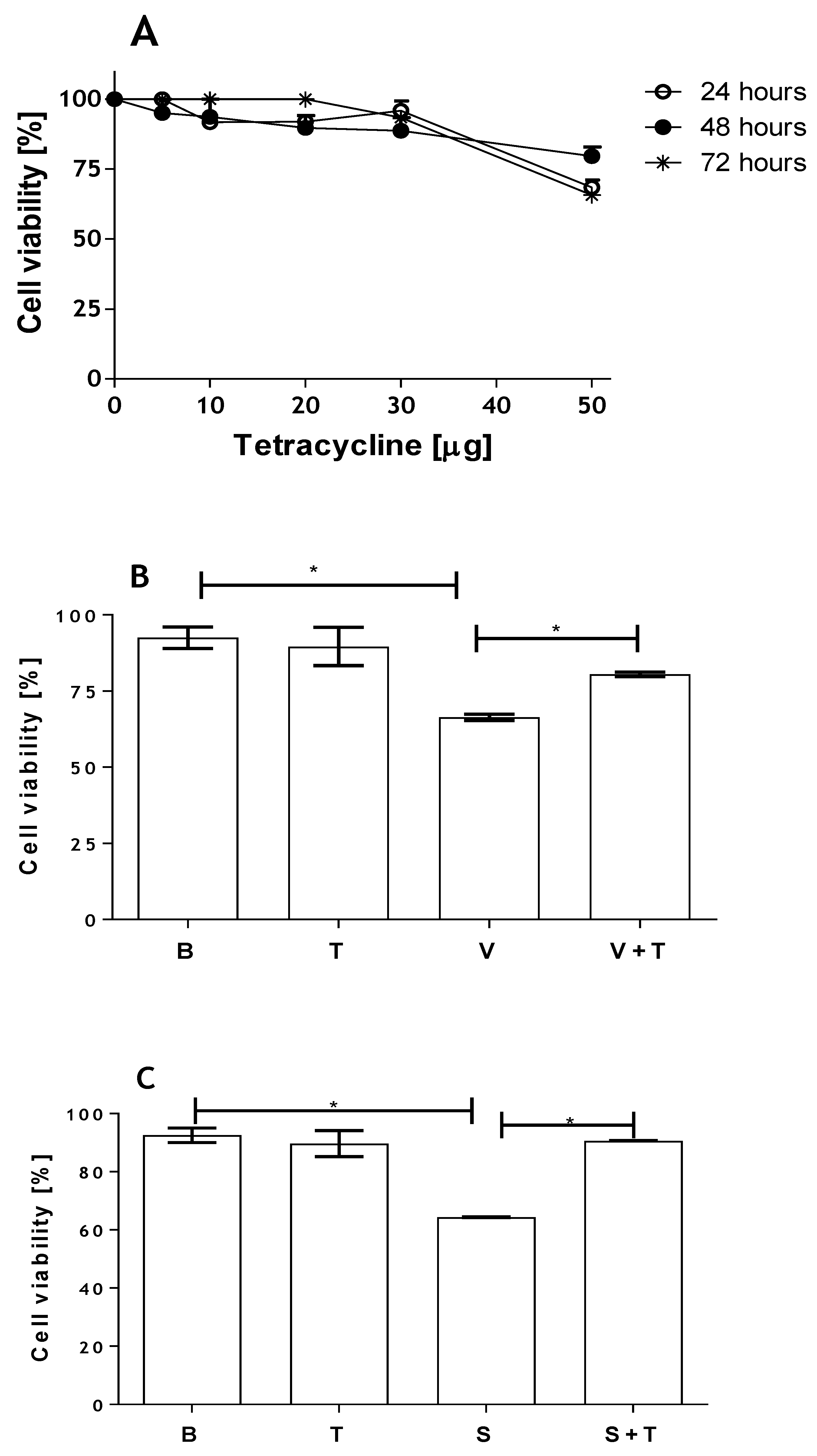
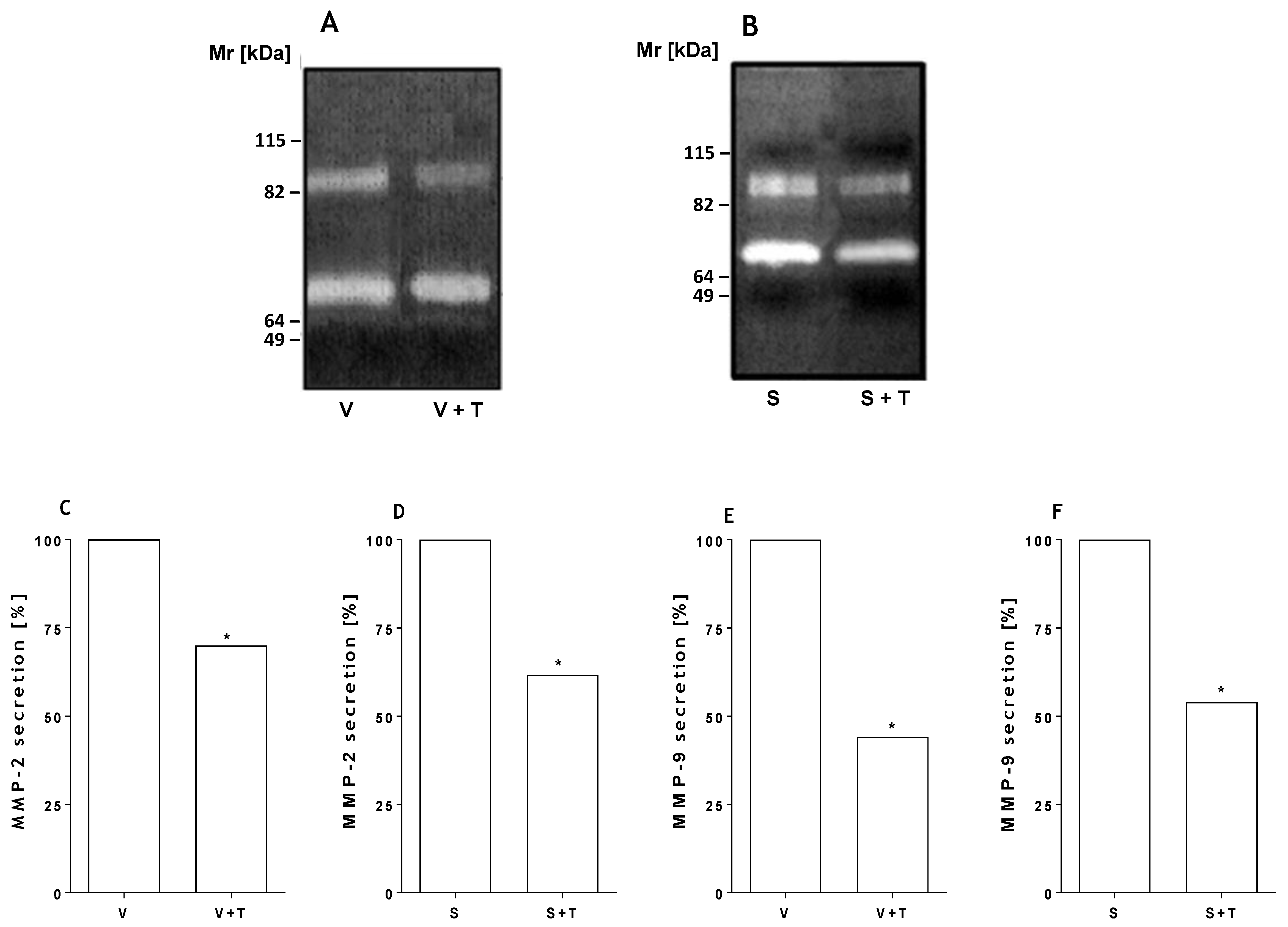
2.3. Tetracycline Prevents Venom and SMase D Increased Expression of MMPs and Protects Kidney Cells from Death
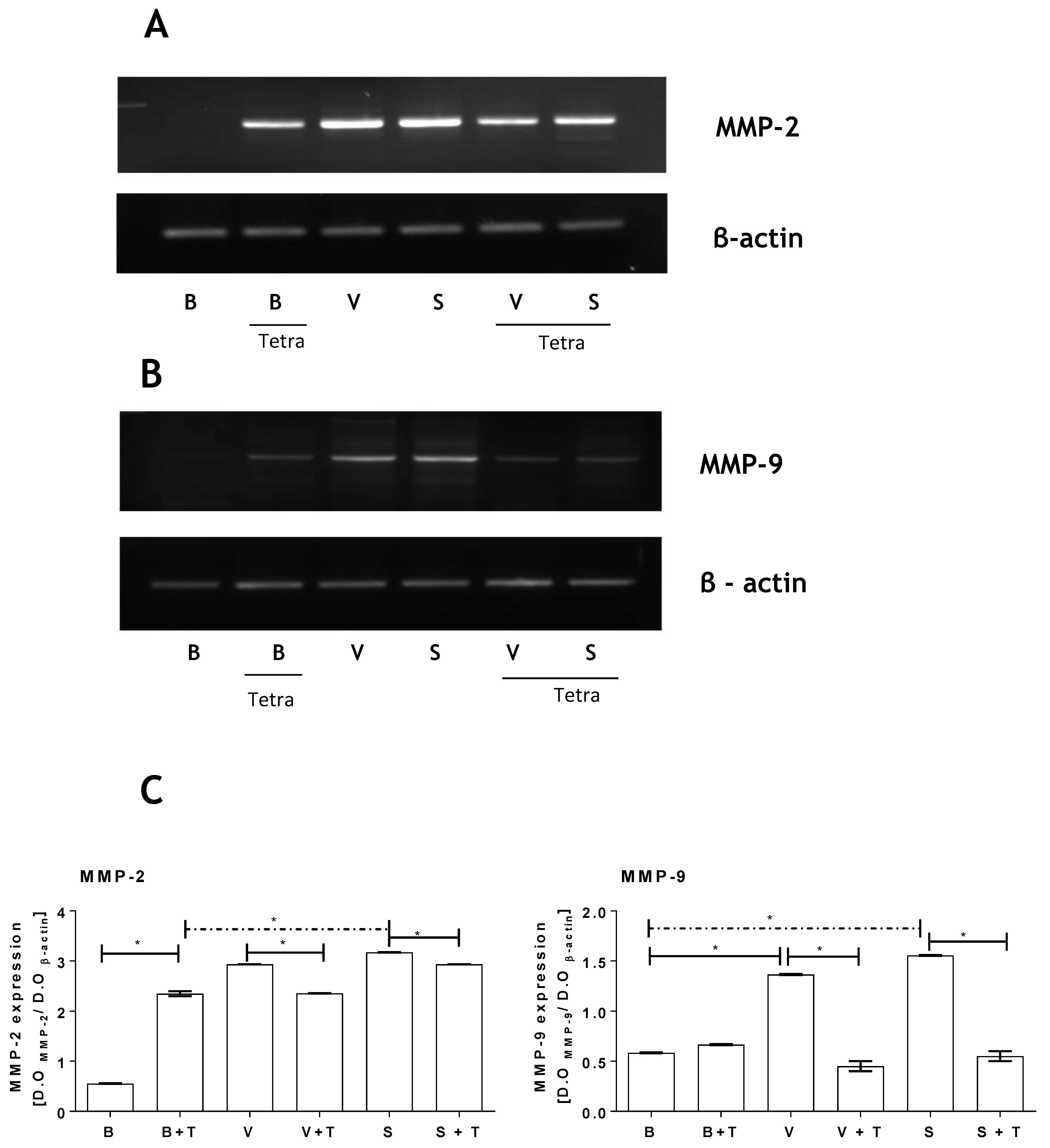
2.4. Loxosceles Venom and SMase D Induce an Increase in MMP Gene Expression in HK-2 Cells
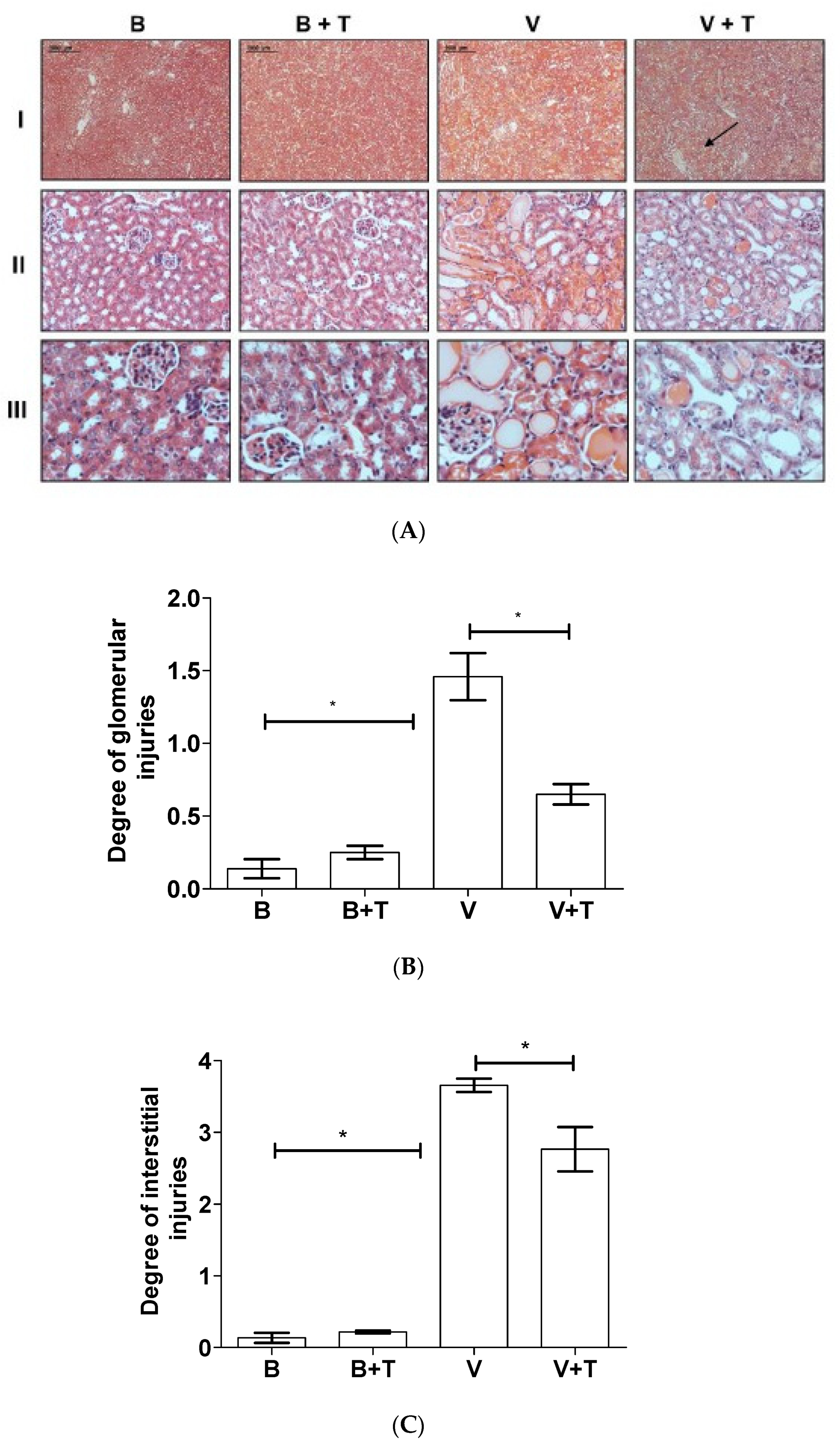
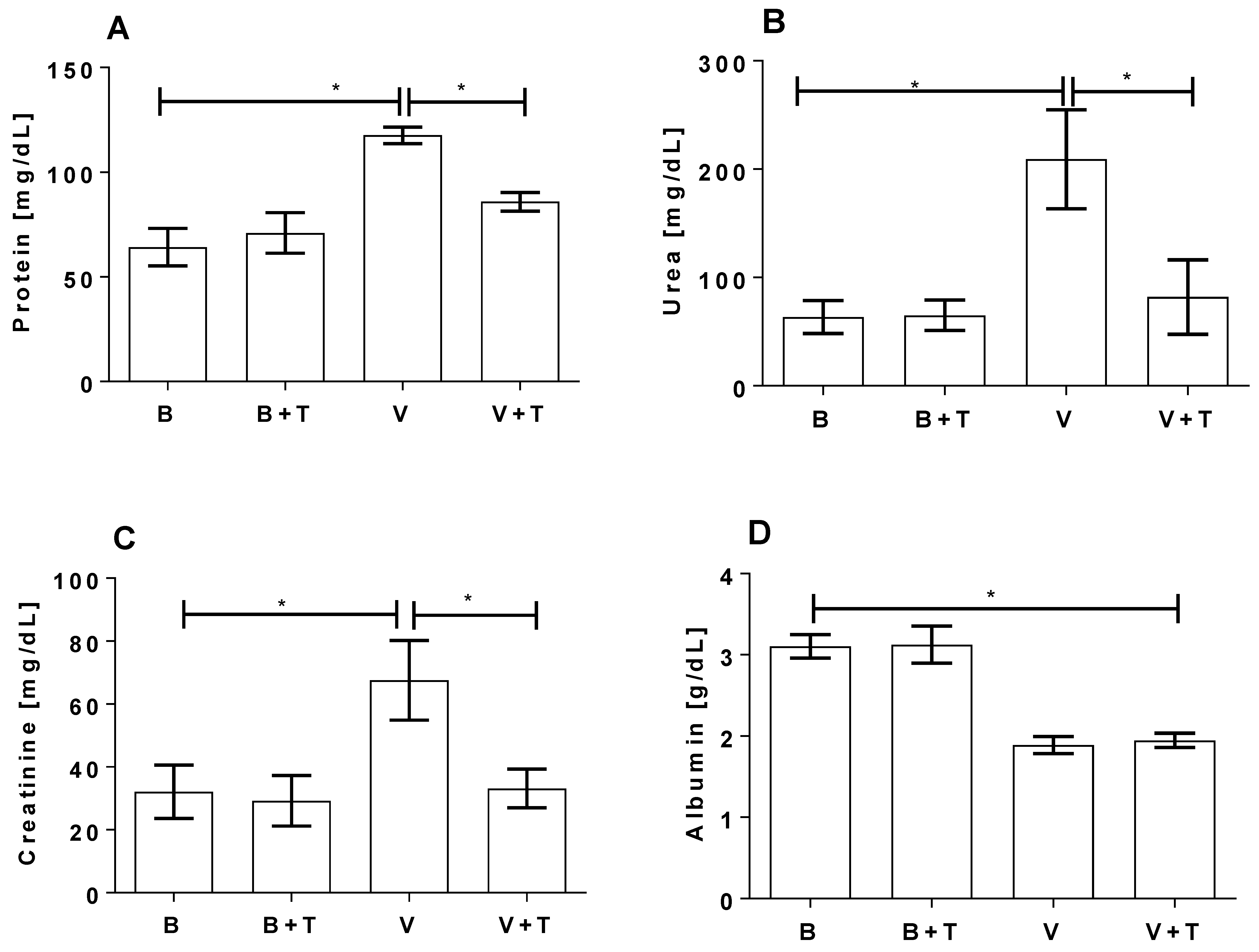
2.5. Tetracycline Prevents Loxosceles Venom Induced Renal Injury in Mice
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Chemicals, Reagents, and Buffers
5.2. Venom
5.3. Sphingomyelinase D Expression
5.4. Cell Culture and Maintenance
5.5. Cell Viability
5.6. Caspase-3 Activity Assay
5.7. Gelatin Zymography
5.8. RNA Extraction
5.9. RT-PCR and PCR
5.10. Mice
5.11. Treatment of Mice with Loxosceles Venom and Tetracycline
5.12. Biochemical Analysis
5.13. Histological Analysis
5.14. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sistema de Informação Agravos de Notificação (SINAN). Ministério da Saúde (BR). 2015. Available online: http://portal.saude.gov.br/portal/saude (accessed on 26 April 2016).
- Futrell, J. Loxoscelism. Am. J. Med. 1992, 304, 261–267. [Google Scholar] [CrossRef]
- Ministério da Saúde. Manual de Acidentes por Animais Peçonhentos; Fundação Nacional da Saúde: Brasília, Brasil, 2001.
- Silva, P.H.; Silveira, R.B.; Appel, M.H.; Mangili, O.C.; Gremski, W.; Veiga, S.S. Brown spiders and loxoscelism. Toxicon 2004, 44, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Schenone, H. Cutaneous loxoscelism with edematous predominance. Bol. Chil. Parasitol. 1998, 53, 78–83. [Google Scholar] [PubMed]
- Fernandes-Pedrosa, M.F.; Junqueira-de-Azevedo, I.L.; Gonçalves-de-Andrade, R.M.; Kobashi, L.S.; Almeida, D.D.; Ho, P.L.; Tambourgi, D.V. Transcriptome analysis of Loxosceles laeta (Araneae, Sicariidae) spider venomous gland using expressed sequence tags. BMC Genom. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.V.; Gonçalves-de-Andrade, R.M.; van den Berg, C.W. Loxoscelism: From basic research to the proposal of new therapies. Toxicon 2010, 56, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Forrester, L.J.; Barrett, J.T.; Campbell, B.J. Red blood cell lysis induced by the venom of the brown recluse spider: The role of sphingomyelinase D. Arch. Biochem. Biophys. 1978, 187, 355–365. [Google Scholar] [CrossRef]
- Kurpiewski, G.; Forrester, L.J.; Barrett, J.T.; Campbell, B.J. Platelet aggregation and sphingomyelinase D activity of a purified toxin from the venom of Loxosceles reclusa. Biochim. Biophys. Acta 1981, 678, 467–476. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Magnoli, F.C.; van den Berg, C.W.; Morgan, B.P.; de Araújo, P.S.; Alves, E.W.; da Silva, W.D. Sphingomyelinases in the venom of the spider Loxosceles intermedia are responsible for both dermonecrosis and complement-dependent hemolysis. Biochem. Biophys. Res. Commun. 1998, 251, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.G.; Ordoñez, M.; Presa, N.; Gomez-Larrauri, A.; Simón, J.; Trueba, M.; Gomez-Muñoz, A. Sphingomyelinase D/ceramide 1-phosphate in cell survival and inflammation. Toxins 2015, 7, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Van Meeteren, L.A.; Frederiks, F.; Giepmans, B.N.; Pedrosa, M.F.; Billington, S.J.; Jost, B.H.; Tambourgi, D.V.; Moolenaar, W.H. Spider and bacterial sphingomyelinases D target cellular lysophosphatidic acid receptors by hydrolyzing lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 10833–10836. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, W.H. Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 1999, 253, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ishii, I.; Kingsbury, M.A.; Chun, J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta 2002, 1585, 108–113. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Paixão-Cavalcante, D.; Gonçalves-de-Andrade, R.M.; Fernandes-Pedrosa, M.; Magnoli, F.C.; Morgan, B.P.; van den Berg, C.W. Loxosceles sphingomielinase induces complement-dependent dermonecrosis, neutrophil infiltration and endogenous gelatinase expression. J. Investig. Dermatol. 2005, 124, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Paixão-Cavalcante, D.; van den Berg, C.W.; Fernandes-Pedrosa, M.; Gonçalves-de Andrade, R.M.; Tambourgi, D.V. Role of matrix metalloproteinases in HaCat keratinocytes apoptosis induced by Loxosceles venom sphingomyelinase D. J. Investig. Dermatol. 2006, 126, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Paixão-Cavalcante, D.; van den Berg, C.W.; Gonçalves-de-Andrade, R.M.; Fernandes-Pedrosa, M.; Okamoto, C.K.; Tambourgi, D.V. Tetracycline protects against dermonecrosis induced by Loxosceles spider venom. J. Investig. Dermatol. 2007, 127, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Fredrich, K.; Weihrauch, D.; Hattan, N.; Chilian, W.M. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am. J. Physiol. Renal Physiol. 2004, 286, F893–F902. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shimizu, A.; Masuda, Y.; Kuwahara, N.; Arai, T.; Kataoka, M.; Uchiyama, M.; Kaneko, T.; Akimoto, T.; Iino, Y.; et al. Involvement of matrix metalloproteinase-2 in the development of renal interstitial fibrosis in mouse obstructive nephropathy. Lab. Investig. 2012, 92, 1149–1160. [Google Scholar]
- Sutton, T.A.; Kelly, K.J.; Mang, H.E.; Plotkin, Z.; Sandoval, R.M.; Dagher, P.C. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am. J. Physiol. Renal Physiol. 2005, 288, F91–F97. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Stanton, H.; Cowell, S.; Butler, G.; Kñauper, V.; Atkinson, S.; Gavrilovic, J. Mechanism for pro matrix metalloproteinase activation. APMIS 1999, 107, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Kandasamy, A.D.; Youssef, N.; Schulz, R. Matrix metalloproteinase inhibitor properties of tetracyclines: Therapeutic potential in cardiovascular diseases. Pharmacol. Res. 2011, 64, 551–560. [Google Scholar]
- Ataie-Kachoie, P.; Morris, D.L.; Pourgholami, M.H. Minocycline suppresses interleukine-6, its receptor system and signaling pathways and impairs migration, invasion and adhesion capacity of ovarian cancer cells: In vitro and in vivo studies. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Holmuhamedov, E.; Zhang, X.; Lovelace, G.L.; Smith, C.D.; Lemasters, J.J. Minocycline and doxycycline, but not other tetracycline-derived compounds, protect liver cells from chemical hypoxia and ischemia/reperfusion injury by inhibition of the mitochondrial calcium uniporter. Toxicol. Appl. Pharmacol. 2013, 273, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.R.; Venitz, J.; Figg, W.D.; Sparreboom, A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist. Updates 2004, 7, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.V.; Petricevich, V.L.; Magnoli, F.C.; Assaf, S.L.; Jancar, S.; da Silva, W.D. Endotoxemic-like shock induced by Loxosceles spider venoms: Pathological changes and putative cytokine mediators. Toxicon 1998, 36, 391–403. [Google Scholar]
- Luciano, M.N.; da Silva, P.H.; Chaim, O.M.; dos Santos, V.L.; Franco, C.R.; Soares, M.F.; Zanata, S.M.; Mangili, O.C.; Gremski, W.; Veiga, S.S. Experimental evidence for a direct cytotoxicity of Loxosceles intermedia (brown spider) venom in renal tissue. J. Histochem. Cytochem. 2004, 52, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Lung, J.M.; Mallory, S.B. A child with spider bite and glomerulonephritis: A diagnostic challenge. Int. J. Dermatol. 2000, 39, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Chaim, O.M.; Sade, Y.B.; da Silveira, R.B.; Toma, L.; Kalapothakis, E.; Chavez-Olórtegui, C.; Mangili, O.C.; Gremski, W.; von Dietrich, C.P.; Nader, H.B.; et al. Brown spider dermonecrotic toxin directly induces nephrotoxicity. Toxicol. Appl. Pharmacol. 2006, 211, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kusma, J.; Chaim, O.M.; Wille, A.C.; Ferrer, V.P.; Sade, Y.B.; Donatti, L.; Gremski, W.; Mangili, O.C.; Veiga, S.S. Nephrotoxicity caused by brown spider venom phospholipase D (dermonecrotic toxin) depends on catalytic activity. Biochimie 2008, 90, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Lucato, R.V., Jr.; Abdulkader, R.C.; Barbaro, K.C.; Mendes, G.E.; Castro, I.; Baptista, M.A.; Cury, P.M.; Malheiros, D.M.; Schor, N.; Yu, L.; et al. Loxosceles gaucho venom-induced acute kidney injury—In vivo and in vitro studies. PLoS Negl. Trop. Dis. 2011, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.O.; Chaim, O.M.; da Silveira, R.B.; Gremski, L.H.; Sade, Y.B.; Paludo, K.S.; Senff-Ribeiro, A.; de Moura, J.; Chavez-Olórtegui, C.; Gremski, W.; et al. Biological e structural comparison of recombinant phospholipase D toxins from Loxosceles intermedia (brown spider) venom. Toxicon 2007, 50, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; de Carlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, K.; Kabadere, S.; Tosun, M.; Koken, T.; Kinaci, M.K.; Isikli, B.; Erkasap, N. Protective effects of doxycycline in ischemia/reperfusion injury on kidney. J. Phisiol. Biochem. 2009, 65, 183–191. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhang, Y.; Harris, D.C.; Zheng, G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013, 6, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Nee, L.E.; McMorrow, T.; Campbell, E.; Slattery, C.; Ryan, M.P. TNF-alpha and IL-1 beta-mediated regulation of MMP-9 and TIMP-1 in renal proximal tubular cells. Kidney Int. 2004, 66, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.A.; Hengartner, M.O. The molecular mechanism of programmed cell death in C. elegans. Ann. N. Y. Acad. Sci. 1999, 887, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in development. Nature 2000, 407, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, M.; Rivera, I.G.; Presa, N.; Gomez-Muñoz, A. Implication of matrix metalloproteinases 2 and 9 in ceramide 1-phosphate-stimulated macrophage migration. Cell Signal. 2016, 28, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Blom, T. Trafficking and functions of bioactive sphingolipids: Lessons from cells and model membranes. Lipid Insights 2015, 8 (Suppl. 1), 11–20. [Google Scholar] [PubMed]
- Schrier, R.W.; Wang, W.; Poole, B.; Mitra, A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J. Clin. Investig. 2004, 114, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bucherl, W. Biology and venoms of the most important South American spiders of the genera Phoneutria, Loxosceles, Lycosa, and Latrodectus. Am. Zool. 1969, 9, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.V.; Fernandes-Pedrosa, M.F.; van den Berg, C.W.; Gonçalves-de-Andrade, R.M.; Ferracini, M.; Paixão-Cavalcante, D.; Morgan, B.P.; Rushmere, N.K. Molecular cloning, expression, function and immunoreactivities of members of a gene family of sphingomyelinases from Loxosceles venom glands. Mol. Immunol. 2004, 41, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal. Biochem. 1994, 218, 325–329. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, C.K.; Van den Berg, C.W.; Masashi, M.; Gonçalves-de-Andrade, R.M.; Tambourgi, D.V. Tetracycline Reduces Kidney Damage Induced by Loxosceles Spider Venom. Toxins 2017, 9, 90. https://doi.org/10.3390/toxins9030090
Okamoto CK, Van den Berg CW, Masashi M, Gonçalves-de-Andrade RM, Tambourgi DV. Tetracycline Reduces Kidney Damage Induced by Loxosceles Spider Venom. Toxins. 2017; 9(3):90. https://doi.org/10.3390/toxins9030090
Chicago/Turabian StyleOkamoto, Cinthya Kimori, Carmen W. Van den Berg, Mizuno Masashi, Rute M. Gonçalves-de-Andrade, and Denise V. Tambourgi. 2017. "Tetracycline Reduces Kidney Damage Induced by Loxosceles Spider Venom" Toxins 9, no. 3: 90. https://doi.org/10.3390/toxins9030090
APA StyleOkamoto, C. K., Van den Berg, C. W., Masashi, M., Gonçalves-de-Andrade, R. M., & Tambourgi, D. V. (2017). Tetracycline Reduces Kidney Damage Induced by Loxosceles Spider Venom. Toxins, 9(3), 90. https://doi.org/10.3390/toxins9030090





