Image-Based Feedback of Multi-Component Microdroplets for Ultra-Monodispersed Library Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Device Fabrication
2.1.2. Device Operation
2.1.3. Software Setup
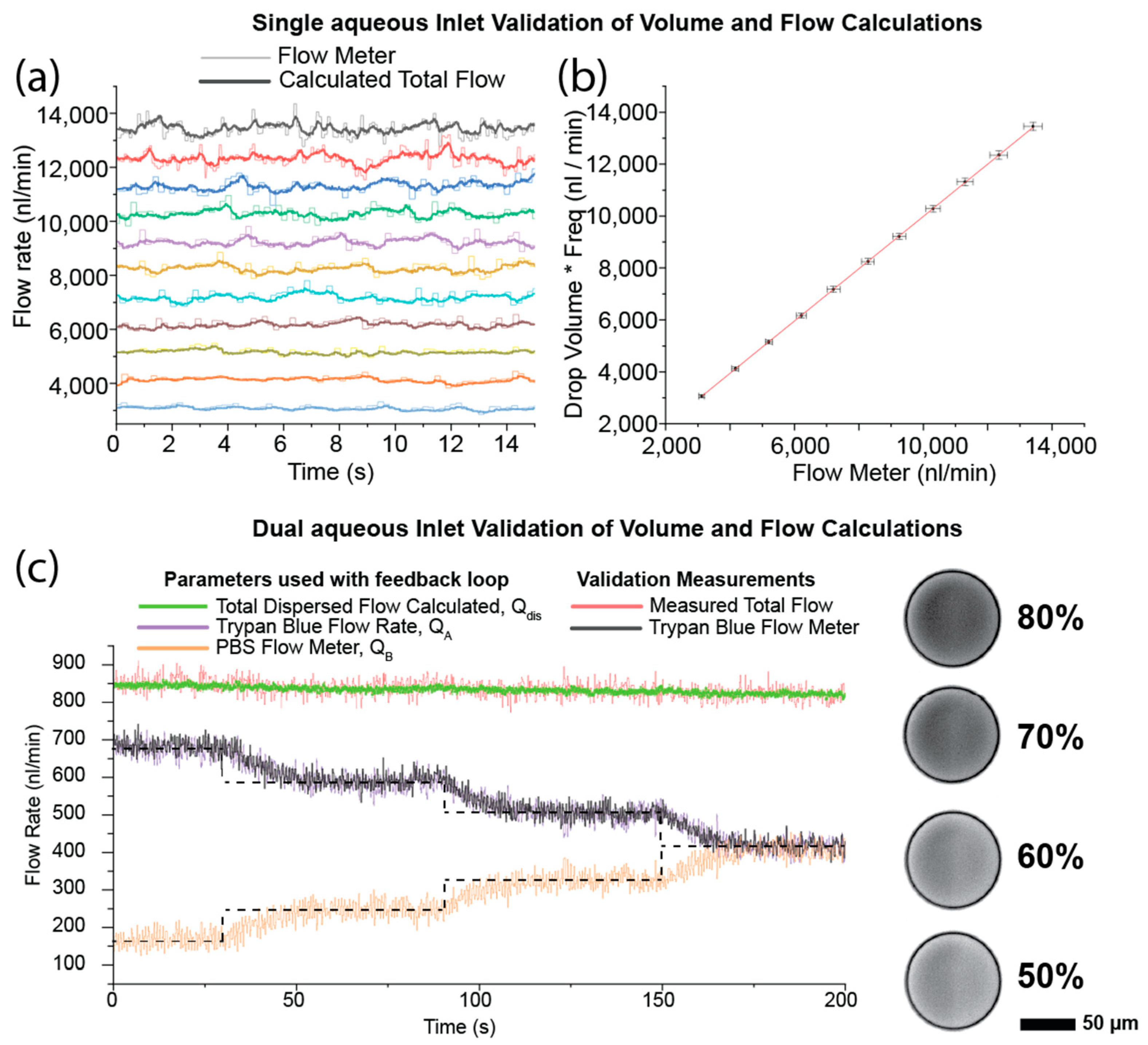
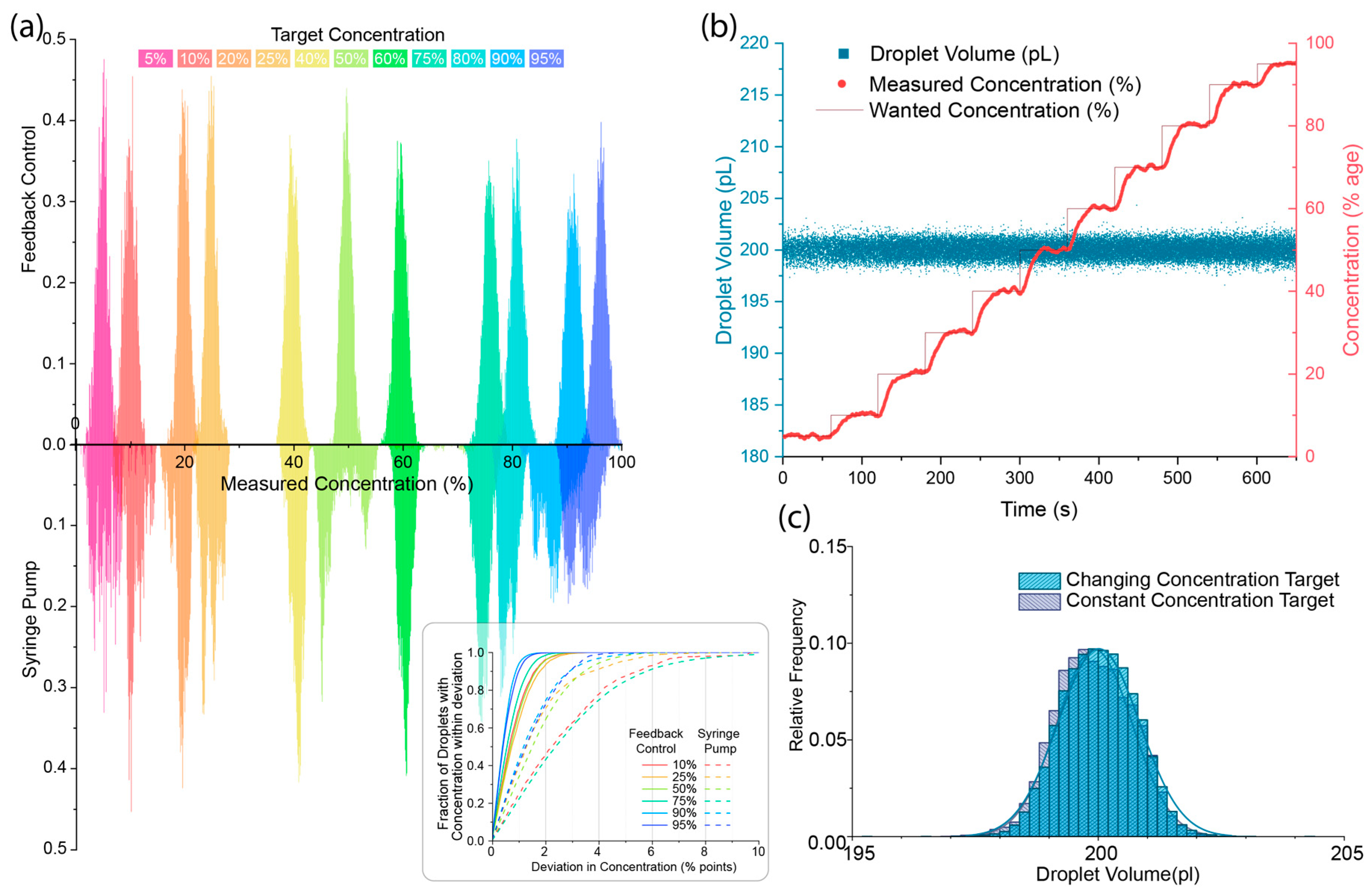
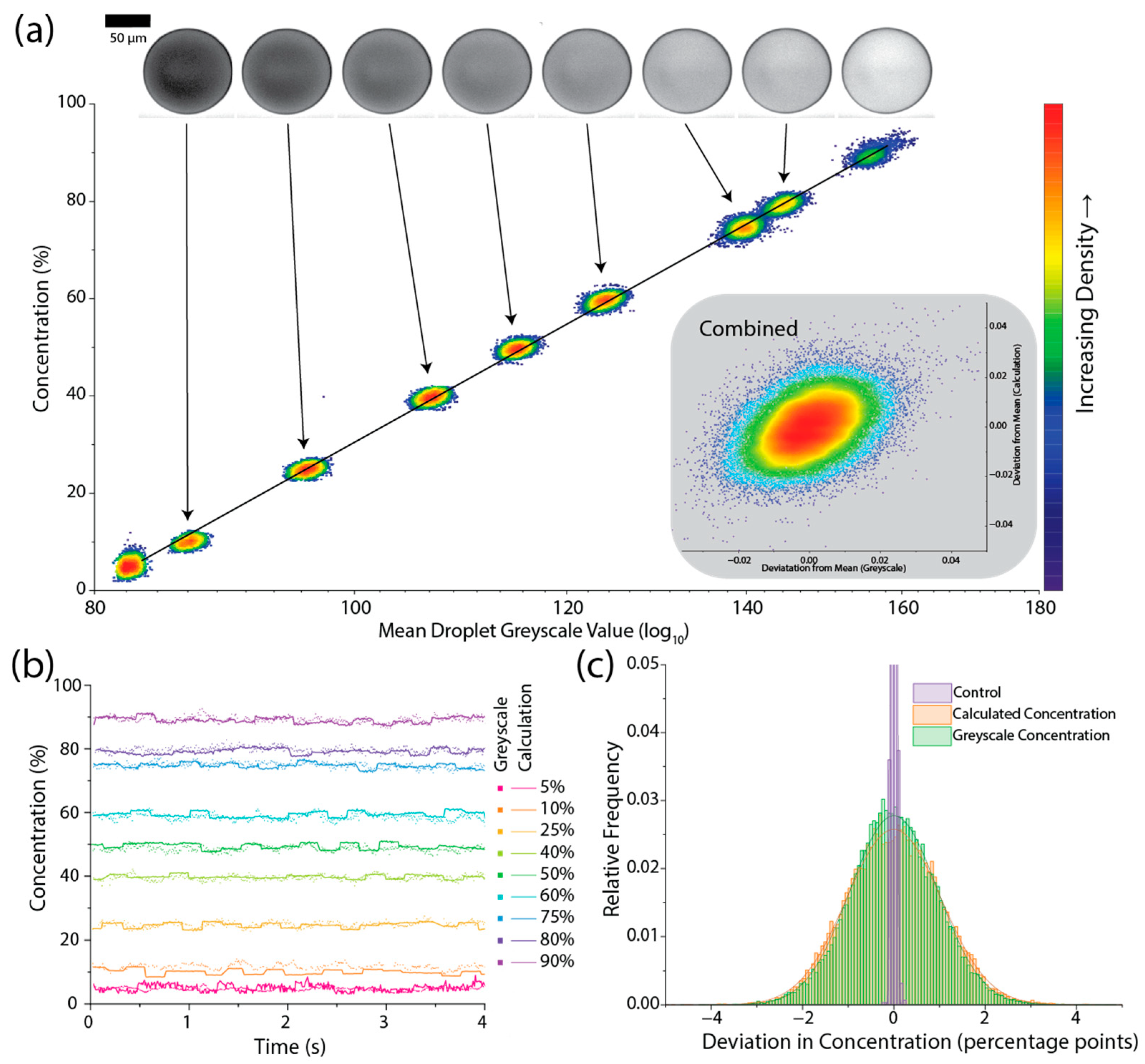
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; Demello, A.J. Microdroplets: A Sea of Applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Theberge, A.B.; Whyte, G.; Huck, W.T.S. Generation of Picoliter Droplets with Defined Contents and Concentration Gradients from the Separation of Chemical Mixtures. Anal. Chem. 2010, 82, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Courtois, F.; Olguin, L.F.; Whyte, G.; Bratton, D.; Huck, W.T.S.; Abell, C.; Hollfelder, F. An Integrated Device for Monitoring Time-Dependent in Vitro Expression from Single Genes in Picolitre Droplets. ChemBioChem 2008, 9, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Olguin, L.F.; Bratton, D.; Whyte, G.; Huck, W.T.S.; de Mello, A.J.; Edel, J.B.; Abell, C.; Hollfelder, F. Development of Quantitative Cell-Based Enzyme Assays in Microdroplets. Anal. Chem. 2008, 80, 3890–3896. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Duraiswamy, S.; Khan, S.A. Plasmonic Nanoshell Synthesis in Microfluidic Composite Foams. Nano Lett. 2010. [Google Scholar] [CrossRef]
- Lee, J.K.; Banerjee, S.; Nam, H.G.; Zare, R.N. Acceleration of Reaction in Charged Microdroplets. Q. Rev. Biophys. 2015, 48, 437–444. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Cooks, R.G.; Yan, X. Accelerated Reaction Kinetics in Microdroplets: Overview and Recent Developments. Annu. Rev. Phys. Chem. 2020, 71, 31–51. [Google Scholar] [CrossRef]
- Garstecki, P.; Stone, H.A.; Whitesides, G.M. Mechanism for Flow-Rate Controlled Breakup in Confined Geometries: A Route to Monodisperse Emulsions. Phys. Rev. Lett. 2005, 94, 164501. [Google Scholar] [CrossRef]
- Shim, J.; Ranasinghe, R.T.; Smith, C.A.; Ibrahim, S.M.; Hollfelder, F.; Huck, W.T.S.; Klenerman, D.; Abell, C. Ultrarapid Generation of Femtoliter Microfluidic Droplets for Single-Molecule-Counting Immunoassays. ACS Nano 2013, 7, 5955–5964. [Google Scholar] [CrossRef]
- Klein, A.M.; Mazutis, L.; Akartuna, I.; Tallapragada, N.; Veres, A.; Li, V.; Peshkin, L.; Weitz, D.A.; Kirschner, M.W. Droplet Barcoding for Single-Cell Transcriptomics Applied to Embryonic Stem Cells. Cell 2015, 161, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Painter, R.E.; Enesa, K.; Holmes, D.; Whyte, G.; Garlisi, C.G.; Monsma, F.J.; Rehak, M.; Craig, F.F.; Smith, C.A. High-Throughput Screening of Antibiotic-Resistant Bacteria in Picodroplets. Lab Chip 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Neild, A.; deMello, A.; Liu, A.-Q.; Ai, Y. The Poisson Distribution and beyond: Methods for Microfluidic Droplet Production and Single Cell Encapsulation. Lab Chip 2015, 15, 3439–3459. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.F.; Smith, C.A.; Whyte, G. Image-Based Closed-Loop Feedback for Highly Mono-Dispersed Microdroplet Production. Sci. Rep. 2017, 7, 10545. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yang, S.; Liu, Y.; Yang, T.; Tong, Z.; Shan, X.; Fu, H. Precise Monodisperse Droplet Generation by Pressure-Driven Microfluidic Flows. Chem. Eng. Sci. 2022, 248, 117206. [Google Scholar] [CrossRef]
- Kalantarifard, A.; Alizadeh-Haghighi, E.; Elbuken, C. A Microfluidic Droplet System for Ultra-Monodisperse Droplet Generation: A Universal Approach. Chem. Eng. Sci. 2022, 261, 117947. [Google Scholar] [CrossRef]
- Howell, L.; Anagnostidis, V.; Gielen, F. Multi-Object Detector YOLOv4-Tiny Enables High-Throughput Combinatorial and Spatially-Resolved Sorting of Cells in Microdroplets. Adv. Mater. Technol. 2022, 7, 2101053. [Google Scholar] [CrossRef]
- Anagnostidis, V.; Sherlock, B.; Metz, J.; Mair, P.; Hollfelder, F.; Gielen, F. Deep Learning Guided Image-Based Droplet Sorting for on-Demand Selection and Analysis of Single Cells and 3D Cell Cultures. Lab Chip 2020, 20, 889–900. [Google Scholar] [CrossRef]
- White, A.M.; Zhang, Y.; Shamul, J.G.; Xu, J.; Kwizera, E.A.; Jiang, B.; He, X. Deep Learning-Enabled Label-Free On-Chip Detection and Selective Extraction of Cell Aggregate-Laden Hydrogel Microcapsules. Small 2021, 17, 2100491. [Google Scholar] [CrossRef]
- Gelado, S.H.; Quilodrán-Casas, C.; Chagot, L. Enhancing Microdroplet Image Analysis with Deep Learning. Micromachines 2023, 14, 1964. [Google Scholar] [CrossRef] [PubMed]
- Sesen, M.; Whyte, G. Image-Based Single Cell Sorting Automation in Droplet Microfluidics. Sci. Rep. 2020, 10, 8736. [Google Scholar] [CrossRef]
- Gardner, K.; Uddin, M.M.; Tran, L.; Pham, T.; Vanapalli, S.; Li, W. Deep Learning Detector for High Precision Monitoring of Cell Encapsulation Statistics in Microfluidic Droplets. Lab Chip 2022, 22, 4067–4080. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.; Albert, S.; Haug, M.; Whyte, G. Dynamically Reconfigurable Fibre Optical Spanner. Lab Chip 2014, 14, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Feng, L.; Lin, T. Fluid Mixing in Droplet-Based Microfluidics with a Serpentine Microchannel. RSC Adv. 2015, 5, 104138–104144. [Google Scholar] [CrossRef]
- Musterd, M.; van Steijn, V.; Kleijn, C.R.; Kreutzer, M.T. Calculating the Volume of Elongated Bubbles and Droplets in Microchannels from a Top View Image. RSC Adv. 2015, 5, 16042–16049. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantwell, C.; McGrath, J.S.; Smith, C.A.; Whyte, G. Image-Based Feedback of Multi-Component Microdroplets for Ultra-Monodispersed Library Preparation. Micromachines 2024, 15, 27. https://doi.org/10.3390/mi15010027
Cantwell C, McGrath JS, Smith CA, Whyte G. Image-Based Feedback of Multi-Component Microdroplets for Ultra-Monodispersed Library Preparation. Micromachines. 2024; 15(1):27. https://doi.org/10.3390/mi15010027
Chicago/Turabian StyleCantwell, Christy, John S. McGrath, Clive A. Smith, and Graeme Whyte. 2024. "Image-Based Feedback of Multi-Component Microdroplets for Ultra-Monodispersed Library Preparation" Micromachines 15, no. 1: 27. https://doi.org/10.3390/mi15010027




