Patterning Perfluorinated Surface with Graphene Oxide and the Microarray Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
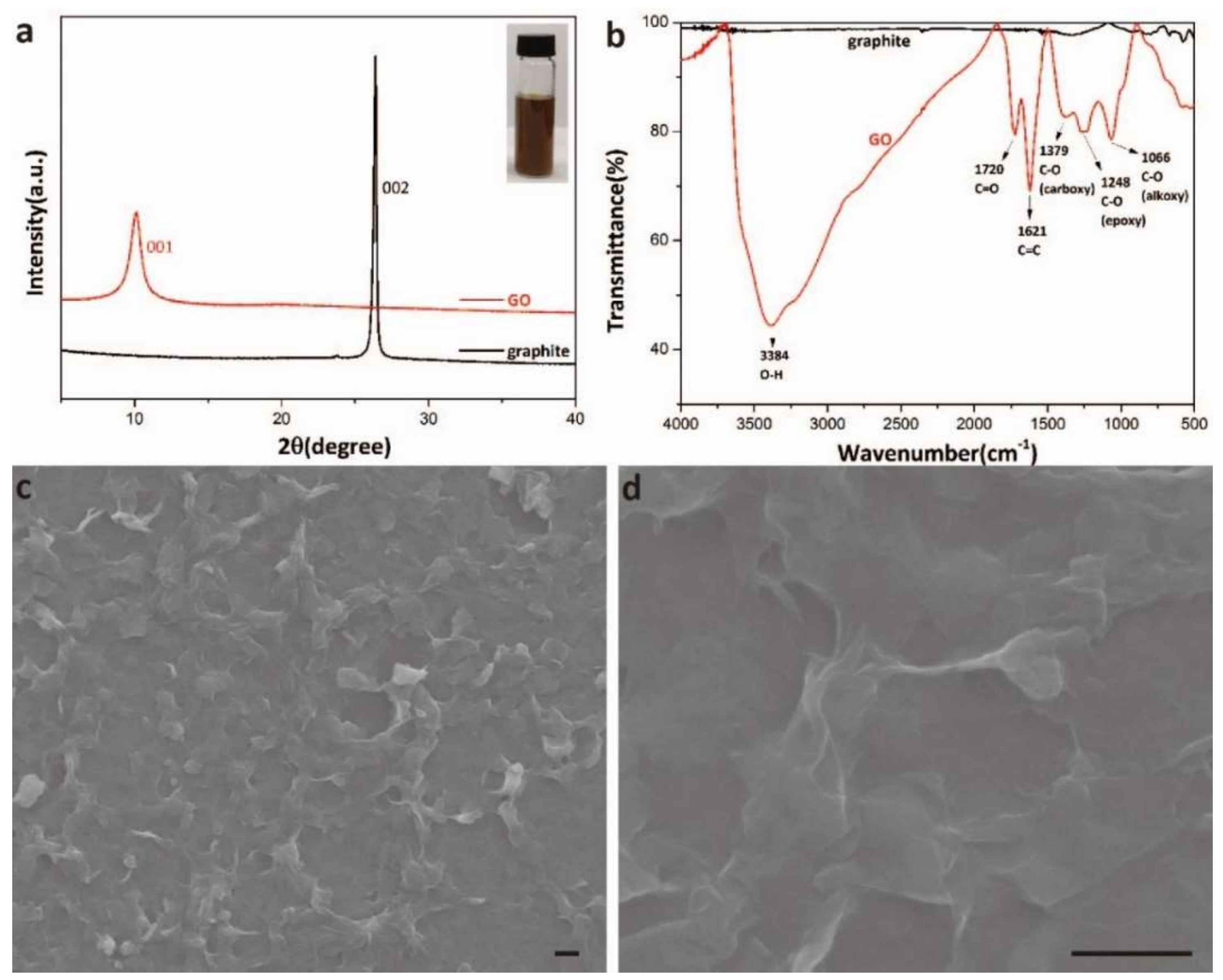
2.2. Synthesis of Graphene Oxide
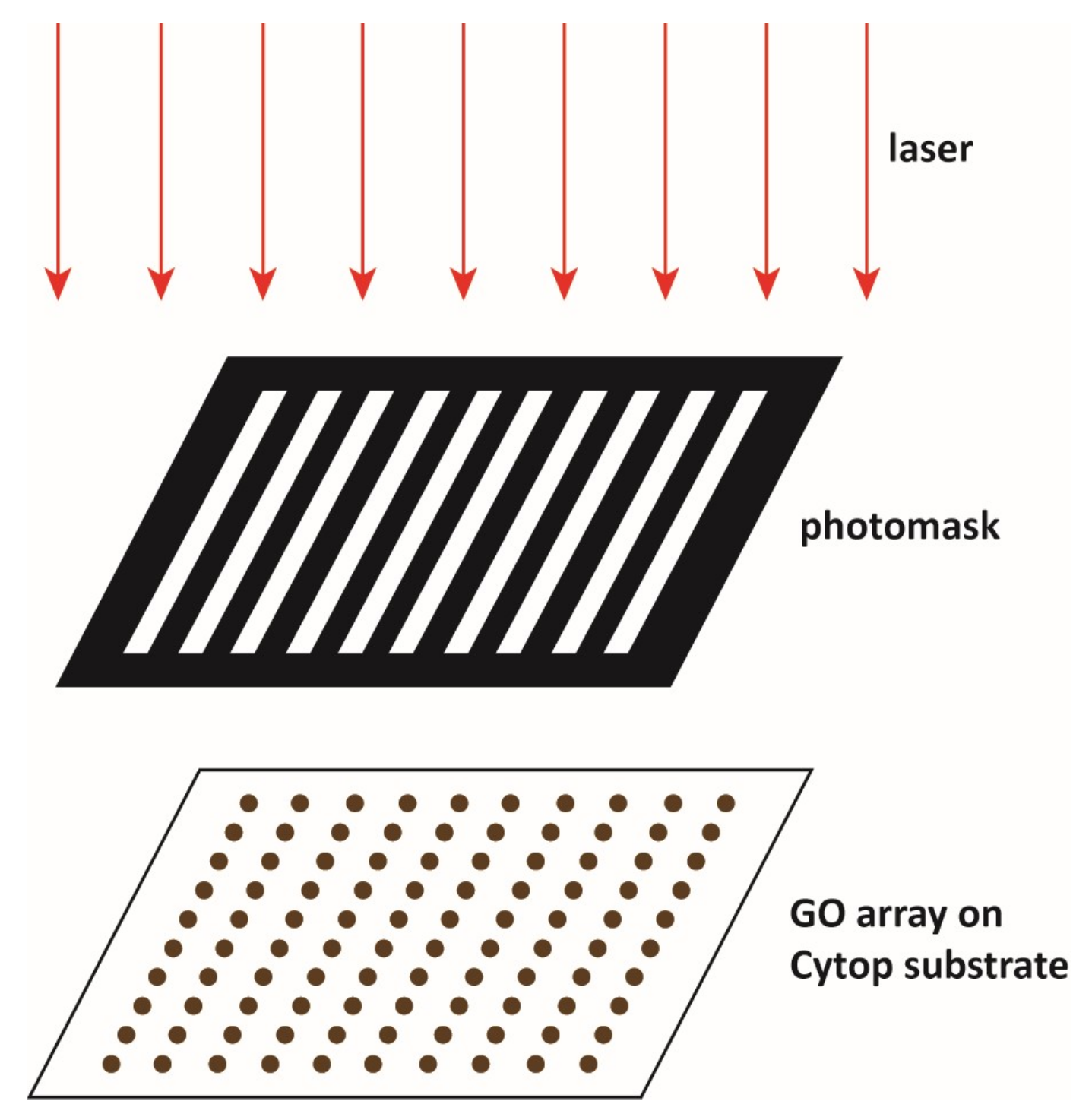
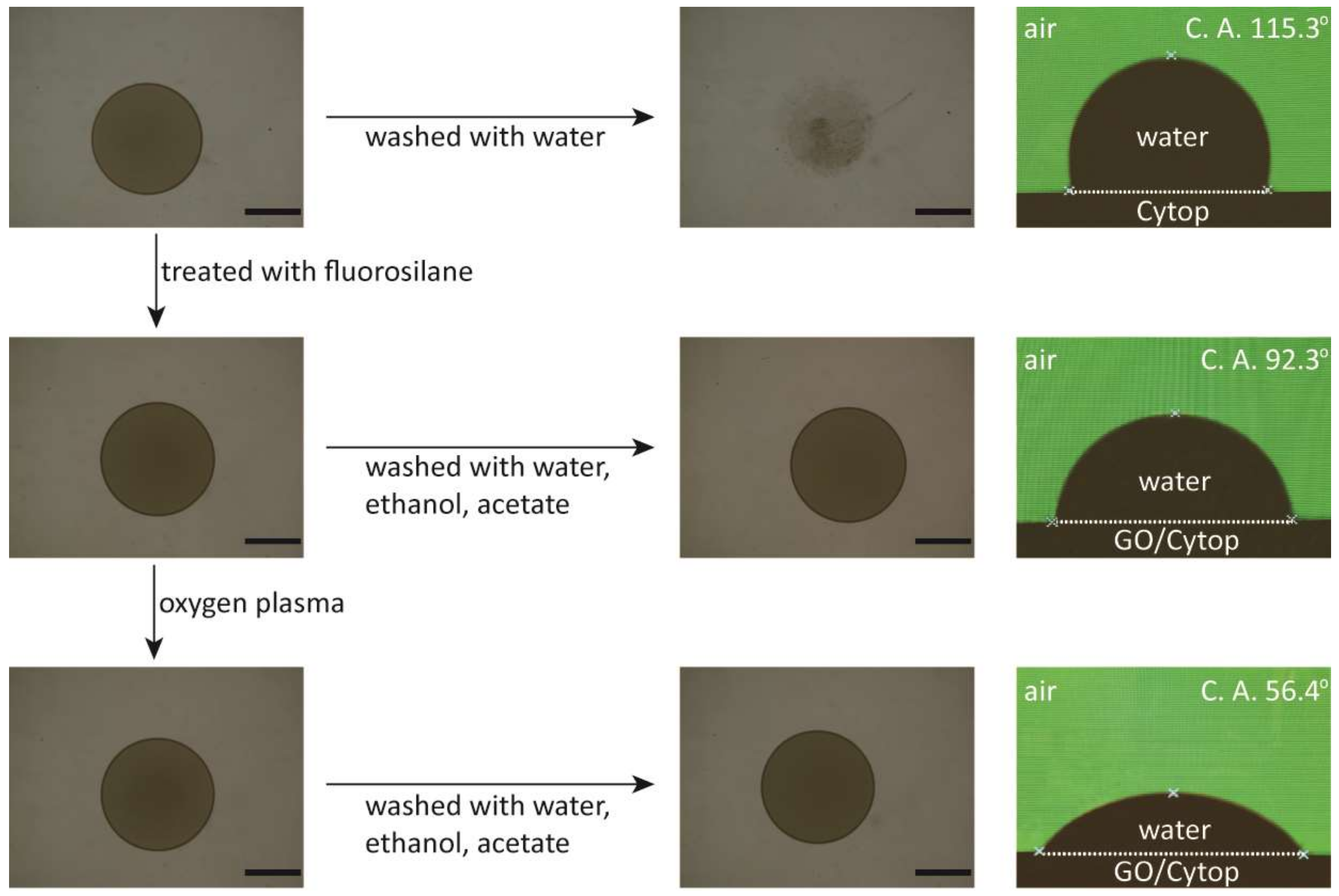
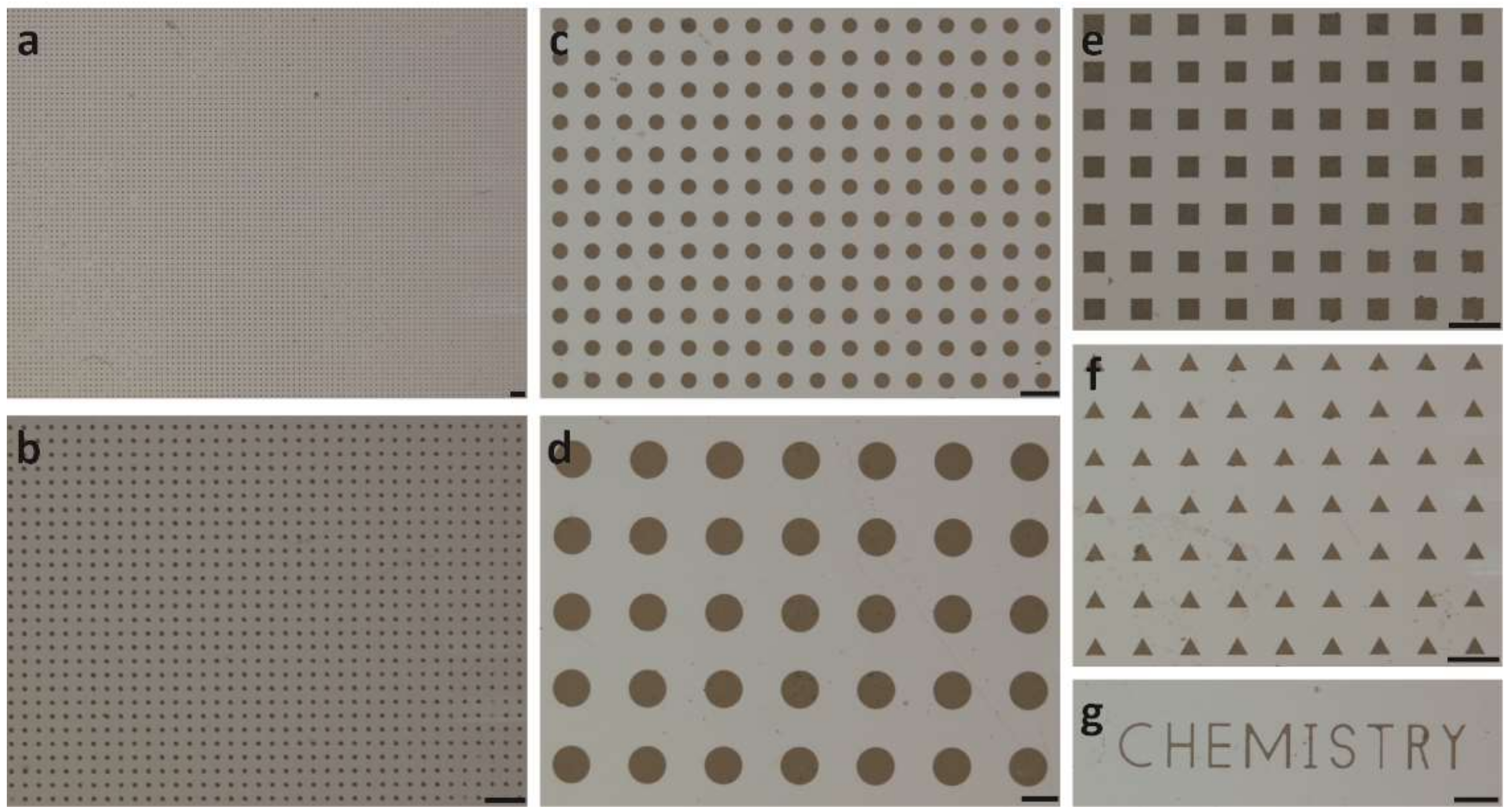
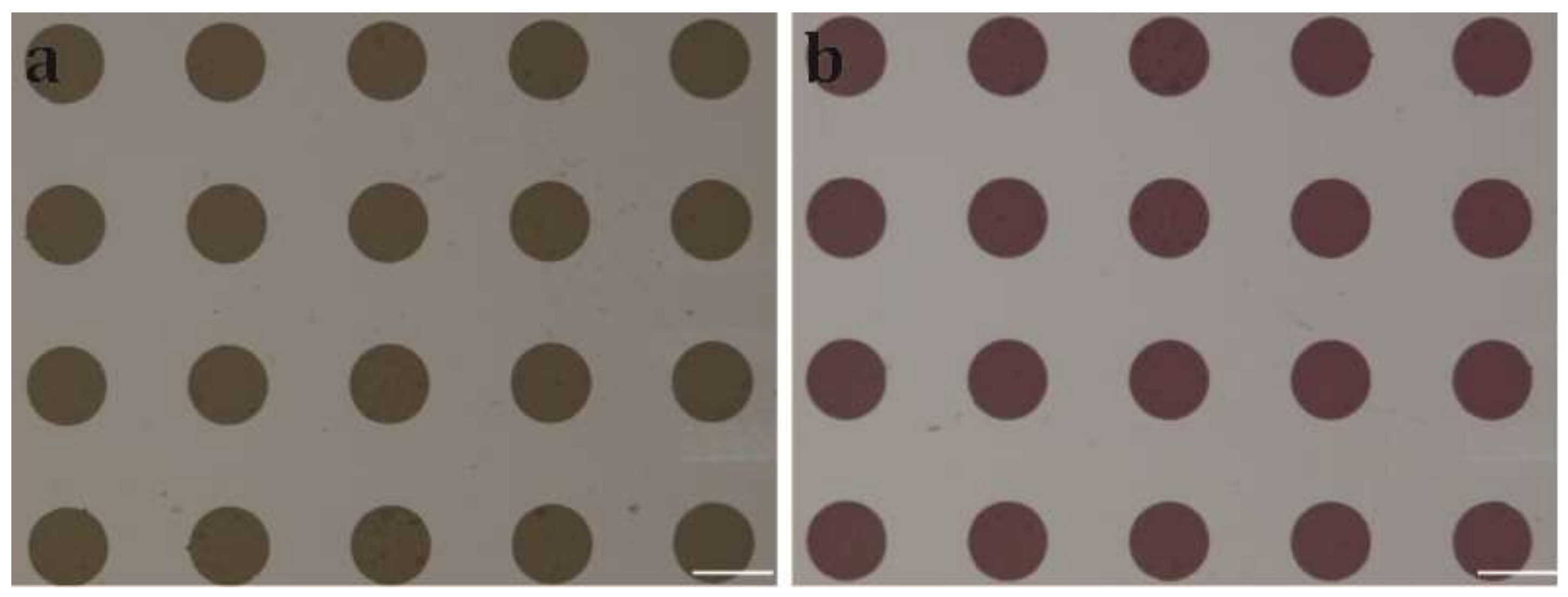
2.3. Patterning Cytop Surface with Graphene Oxide Thin Film
2.4. Surface Testing and Contact Angle Measurement
2.5. Scanning Electron Microscopy (SEM)
2.6. Confocal Microscopy
2.7. X-ray Diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), and Infrared (IR) Spectroscopy
2.8. Stem Cells Culture and Harvest
2.9. Culturing Stem Sells on Graphene Oxide and Irradiation with a Low-Level Laser
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Weber, S.G. Teflon AF Materials BT. In Fluorous Chemistry; Horváth, I.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–337. ISBN 978-3-642-25234-1. [Google Scholar]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Xiong, B.; Wu, H. Convenient surface functionalization of whole-Teflon chips with polydopamine coating. Biomicrofluidics 2015, 9, 44111. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, Q.; Ma, H.; Zheng, B. An ultralow background substrate for protein microarray technology. Analyst 2015, 140, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wu, Y.; Yang, X.; Liu, X.; He, J.; Fu, L.; Wang, J.; Xu, H.; Shi, Y.; Zhong, R. Integrated poly(dimethysiloxane) with an intrinsic nonfouling property approaching “absolute” zero background in immunoassays. Anal. Chem. 2010, 82, 6338–6342. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Nakafuji, T. Fabrication of Cotton-like Superhydrophobic Surface on Teflon Using a Pulsed Ultraviolet Laser. J. Laser Micro Nanoeng. 2010, 5, 134–137. [Google Scholar] [CrossRef]
- Cho, C.-C.; Wallace, R.M.; Files-Sesler, L.A. Patterning and etching of amorphous teflon films. J. Electron. Mater. 1994, 23, 827–830. [Google Scholar] [CrossRef]
- Kitamura, A.; Satoh, T.; Koka, M.; Kamiya, T.; Kobayashi, T. Modification of Teflon surface by proton microbeam and nitrogen ion beam. Nucl. Instruments Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2013, 314, 82–85. [Google Scholar] [CrossRef]
- Boittiaux, V.; Boucetta, F.; Combellas, C.; Kanoufi, F.; Thiébault, A.; Delamar, M.; Bertrand, P. Surface modification of halogenated polymers: 3. Influence of additives such as alkali cations or nucleophiles on the magnesium reductive treatment of polytetrafluoroethylene. Polymer (Guildf). 1999, 40, 2011–2026. [Google Scholar] [CrossRef]
- Nelson, E.; Kilduff, T.J.; Benderly, A.A. Bonding of Teflon. Ind. Eng. Chem. 1958, 50, 329–330. [Google Scholar] [CrossRef]
- Bart, J.; Tiggelaar, R.; Yang, M.; Schlautmann, S.; Zuilhof, H.; Gardeniers, H. Room-temperature intermediate layer bonding for microfluidic devices. Lab Chip 2009, 9, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feng, H.; Guo, D.; Zheng, B. Measuring the adhesion strength of a thin film to a substrate by centrifugation. RSC Adv. 2014, 4, 60002–60006. [Google Scholar] [CrossRef]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene oxide: from fundamentals to applications. J. Phys. Condens. Matter 2015, 27, 13002. [Google Scholar] [CrossRef] [PubMed]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The enzymatic oxidation of graphene oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, M.; He, H.; Zhang, L.; Huang, J.; Chong, Y.; Dai, J.; Zhang, Z. PEGylated graphene oxide-mediated protein delivery for cell function regulation. ACS Appl. Mater. Interfaces 2012, 4, 6317–6323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Garcia-Bernal, D.; Aznar-Cervantes, S.; Ros-Roca, M.A.; Alguero, M.C.; Atucha, N.M.; Lozano-Garcia, A.A.; Moraleda, J.M.; Cenis, J.L. Effects of composite films of silk fibroin and graphene oxide on the proliferation, cell viability and mesenchymal phenotype of periodontal ligament stem cells. J. Mater. Sci. Med. 2014, 25, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, H.; Fang, Y.X.; Cao, Y.H.; Huang, J.; Zhang, M.X.; Dai, J.W.; Shi, X.Y.; Zhang, Z.J. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, Y.; Kim, D.H.; Choung, P.H.; Cho, C.S.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. Part A 2013, 101, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Pang, D.W.P.; Hwang, S.M.; Tuan, H.Y.; Hu, Y.C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Ren, J.; Qu, X. A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials 2011, 32, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide 1: High-yield synthesis and flexibility of the particles. Carbon N. Y. 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Zhao, G.X.; Li, J.X.; Ren, X.M.; Chen, C.L.; Wang, X.K. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Hair, M.L.; Hertl, W. Reactions of chlorosilanes with silica surfaces. J. Phys. Chem. 1969, 73, 2372–2378. [Google Scholar] [CrossRef]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Niembro, S.; Prastaro, A.; Shafir, A.; Vallribera, A. Perfluoro-tagged gold nanoparticles immobilized on fluorous silica gel: A reusable catalyst for the benign oxidation and oxidative esterification of alcohols. ChemSusChem 2009, 2, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Boswell, P.G.; Bühlmann, P. Fluorous bulk membranes for potentiometric sensors with wide selectivity ranges: Observation of exceptionally strong ion pair formation. J. Am. Chem. Soc. 2005, 127, 8958–8959. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-M. Recent advances of fluorous chemistry in material sciences. Chem. Commun. 2012, 48, 11382–11391. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene oxide as a matrix for enzyme immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Aphale, A.N.; Macwan, I.G.; Patra, P.K.; Gonzalez, W.G.; Miksovska, J.; Leblanc, R.M. Graphene oxide as a quencher for fluorescent assay of amino acids, peptides, and proteins. ACS Appl. Mater. Interfaces 2012, 4, 7069–7075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, T.; Zhou, C. Low-Level Laser Therapy and Stem Cells. In Low-Level Light Therapy: Photobiomodulation; Hamblin, M.R., Ferraresi, C., Huang, Y.-Y., de Freitas, L.F., Carroll, J.D., Eds.; SPIE: Bellingham, WA, USA, 2018; pp. 117–129. [Google Scholar]
- Hou, J.; Zhang, H.; Yuan, X.; Li, J.; Wei, Y.; Hu, S. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: Proliferation, growth factors secretion and myogenic differentiation. Lasers Surg. Med. 2008, 40, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Chellini, F.; Sassoli, C.; Francini, F.; Pini, A.; Squecco, R.; Nosi, D.; Bani, D.; Zecchi-Orlandini, S.; Formigli, L. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: Effects and mechanisms of action. J. Cell. Physiol. 2013, 228, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Chen, A.C.-H.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose Response 2009, 7, 358–383. [Google Scholar] [CrossRef] [PubMed]
- Wojtoniszak, M.; Chen, X.; Kalenczuk, R.J.; Wajda, A.; Łapczuk, J.; Kurzewski, M.; Drozdzik, M.; Chu, P.K.; Borowiak-Palen, E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B Biointerfaces 2012, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Luzo, A.C.M.; de Souza, J.G.; Favaro, W.J.; Garcia, P.; Duran, N. Graphene oxide as scaffolds for stem cells: An overview. Curr. Mol. Med. 2017, 17, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A mini review focused on the recent applications of graphene oxide in stem cell growth and differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Castro Neto, A.H. Graphene oxide-based substrate: physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alegria, E.; Iliut, M.; Stefanska, M.; Silva, C.; Heeg, S.; Kimber, S.J.; Kouskoff, V.; Lacaud, G.; Vijayaraghavan, A.; Batta, K. Graphene Oxide promotes embryonic stem cell differentiation to haematopoietic lineage. Sci. Rep. 2016, 6, 25917. [Google Scholar] [CrossRef] [PubMed]
- Hanada, Y.; Sugioka, K.; Kawano, H.; Tsuchimoto, T.; Miyamoto, I.; Miyawaki, A.; Midorikawa, K. Selective cell culture on UV transparent polymer by F2 laser surface modification. Appl. Surf. Sci. 2009, 255, 9885–9888. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Liu, B.; Zhu, M.; Guo, D.; Wu, H.; Bian, L.; Zheng, B. Patterning Perfluorinated Surface with Graphene Oxide and the Microarray Applications. Micromachines 2019, 10, 173. https://doi.org/10.3390/mi10030173
Wu L, Liu B, Zhu M, Guo D, Wu H, Bian L, Zheng B. Patterning Perfluorinated Surface with Graphene Oxide and the Microarray Applications. Micromachines. 2019; 10(3):173. https://doi.org/10.3390/mi10030173
Chicago/Turabian StyleWu, Liang, Baishu Liu, Meiling Zhu, Dameng Guo, Han Wu, Liming Bian, and Bo Zheng. 2019. "Patterning Perfluorinated Surface with Graphene Oxide and the Microarray Applications" Micromachines 10, no. 3: 173. https://doi.org/10.3390/mi10030173




