Fabrication of a Gelatin-Based Microdevice for Vascular Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modification of Coverslips with APTES-Glutaraldehyde (GA)
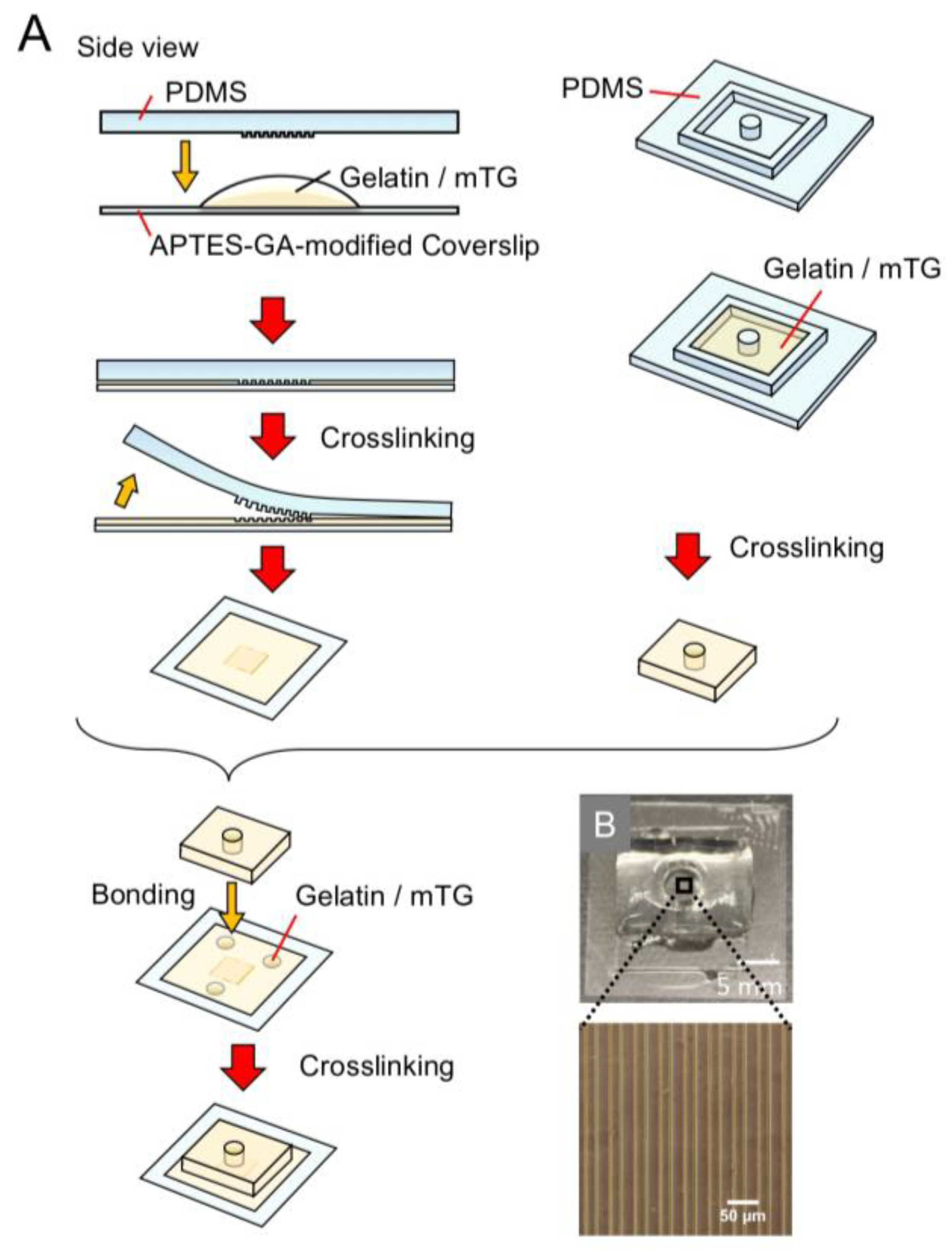
2.2. Fabrication of PDMS Molds
2.3. Fabrication of Crosslinked Gelatin Microfluidic Devices
2.4. Fabrication of PDMS Microfluidic Devices
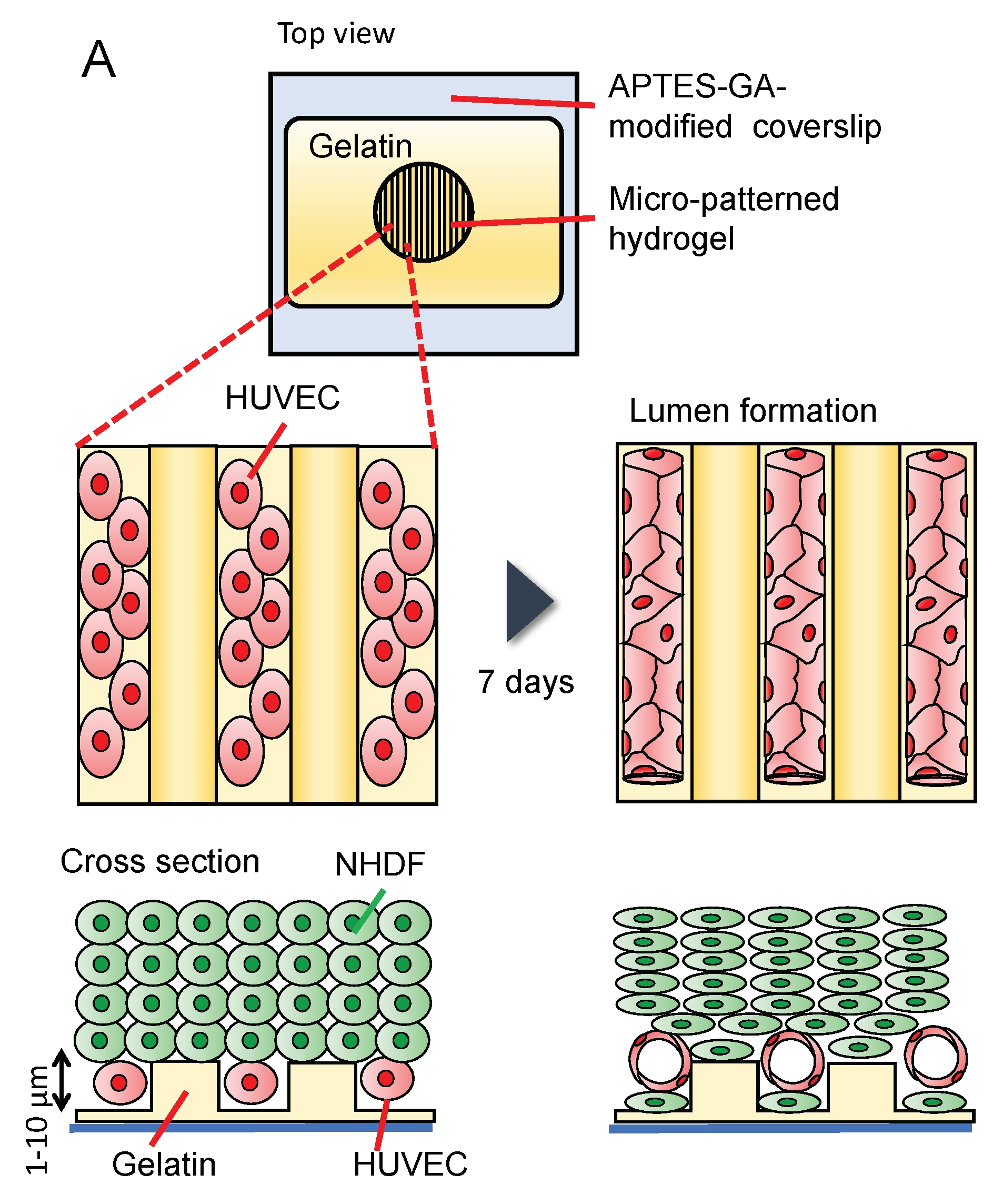
2.5. Fabrication of Stripe-Micropatterned Gelatin Wells
2.6. Gel Indentation Assay
2.7. Scanning Electron Microscopy (SEM) Imaging of Gelatin and PDMS Surface
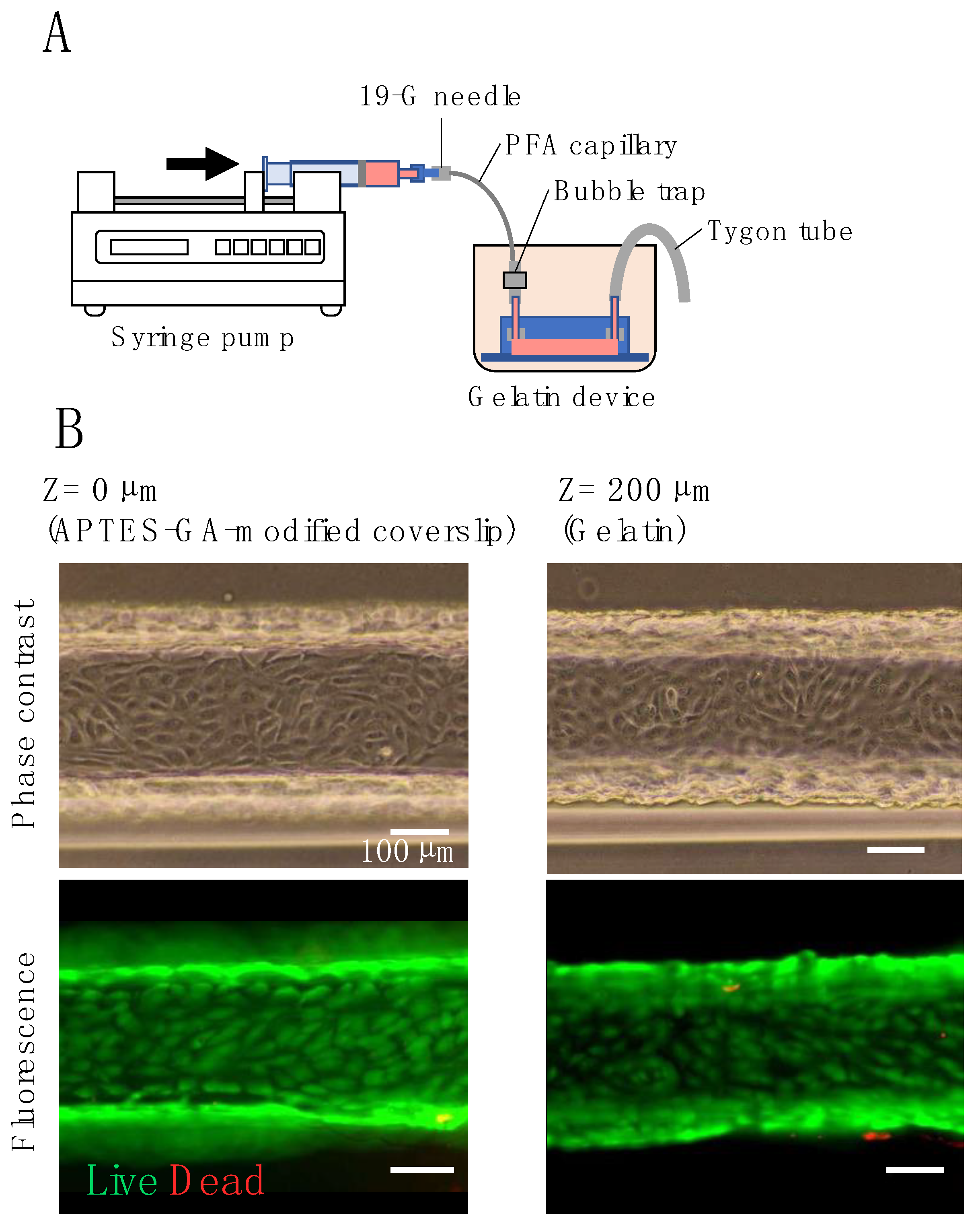
2.8. Microfluidic Cell Culture
2.9. Microfluidic Perfusion
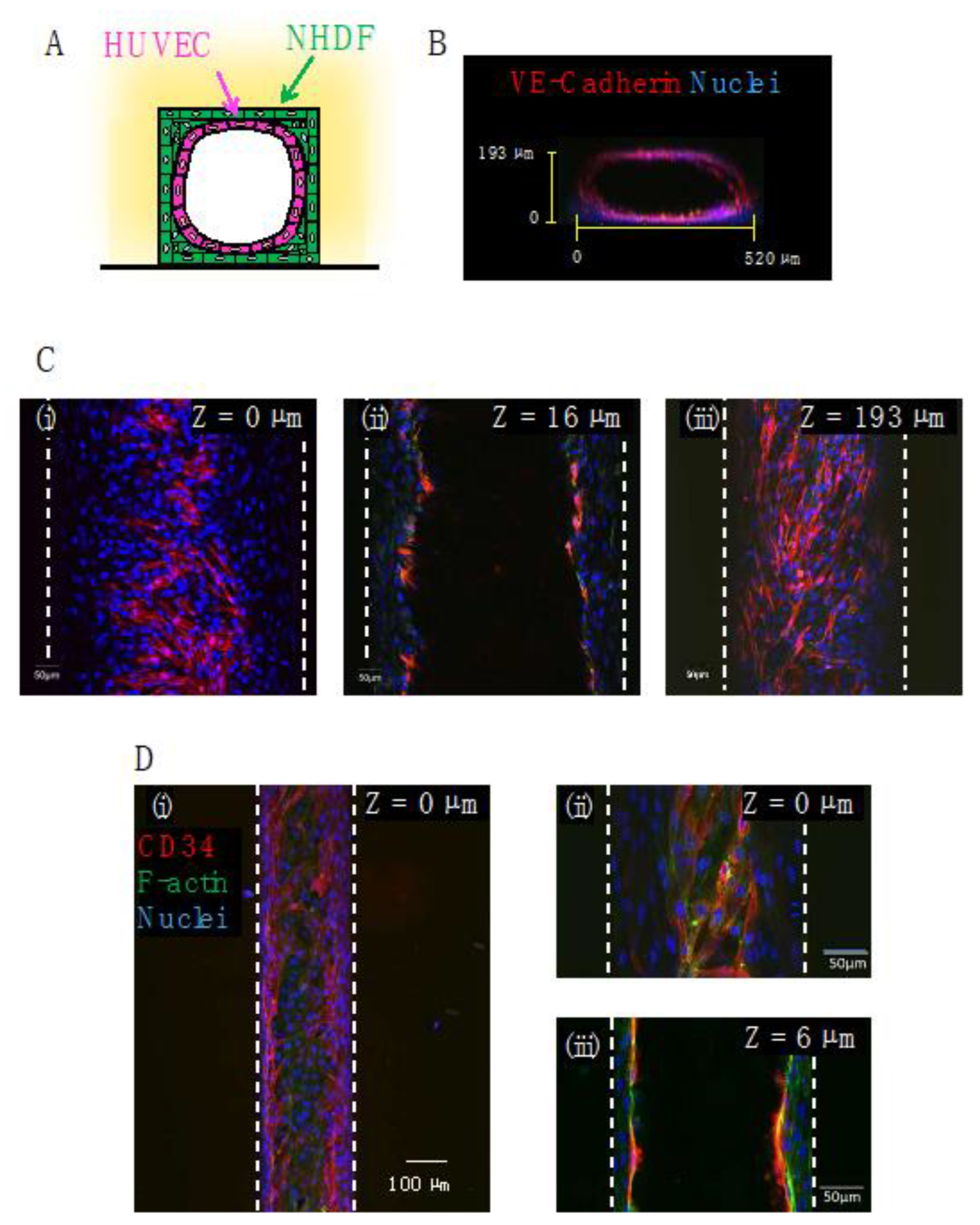
2.10. Co-Culture on Stripe-Micropatterned Surfaces
2.11. Cell Viability Assay
2.12. Cell Staining
3. Results and Discussion
3.1. Characterization of Gelatin-Based Devices
3.2. Monolayer Cell Culture in the Gelatin Channel
3.3. Observation of Fibronectin by Confocal Microscopy and SEM
3.4. Microfluidic Culture of HUVECs
3.5. Co-Culture of HUVECs and NHDFs in the Gelatin Channel
3.6. Formation of Capillary/Vessel-like Structure in the Gelatin Microstructure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Shinjo, M.; Hirakawa, S.; Nishinaka, M.; Tanaka, Y.; Mawatari, K.; Kitamori, T.; Sato, K. A palmtop-sized microfluidic cell culture system driven by a miniaturized infusion pump. Electrophoresis 2012, 33, 1729–1735. [Google Scholar] [CrossRef]
- Sato, M.; Sasaki, N.; Ato, M.; Hirakawa, S.; Sato, K.; Sato, K. Microcirculation-on-a-Chip: A Microfluidic Platform for Assaying Blood- and Lymphatic-Vessel Permeability. PLoS ONE 2015, 10, e0137301. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nakajima, M.; Tokuda, S.; Ogawa, A. Fluidic Culture and Analysis of Pulmonary Artery Smooth Muscle Cells for the Study of Pulmonary Hypertension. Anal. Sci. 2016, 32, 1217–1221. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Sato, K. Recent Progress in the Development of Microfluidic Vascular Models. Anal. Sci. 2018, 34, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Sato, M.; Yokoyama, M.; Hirai, M.; Furuta, A. Influence of Culture Conditions on Cell Proliferation in a Microfluidic Channel. Anal. Sci. 2019, 35, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Maeda, M.; Kamata, E.; Ishii, S.; Yanagisawa, K.; Kitajima, K.; Hara, T. Nitric Oxide and a Conditioned Medium Affect the Hematopoietic Development in a Microfluidic Mouse Embryonic Stem Cell/OP9 Co-Cultivation System. Micromachines 2020, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Xu, Z.; Xiao, L.; Shi, T.; Xiao, H.; Wang, Y.; Li, Y.; Xue, F.; Zeng, W. Review on the Vascularization of Organoids and Organoids-on-a-Chip. Front. Bioeng. Biotechnol. 2021, 9, 637048. [Google Scholar] [CrossRef]
- Naeimi, M.; Karkhaneh, A.; Barzin, J.; Khorasani, M.T.; Ghaffarieh, A. Novel PDMS-based membranes: Sodium chloride and glucose permeability. J. Appl. Polym. Sci. 2013, 127, 3940–3947. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, A.; Trosper, N.E.; Yang, H.S.; Kim, J.; Tsui, J.H.; Frankel, S.D.; Murry, C.E.; Kim, D.H. Thermoresponsive nanofabricated substratum for the engineering of three-dimensional tissues with layer-by-layer architectural control. ACS Nano 2014, 8, 4430–4439. [Google Scholar] [CrossRef]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef] [Green Version]
- Bettadapur, A.; Suh, G.C.; Geisse, N.A.; Wang, E.R.; Hua, C.; Huber, H.A.; Viscio, A.A.; Kim, J.Y.; Strickland, J.B.; McCain, M.L. Prolonged Culture of Aligned Skeletal Myotubes on Micromolded Gelatin Hydrogels. Sci. Rep. 2016, 6, 28855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, G.C.; Bettadapur, A.; Santoso, J.W.; McCain, M.L. Fabrication of micromolded gelatin hydrogels for long-term culture of aligned skeletal myotubes. In Skeletal Muscle Development; Springer: Berlin, Germany, 2017; pp. 147–163. [Google Scholar]
- Denes, L.T.; Riley, L.A.; Mijares, J.R.; Arboleda, J.D.; McKee, K.; Esser, K.A.; Wang, E.T. Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet. Muscle 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin-laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef]
- Rexius-Hall, M.L.; Khalil, N.N.; Andres, A.M.; McCain, M.L. Mitochondrial division inhibitor 1 (mdivi-1) increases oxidative capacity and contractile stress generated by engineered skeletal muscle. FASEB J. 2020, 34, 11562–11576. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Santoso, J.W.; McCain, M.L. Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates. Bioengineering 2021, 8, 6. [Google Scholar] [CrossRef]
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2018, 10, 025003. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kumar, P.; Lv, S.; Xiong, D.; Zhao, H.; Cai, Z.; Zhao, X. Recent advances in 3D bioprinting of vascularized tissues. Mater. Des. 2021, 199, 109398. [Google Scholar] [CrossRef]
- Wang, D.; Maharjan, S.; Kuang, X.; Wang, Z.; Mille, L.S.; Tao, M.; Yu, P.; Cao, X.; Lian, L.; Lv, L.; et al. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci. Adv. 2022, 8, eabq6900. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Dance, Y.W. Microfluidic biomaterials. Adv. Health Mater. 2021, 10, e2001028. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Young, E.W.; Mader, B.R.; Beebe, D.J. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 2013, 34, 1471–1477. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.L.; Margolis, E.A.; Ryan, T.J.; Coisman, B.J.; Price, G.M.; Wong, K.H.K.; Tien, J. Design principles for lymphatic drainage of fluid and solutes from collagen scaffolds. J. Biomed. Mater. Res. A 2018, 106, 106–114. [Google Scholar] [CrossRef]
- Gong, M.M.; Lugo-Cintron, K.M.; White, B.R.; Kerr, S.C.; Harari, P.M.; Beebe, D.J. Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function. Biomaterials 2019, 214, 119225. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Yamada, M.; Yamada, E.; Iwase, M.; Seki, M. Facile fabrication processes for hydrogel-based microfluidic devices made of natural biopolymers. Biomicrofluidics 2014, 8, 024115. [Google Scholar] [CrossRef] [PubMed]
- Paguirigan, A.; Beebe, D.J. Gelatin based microfluidic devices for cell culture. Lab Chip 2006, 6, 407–413. [Google Scholar] [CrossRef]
- Paguirigan, A.L.; Beebe, D.J. Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture. Nat. Protoc. 2007, 2, 1782–1788. [Google Scholar] [CrossRef]
- Yang, C.W.; Ouellet, E.; Lagally, E.T. Using inexpensive Jell-O chips for hands-on microfluidics education. Anal. Chem. 2010, 82, 5408–5414. [Google Scholar] [CrossRef]
- He, J.; Chen, R.; Lu, Y.; Zhan, L.; Liu, Y.; Li, D.; Jin, Z. Fabrication of circular microfluidic network in enzymatically-crosslinked gelatin hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Gao, Q.; Wang, Y.; Zeng, J.; Zhao, H.; Sun, Y.; Shen, J.; Ramezani, H.; Fu, Z.; Liu, Z.; et al. Vessel-on-a-chip with hydrogel-based microfluidics. Small 2018, 14, e1802368. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Gelatin-based microfluidics device with the feature sizes smaller than 100 µm for production of oil-in-water emulsions. Microfluid. Nanofluidics 2019, 23, 35. [Google Scholar] [CrossRef]
- Shimizu, A.; Goh, W.H.; Itai, S.; Hashimoto, M.; Miura, S.; Onoe, H. ECM-based microchannel for culturing in vitro vascular tissues with simultaneous perfusion and stretch. Lab Chip 2020, 20, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Goh, W.H.; Itai, S.; Karyappa, R.; Hashimoto, M.; Onoe, H. ECM-based microfluidic gradient generator for tunable surface environment by interstitial flow. Biomicrofluidics 2020, 14, 044106. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zou, T.; Dissanayaka, W.L.; Wong, H.M.; Bertassoni, L.E.; Zhang, C. Fabrication of Tapered Fluidic Microchannels Conducive to Angiogenic Sprouting within Gelatin Methacryloyl Hydrogels. J. Endod. 2021, 47, 52–61. [Google Scholar] [CrossRef]
- Makita, R.; Akasaka, T.; Tamagawa, S.; Yoshida, Y.; Miyata, S.; Miyaji, H.; Sugaya, T. Preparation of micro/nanopatterned gelatins crosslinked with genipin for biocompatible dental implants. Beilstein J. Nanotechnol. 2018, 9, 1735–1754. [Google Scholar] [CrossRef] [Green Version]
- Ying, G.; Jiang, N.; Yu, C.; Zhang, Y.S. Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Biodes. Manuf. 2018, 1, 215–224. [Google Scholar] [CrossRef]
- Hasan, A.; Paul, A.; Memic, A.; Khademhosseini, A. A multilayered microfluidic blood vessel-like structure. Biomed. Microdevices 2015, 17, 88. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A. Potential role of genipin in cancer therapy. Pharmacol. Res. 2018, 133, 195–200. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, M.; Wei, W.; Zhu, H.; Fan, X. Preparation of a genipin blue from egg protein and genipin. Nat. Prod. Res. 2012, 26, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xiao, Z.; Gao, L.; Zhang, J.; He, L.; Zhang, H.; Liu, Q.; Yang, G. Assessment of the effects of four crosslinking agents on gelatin hydrogel for myocardial tissue engineering applications. Biomed. Mater. 2021, 16, 045026. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Strop, P. Versatility of microbial transglutaminase. Bioconjug. Chem. 2014, 25, 855–862. [Google Scholar] [CrossRef]
- Ikeda, S.; Sekine, S.; Bessho, T.; Otsuki, H.; Sibata, S.; Nakano, M.; Sato, K. Development of Gelatin Well Device for Cell Culture. Bunseki Kagaku 2022, 71, 289–296. [Google Scholar] [CrossRef]
- Morikawa, K.; Ohta, R.; Mawatari, K.; Kitamori, T. Metal-Free Fabrication of Fused Silica Extended Nanofluidic Channel to Remove Artifacts in Chemical Analysis. Micromachines 2021, 12, 917. [Google Scholar] [CrossRef]
- Long, R.; Hall, M.S.; Wu, M.; Hui, C.Y. Effects of gel thickness on microscopic indentation measurements of gel modulus. Biophys. J. 2011, 101, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med. Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, S.J.; Lee, E.-J.; Lee, D.H.; Kim, J.Y.; Lee, S.-H. Increased poly (dimethylsiloxane) stiffness improves viability and morphology of mouse fibroblast cells. BioChip J. 2010, 4, 230–236. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Rosenwasser, M.; Buckwalter, J.; Malinin, T.; Mow, V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef]
- Seal, A.; Dalui, A.; Banerjee, M.; Mukhopadhyay, A.; Phani, K. Mechanical properties of very thin cover slip glass disk. Bull. Mater. Sci. 2001, 24, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Bingham, R.J.; Potts, J.R. Fibronectin structure: A new piece of the puzzle emerges. Structure 2010, 18, 660–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nishiguchi, A.; Yoshida, H.; Matsusaki, M.; Akashi, M. Rapid construction of three-dimensional multilayered tissues with endothelial tube networks by the cell-accumulation technique. Adv. Mater. 2011, 23, 3506–3510. [Google Scholar] [CrossRef] [PubMed]





| Cost Effectiveness | Low Cytotoxicity | Ease of Observation | |
|---|---|---|---|
| mTG | ✓ | ✓ | ✓ |
| Genipin | ✓ | - | - |
| GelMa | - | - | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, S.; Suzuki, T.; Morikawa, K.; Matsusaki, M.; Sato, K. Fabrication of a Gelatin-Based Microdevice for Vascular Cell Culture. Micromachines 2023, 14, 107. https://doi.org/10.3390/mi14010107
Sasaki S, Suzuki T, Morikawa K, Matsusaki M, Sato K. Fabrication of a Gelatin-Based Microdevice for Vascular Cell Culture. Micromachines. 2023; 14(1):107. https://doi.org/10.3390/mi14010107
Chicago/Turabian StyleSasaki, Satoko, Tomoko Suzuki, Kyojiro Morikawa, Michiya Matsusaki, and Kae Sato. 2023. "Fabrication of a Gelatin-Based Microdevice for Vascular Cell Culture" Micromachines 14, no. 1: 107. https://doi.org/10.3390/mi14010107





