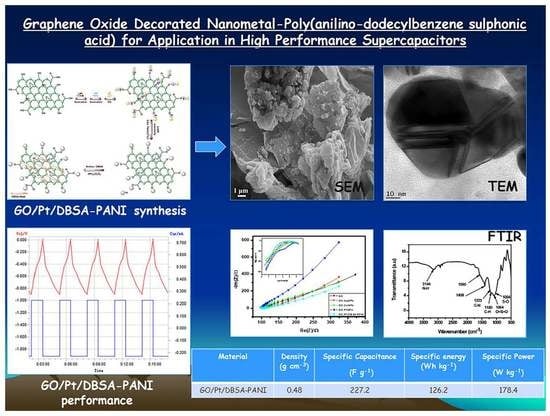
Graphene Oxide Decorated Nanometal-Poly(Anilino-Dodecylbenzene Sulfonic Acid) for Application in High Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Experimental
2.3.1. Synthesis of GO
2.3.2. Synthesis of Graphene Oxide Loaded with Pt, Ag and Cu NPs
2.3.3. Synthesis of GO/Pt NPs, GO/Ag NPs and GO/Cu NPs Anchored DBSA-Doped PANI
2.4. Characterization of Electrode Materials
2.5. Fabrication of the Electrode Material
2.5.1. Preparation of the Electrode Materials
2.5.2. Construction of Supercapacitor Cell
3. Results and Discussion
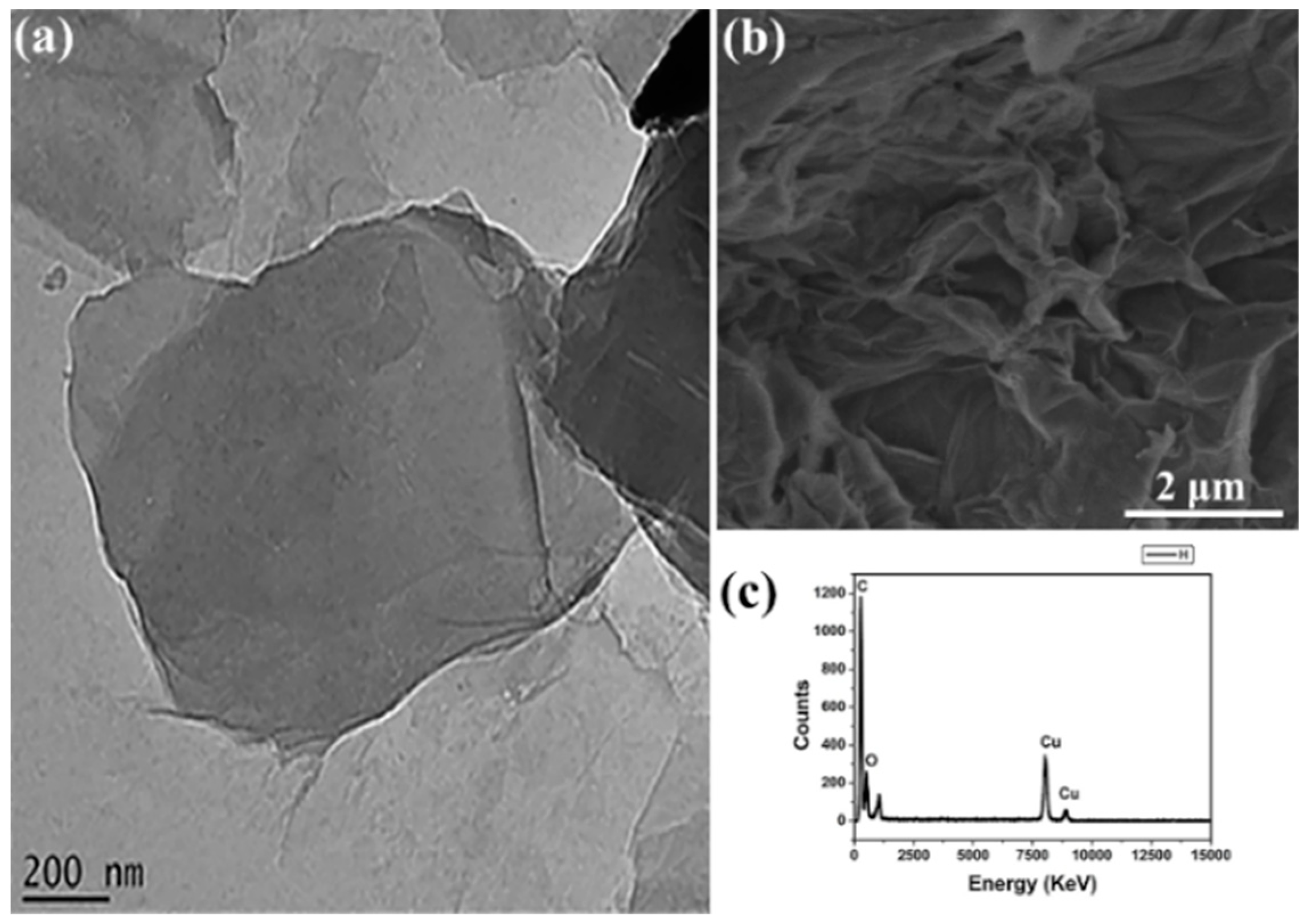
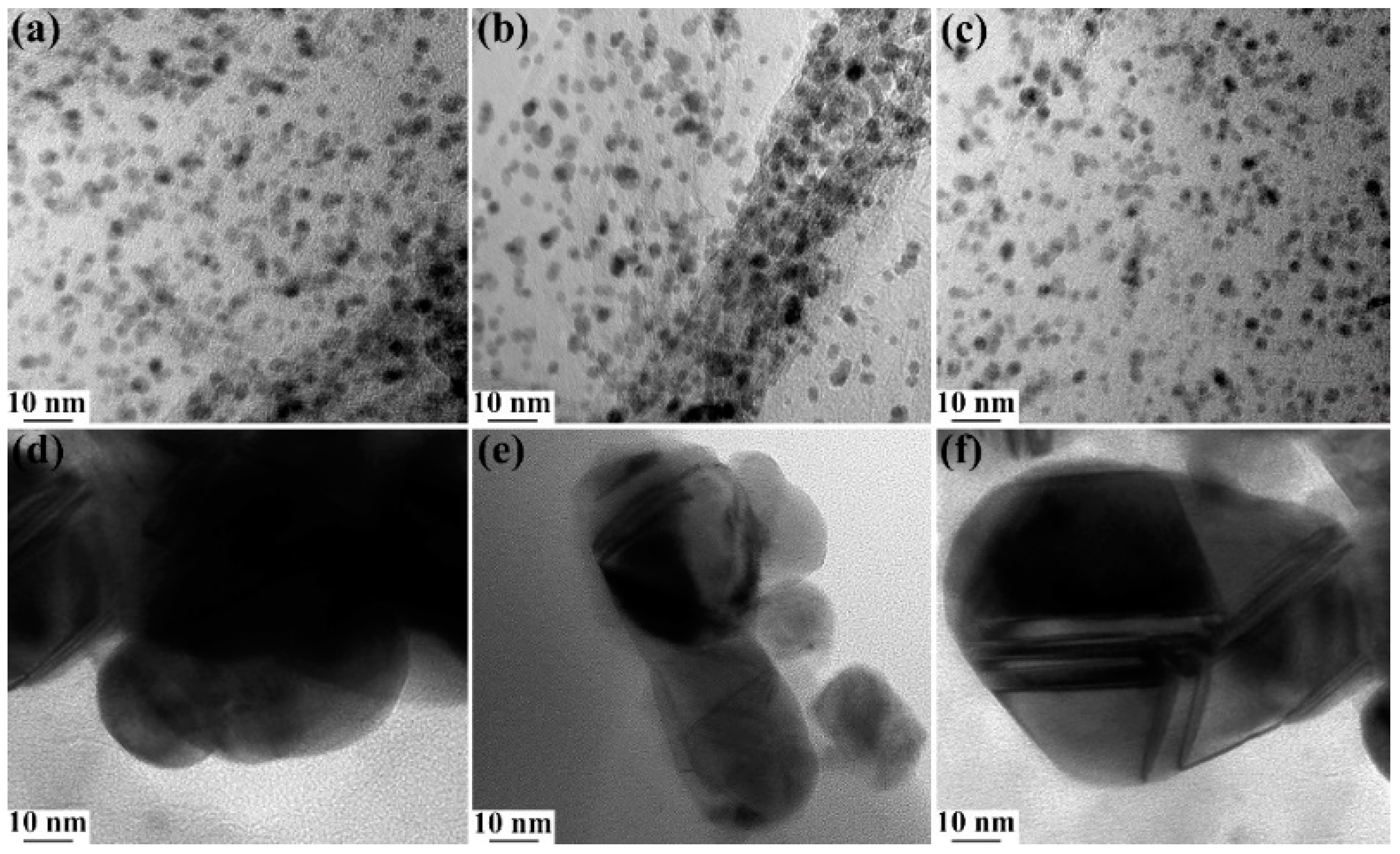
3.1. Morphology Characterization
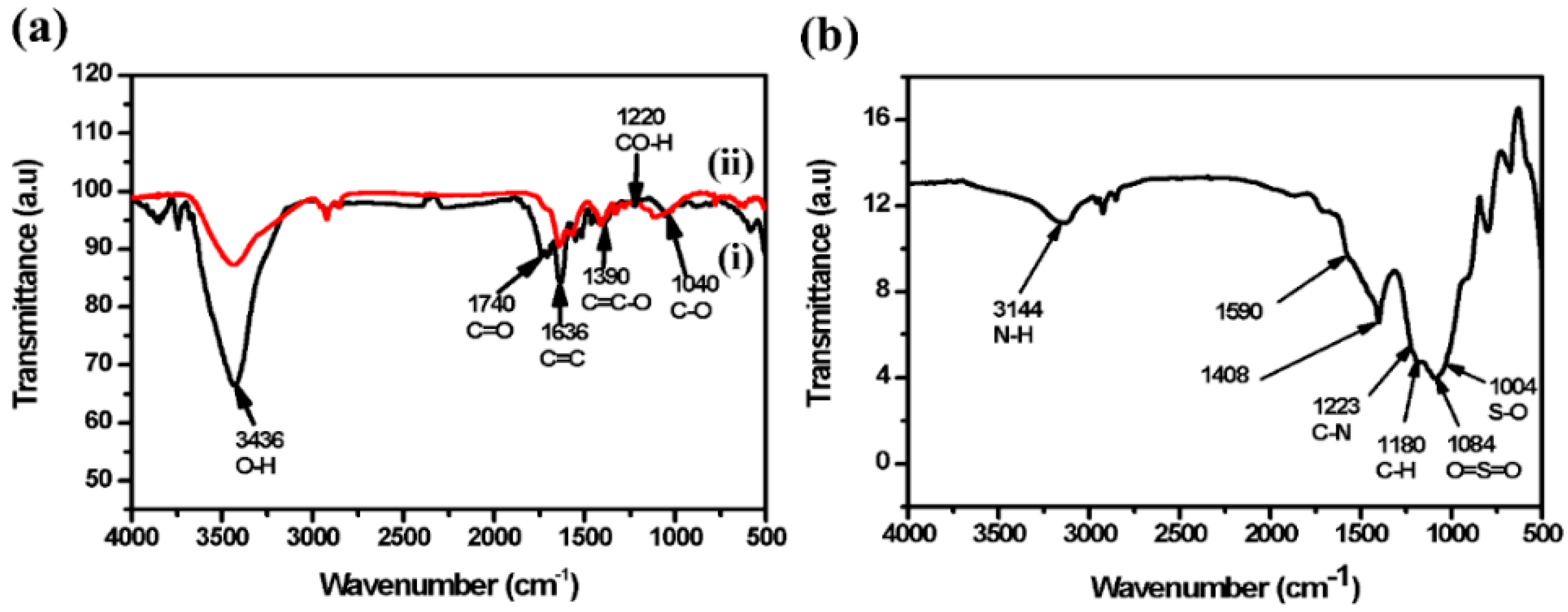
3.2. Molecular Structure Characterization—Fourier Transform Infrared (FTIR)
3.3. Electrochemistry
3.3.1. Cyclic Voltammetry
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
3.4. Galvanostatic-Charge Discharge Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colgan, J. The International Energy Agency; Global Public Policy Institute: Berlin Germany, 2009. [Google Scholar]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, S.-H.; Jeong, S.M.; Son, K.Y.; Lim, S.K. Colorimetric hydrogen gas sensor based on PdO /metal oxides hybrid nanoparticles. Talanta 2018, 188, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; McRae, L.; Firby, C.J.; Al-Hussein, M.; Elezzabi, A.Y. Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nanoenergy 2018, 47, 130–139. [Google Scholar] [CrossRef]
- Xie, S.; Bi, Z.; Chen, Y.; He, X.; Guo, X.; Gao, X.; Li, X. Electrodeposited Mo-doped WO3 film with large optical modulation and high areal capacitance toward electrochromic energy-storage applications. Appl. Surf. Sci. 2018, 459, 774–781. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-based electron transporting layer materials for perovskite solar cells: A review of recent progress. J. Energy. Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Bella, F.; Muñoz-García, A.B.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Unveiling the controversial mechanism of reversible Na storage in TiO2 nanotube arrays: Amorphous versus anatase TiO2. Nano Res. 2017. [Google Scholar] [CrossRef]
- Perreault, L.L.; Colò, F.; Meligrana, G.; Kim, K.; Fiorilli, S.; Bella, F.; Nair, J.R.; Vitale-Brovarone, V.; Florek, J.; Kleitz, F.; et al. Spray-Dried Mesoporous Mixed Cu-Ni Oxide@Graphene Nanocomposite Microspheres for High Power and Durable Li-Ion Battery Anodes. Adv. Energy Mater. 2018, 8, 1802438. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 10628–10643. [Google Scholar] [CrossRef]
- Chen, Y.T.; Ma, C.W.; Chang, C.M.; Yang, Y.J. Micromachined Planar Supercapacitor with Interdigital Buckypaper Electrodes. Micromachines 2018, 9, 242. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Lu, P.; Xue, D.; Yang, H.; Liu, Y. Supercapacitor and nanoscale research towards electrochemical energy storage. Int. J. Smart Nano Mater. 2013, 4, 2–26. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Simple Capacitors to Supercapacitors—An Overview. Int. J. Electrochem. Sci. 2008, 3, 1196–1217. [Google Scholar]
- Snook, G.A.; Peng, C.; Fray, D.J.; Chen, G.Z. Chieving high electrode specific capacitance with materials of low mass specific capacitance: Potentiostatically grown thick micro-nanoporous PEDOT films. Electrochem. Commun. 2007, 9, 83–88. [Google Scholar] [CrossRef]
- Burke, A.; Zhao, H. Present and future applications of supercapacitors in electric and hybrid vehicles. In Proceedings of the 2014 IEEE International Electric Vehicle Conference (IEVC), Florence, Italy, 17–19 December 2014; pp. 1–8. [Google Scholar] [CrossRef]
- Gao, W. The Chemistry of Graphene Oxide. Graphene Oxide Reduct. Recipes, Spectrosc. Appl. 2015, 61–95. [Google Scholar] [CrossRef]
- Hang, L.; Zhao, Y.; Zhang, H.; Liu, G.; Cai, W. Copper nanoparticle@graphene composite arrays and their enhanced catalytic performance. Acta Mater. 2016, 105, 59–67. [Google Scholar] [CrossRef]
- Hu, J.; He, B.; Lu, J.; Hong, L.; Yuan, J.; Song, J.; Niu, L. Facile Preparation of Pt/Polyallylamine/Reduced Graphene Oxide Composites and Their Application in the Electrochemical Catalysis on Methanol Oxidation. Int. J. Electrochem. Sci. 2012, 7, 10094–10107. [Google Scholar]
- Shen, C.; Barrios, E.; McInnis, M.; Zuyus, J.; Zhai, L. Fabrication of graphene aerogols with heavily loaded metallic nanoparticles. Micromachines 2017, 8, 47. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Huan, W.; Qiao, W.; Zhou, Y.; Ye, Y.; Chen, L.S. Towards flexible transparent electrodes based on carbon and metallic materials. Micromachines 2017, 8, 12. [Google Scholar] [CrossRef]
- Grinou, A.; Yun, Y.S.; Cho, S.Y.; Park, H.H.; Jin, H.J. Dispersion of Pt Nanoparticle-Doped Reduced Graphene Oxide Using Aniline as a Stabilizer. Materials 2012, 5, 2927–2936. [Google Scholar] [CrossRef]
- Bonet, F.; Delmas, V.; Grugeon, S.; Herrera Urbina, R.; Silvert, P.Y.; Tekaia-Elhsissen, K. Synthesis of Monodisperse Au, Pt, Pd, Ru and Ir Nanoparticles in Ethylene Glycol. Nanostructured Mater. 1999, 11, 1277–1284. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, M.; Huang, H.; Xu, H.-M.; Hong, R.-J.; Shen, H. Multifunctional TiO2 nanowires-modified nanoparticles bilayer film for 3D dye-sensitized solar cells. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 1166–1169. [Google Scholar]
- Zou, Y.; Wang, Q.; Xiang, C.; Tang, C. Doping composite of polyaniline and reduced graphene oxide with palladium nanoparticles for room-temperature hydrogen-gas sensing. Int. J. Hydrogen Energy 2016, 41, 5396–5404. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Halper, M.J. Ellenbogen, Supercapacitors: A brief overview. Rep. No. MP 05W0000272 2006, 1–29. [Google Scholar]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Amal, I.; Lee, J.; Cho, M.H. pTSA doped conducting graphene/polyaniline nanocomposite fibers: Thermoelectric behavior and electrode analysis. Chem. Eng. J. 2014, 242, 155–161. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Ramkumar, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015, 44, 6158–6168, doi101039/c5dt00622h. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Njomo, N.; Waryo, T.; Masikini, M.; Ikpo, C.O.; Mailu, S.; Tovide, O.; Ross, N.; Williams, A.; Matinise, N.; Sunday, C.E.; et al. Graphenated tantalum(IV) oxide and poly(4-styrene sulfonic acid)-doped polyaniline nanocomposite as cathode material in an electrochemical capacitor. Electrochim. Acta 2014, 128, 226–237. [Google Scholar] [CrossRef]
- Li, B.; Cheng, J.; Wang, Z.; Li, Y.; Ni, W.; Wang, B. Highly-wrinkled reduced graphene oxide-conductive polymer fibers for flexible fiber-shaped and interdigital-designed supercapacitors. J. Power Sources 2018, 376, 117–124. [Google Scholar] [CrossRef]
- Ndipingwi, M.M.; Ikpo, C.; Hlongwa, N.W.; Ross, N.; Masikini, M.; John, S.V.; Baker, P.; Roos, W.; Iwuoha, E.I. Orthorombic nanostructured Li2MnSiO4/Al2O3 supercapattery electrode with efficient Lithium-ion migratory pathway. Batteries & Supercaps 2018, 1, 223–235. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, Y.-C.; Chen, Y.-F.; Huq, M.M.; Chen, P.-Y.; Jang, B.-S. Microwave synthesis of titania-coated carbon nanotube composites for electrochemical capacitors. J. Power Sources 2014, 269, 526–533. [Google Scholar] [CrossRef]
- Karabatsos, D.; Ntziouni, A.; Efthymiou, G.; Fujisawa, K.; Lei, Y.; Terrones, M.; Kordatos, K. Controlled anchoring of Fe3O4 nanoparticles on graphene oxide and thermally reduced graphene oxide. Nanoscale Adv. (under review).
- Fang, Y.; Guo, S.; Zhu, C.; Zhai, Y.; Wang, E. Self-assembly of cationic polyelectrolyte-functionalized graphene nanosheets and gold nanoparticles: A two-dimensional heterostructure for hydrogen peroxide sensing. Langmuir 2010, 26, 11277–11282. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Barakat, M.A. DBSA doped polyaniline/multi-walled carbon nanotubes composite for high efficiency removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2013, 228, 748–755. [Google Scholar] [CrossRef]
- Karthika, P.; Rajalakshmi, N.; Dhathathreyan, K.S. Functionalized exfoliated graphene oxide as supercapacitor electrodes. Soft Nanosci. Lett. 2012, 2, 59–66. [Google Scholar] [CrossRef]
- Sarlak, N.; Meyer, T.J. Fabrication of completely water-soluble graphene oxides graft poly citric acid using different oxidation methods and comparison of them. J. Mol. Liq. 2017, 243, 654–663. [Google Scholar] [CrossRef]
- Mane, A.T.; Navale, S.T.; Mane, R.S.; Naushad, M.; Patil, V.B. Synthesis and structural, morphological, compositional, optical and electrical properties of DBSA-doped PPy–WO3 nanocomposites. Prog. Org. Coatings 2015, 87, 88–94. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.; Nihtianova, D.; Markov, P.; Staneva, A.D.; Iordanova, R.S.; Dimitriev, Y.B. Structural analysis of reduced graphene oxide by transmission electron microscopy. Bulg. Chem. Commun. 2015, 47, 291–295. [Google Scholar]
- Wilson, N.R.; Pandey, P.A.; Beanland, R.; Young, R.J.; Kinloch, I.A.; Gong, L.; Liu, Z.; Suenaga, K.; Rourke, J.P.; York, S.J.; et al. Graphene oxide: Structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 2009, 3, 2547–2556. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Yang, Y. What is the choice for supercapacitors: graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Xin, L.; Yang, F.; Rasouli, S.; Qiu, Y. Understanding Pt Nanoparticle Anchoring on Graphene Supports through Surface Functionalization. ACS Catal. 2016, 6, 2642–2653. [Google Scholar] [CrossRef]
- Calheiros, L.F.; Soares, B.G.; Barra, G.M.O. DBSA-CTAB mixture as the surfactant system for the one step inverse emulsion polymerization of aniline: Characterization and blend with epoxy resin. Synth. Met. 2017, 226, 139–147. [Google Scholar] [CrossRef]
- Afzal, A.B.; Javed Akhtar, M. Effects of silver nanoparticles on thermal properties of DBSA-doped polyaniline/PVC blends. Iran. Polym. J. 2012, 21, 489–496. [Google Scholar] [CrossRef]
- Haba, Y.; Segal, E.; Narkis, M.; Titelman, G.I.; Siegmann, A. Polymerization of aniline in the presence of DBSA in an aqueous dispersion. Synth. Met. 1999, 106, 59–66. [Google Scholar] [CrossRef]
- Haba, Y.; Segal, E.; Narkis, M.; Titelman, G.I.; Siegmann, A. Polyaniline–DBSA/polymer blends prepared via aqueous dispersions. Synth. Met. 2000, 110, 189–193. [Google Scholar] [CrossRef]
- Long, N.V.; Chien, N.D.; Hayakawa, T.; Hirata, H.; Lakshminarayana, G.; Nogami, M. The synthesis and characterization of platinum nanoparticles: A method of controlling the size and morphology. Nanotechnology 2010, 21, 035605. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-Castro, T.; Castillo-Ortega, M.M.; Villarreal, I.; Brown, F.; Grijalva, H.; Perez-Tello, M.; Nuno-Donlucas, S.M.; Puig, J.E. Synthesis and characterization of composites of DBSA-doped polyaniline and polystyrene-based ionomers. Compos. Part A Appl. Sci. Manuf. 2007, 38, 639–645. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Dong, L.; Gari, R.R.S.; Li, Z.; Craig, M.M.; Hou, S. Graphene-supported platinum and platinum–ruthenium nanoparticles with high electrocatalytic activity for methanol and ethanol oxidation. Carbon 2010, 48, 781–787. [Google Scholar] [CrossRef]
- Yue, Y.; Zhou, B.; Shi, J.; Chen, C.; Li, N.; Xu, Z.; Liu, L.; Kuang, L.; Ma, M.; Fu, H. γ-Irradiation assisted synthesis of graphene oxide sheets supported Ag nanoparticles with single crystalline structure and parabolic distribution from interlamellar limitation. Appl. Surf. Sci. 2017, 403, 282–293. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Karshenas, A. Graphene oxide supported copper coordinated amino acids as novelheterogeneous catalysts for epoxidation of norbornene. Catal. Commun. 2017, 92, 109–113. [Google Scholar] [CrossRef]
- Chew, T.; Daik, R.; Hamid, M. Thermal Conductivity and Specific Heat Capacity of Dodecylbenzenesulfonic Acid-Doped Polyaniline Particles—Water Based Nanofluid. Polymers 2015, 7, 1221–1231. [Google Scholar] [CrossRef]
- Jianming, J.; Wei, P.; Shenglin, Y.; Guang, L. Electrically conductive PANI-DBSA/Co-PAN composite fibers prepared by wet spinning. Synth. Met. 2005, 149, 181–186. [Google Scholar] [CrossRef]
- Yin, H.; Yamamoto, T.; Wada, Y.; Yanagida, S. Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater. Chem. Phys. 2004, 83, 66–70. [Google Scholar] [CrossRef]
- Yin, W.; Ruckenstein, E. Soluble polyaniline co-doped with dodecyl benzene sulfonic acid and hydrochloric acid. Synth. Met. 2000, 108, 39–46. [Google Scholar] [CrossRef]
- Fakhri, P.; Jaleh, B.; Nasrollahzadeh, M. Synthesis and characterization of copper nanoparticles supported on reduced graphene oxide as a highly active and recyclable catalyst for the synthesis of formamides and primary amines. J. Mol. Catal. A Chem. 2014, 383, 17–22. [Google Scholar] [CrossRef]
- Khan, S.; Ali, J.; Husain, M.; Zulfequar, M. Synthesis of reduced graphene oxide and enhancement of its electrical and optical properties by attaching Ag nanoparticles. Phys. E Low-dimensional Syst. Nanostructures 2016, 81, 320–325. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, J.; Xie, K.; Wang, W.; Fang, Y. Aerobic oxidation of cyclohexane catalyzed by graphene oxide: Effects of surface structure and functionalization. Mol. Catal. 2017, 431, 1–8. [Google Scholar] [CrossRef]
- Yang, S.-D.; Shen, C.-M.; Tong, H.; He, W.; Zhang, X.-G.; Gao, H.-J. Highly dispersed Pd nanoparticles on chemically modified graphene with aminophenyl groups for formic acid oxidation. Chinese Phys. B 2011, 20, 113301. [Google Scholar] [CrossRef]
- Han, D.; Chu, Y.; Yang, L.; Liu, Y.; Lv, Z. Reversed micelle polymerization: a new route for the synthesis of DBSA–polyaniline nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 259, 179–187. [Google Scholar] [CrossRef]
- Nasehnia, F.; Mohammadpour Lima, S.; Seifi, M.; Mehran, E. First principles study on optical response of graphene oxides: From reduced graphene oxide to the fully oxidized surface. Comput. Mater. Sci. 2016, 114, 112–120. [Google Scholar] [CrossRef]
- Wang, J.; Caliskan Salihi, E.; Šiller, L. Green reduction of graphene oxide using alanine. Mater. Sci. Eng. 2017, 72, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Ohlan, A.; Dhawan, S.K.; Kong, B.S. Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution. Nanoscale 2013, 5, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Zoladek, S.; Rutkowska, I.A.; Blicharska, M.; Miecznikowski, K.; Ozimek, W.; Orlowska, J.; Negro, E.; Noto, V.D.; Kulesza, P.J. Evaluation of reduced-graphene-oxide-supported gold nanoparticles as catalytic system for electroreduction of oxygen in alkaline electrolyte. Electrochim. Acta 2017, 233, 113–122. [Google Scholar] [CrossRef]
- Bhadra, J.; Madi, N.K.; Al-Thani, N.J.; Al-Maadeed, M.A. Polyaniline/polyvinyl alcohol blends: Effect of sulfonic acid dopants on microstructural, optical, thermal and electrical properties. Synth. Met. 2014, 191, 126–134. [Google Scholar] [CrossRef]
- Xie, H.Q.; Ma, Y.M.; Guo, J.S. Secondary doping phenomena of two conductive polyaniline composites. Synth. Met. 2001, 123, 47–52. [Google Scholar] [CrossRef]
- Guo, Q.; Yi, C.; Zhu, L.; Yang, Q.; Xie, Y. Chemical synthesis of cross-linked polyaniline by a novel solvothermal metathesis reaction of p-dichlorobenzene with sodium amide. Polymer 2005, 46, 3185–3189. [Google Scholar] [CrossRef]
- Chang, K.C.; Yang, G.-W.; Peng, C.-W.; Lin, C.-Y.; Shieh, J.-C.; Yeh, J.M.; Yang, J.-C.; Li, W.T. Comparatively electrochemical studies at different operational temperatures for the effect of nanoclay platelets on the anticorrosion efficiency of DBSA-doped polyaniline/Na+–MMT clay nanocomposite coatings. Electrochim. Acta 2007, 52, 5191–5200. [Google Scholar] [CrossRef]
- Chen, C.H. Thermal Studies of Polyaniline Doped with Dodecyl Benzene Sulfonic Acid Directly Prepared via Aqueous Dispersions. J. Polym. Res. 2002, 9, 195–200. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, G.; Zhang, H.; Li, S. Evaluation and Characterization of Reduced Graphene Oxide Nanosheets as Anode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2013, 8, 6269–6280. [Google Scholar]
- Sharma, V.V.; Gualandi, I.; Vlamidis, Y.; Tonelli, D. Electrochemical behavior of reduced graphene oxide and multi-walled carbon nanotubes composites for catechol and dopamine oxidation. Electrochim. Acta 2017, 246, 415–423. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Jang, H.; Kim, J.; Kang, H.; Bae, D.; Chang, H.; Choi, H. Reduced graphene oxide as a protection layer for Al. Appl. Surf. Sci. 2017, 407, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Engelhard, M.H.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of grapheme oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]







| Material | Specific Capacitance (F·g−1) | Specific Energy (Wh·kg−1) | Specific Power (W·kg−1) | References |
|---|---|---|---|---|
| GO/Pt/DBSA–PANI | 227.2 | 126.2 | 178.4 | This work |
| Graphene/MnO2//CAN | 113.5 | 51.1 | 102.2 | [33] |
| TaO2–PANI–PSSA | 318.4 | 157.0 | 435.0 | [34] |
| MnO2/e–CMG | 389.0 | 44.0 | 250.0 | [35] |
| Li2MnSiO4/Al2O3 | 117.5 | 864.3 | 104.0 | [36] |
| TiO2/CNT | 176.5 | 40.0 | 6428.0 | [37] |
| GO–Pt NPs | GO/Pt/DBSA–PANI | ||
|---|---|---|---|
| Wavenumber (cm−1) | Bond | Wavenumber (cm−1) | Bond |
| 3436 | O-H | 3144 | N-H |
| 1740 | C=O | 1590 | Quinoid |
| 1636 | C=C | 1408 | Benzenoid |
| 1390 | C=C-O | 1223 | C-N |
| 1220 | CO-H | 1180 | C-H |
| 1040 | C-O | 1084 | O=S=O |
| - | 1004 | S-O | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dywili, N.R.; Ntziouni, A.; Ikpo, C.; Ndipingwi, M.; Hlongwa, N.W.; Yonkeu, A.L.D.; Masikini, M.; Kordatos, K.; Iwuoha, E.I. Graphene Oxide Decorated Nanometal-Poly(Anilino-Dodecylbenzene Sulfonic Acid) for Application in High Performance Supercapacitors. Micromachines 2019, 10, 115. https://doi.org/10.3390/mi10020115
Dywili NR, Ntziouni A, Ikpo C, Ndipingwi M, Hlongwa NW, Yonkeu ALD, Masikini M, Kordatos K, Iwuoha EI. Graphene Oxide Decorated Nanometal-Poly(Anilino-Dodecylbenzene Sulfonic Acid) for Application in High Performance Supercapacitors. Micromachines. 2019; 10(2):115. https://doi.org/10.3390/mi10020115
Chicago/Turabian StyleDywili, Nomxolisi R., Afroditi Ntziouni, Chinwe Ikpo, Miranda Ndipingwi, Ntuthuko W. Hlongwa, Anne L. D. Yonkeu, Milua Masikini, Konstantinos Kordatos, and Emmanuel I. Iwuoha. 2019. "Graphene Oxide Decorated Nanometal-Poly(Anilino-Dodecylbenzene Sulfonic Acid) for Application in High Performance Supercapacitors" Micromachines 10, no. 2: 115. https://doi.org/10.3390/mi10020115