A Simple Non-Embedded Single Capillary Device for On-Demand Complex Emulsion Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Emulsion Preparation
2.2. Device
Emulsion and Microparticle Preparation
3. Droplet Formation Mechanism
4. Theoretical Methodology of Droplet Pinch-Off
4.1. Force Balance
4.1.1. Force Balance on the Middle Phase at Region 1: Case 2 of Pinching-Off Mechanism
4.1.2. Force Balance at Region 2: Case 1 of Pinching-Off Mechanism
5. Results
5.1. Final Morphology of Droplets
5.1.1. Complete Engulfment
5.1.2. Partial Engulfment
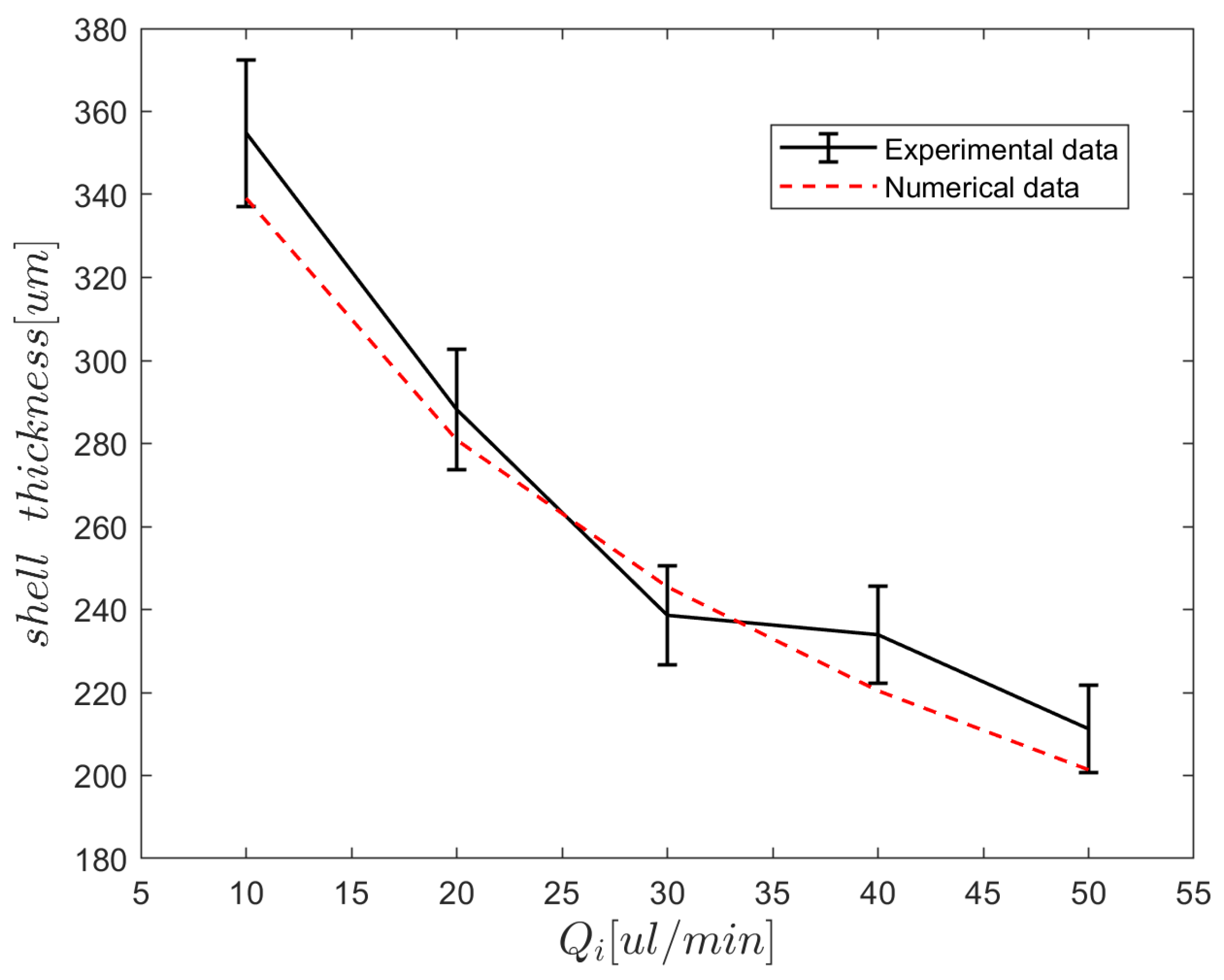
5.2. Droplet Size Prediction
5.3. On-Demand Complex Emulsions
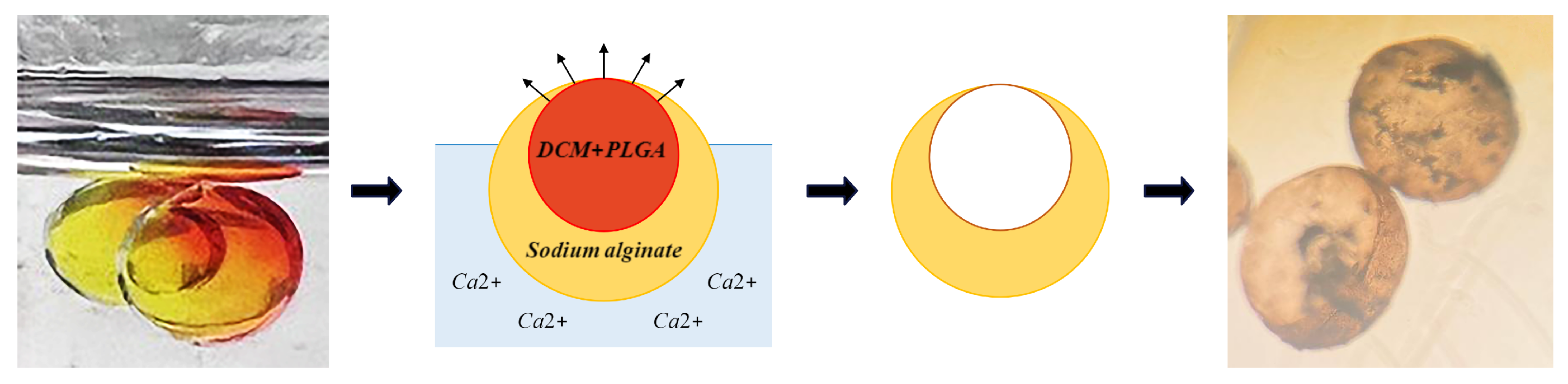
5.4. Hollow Alginate Microparticle
5.5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, S.; Serra, C.; Vandamme, T.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Zhang, M.; Su, Y.; Pan, D.; Liu, Z.; Ju, X.; Xie, R.; Faraj, Y.; Chu, L. Microfluidic emulsification techniques for controllable emulsion production and functional microparticle synthesis. Chem. Eng. J. 2023, 452. [Google Scholar] [CrossRef]
- Choi, C.; Kim, J.; Nam, J.; Kang, S.; Jeong, S.; Lee, C. Microfluidic design of complex emulsions. ChemPhysChem 2014, 15, 21–29. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H. Determination of the Dominating Coalescence Pathways in Double Emulsion Formulations by Use of Microfluidic Emulsions. Processes 2023, 11, 234. [Google Scholar] [CrossRef]
- Tenorio-Garcia, E.; Araiza-Calahorra, A.; Simone, E.; Sarkar, A. Recent advances in design and stability of double emulsions: Trends in Pickering stabilization. Food Hydrocoll. 2022, 128, 107601. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Chu, L. Microfluidic approach for encapsulation via double emulsions. Curr. Opin. Pharmacol. 2014, 18, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dewandre, A.; Rivero-Rodriguez, J.; Vitry, Y.; Sobac, B.; Scheid, B. Microfluidic droplet generation based on non-embedded co-flow-focusing using 3D printed nozzle. Sci. Rep. 2020, 10, 21616. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Um, T.; Ahn, G.; Kim, D.; Lee, H. Robust and scalable production of emulsion-templated microparticles in 3D-printed milli-fluidic device. Chem. Eng. J. 2022, 431, 133998. [Google Scholar] [CrossRef]
- Yang, G.; Tsubaki, N.; Shamoto, J.; Yoneyama, Y.; Zhang, Y. Confinement effect and synergistic function of H-ZSM-5/Cu-ZnO-Al2O3 capsule catalyst for one-step controlled synthesis. J. Am. Chem. Soc. 2010, 132, 8129–8136. [Google Scholar] [CrossRef]
- Takei, T.; Yamasaki, Y.; Yuji, Y.; Sakoguchi, S.; Ohzuno, Y.; Hayase, G.; Yoshida, M. Millimeter-sized capsules prepared using liquid marbles: Encapsulation of ingredients with high efficiency and preparation of spherical core-shell capsules with highly uniform shell thickness using centrifugal force. J. Colloid Interface Sci. 2019, 536, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Gebhardt, S.; Glaeser, R.; Liu, C. Millimeter-scale magnetic spherical metal-organic framework core-shell structured composites for recyclable catalytic applications. Microporous Mesoporous Mater. 2020, 300, 110152. [Google Scholar] [CrossRef]
- Wang, P.; Ding, M.; Zhang, T.; Wu, T.; Qiao, R.; Zhang, F.; Wang, X.; Zhong, J. Electrospraying technique and its recent application advances for biological macromolecule encapsulation of food bioactive substances. Food Rev. Int. 2022, 38, 566–588. [Google Scholar] [CrossRef]
- Panizza, P.; Engl, W.; Hany, C.; Backov, R. Controlled production of hierarchically organized large emulsions and particles using assemblies on line of co-axial flow devices. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 312, 24–31. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Shao, T.; Luo, X.; Zhang, L. Fabrication of Millimeters-Sized Poly (Divinylbenzene) Foam Shells from Controllable Double Emulsion in Microfluidic Device. Int. J. Nanosci. 2018, 17, 1750023. [Google Scholar] [CrossRef]
- Farahmand, A.; Ghorani, B.; Emadzadeh, B.; Sarabi-Jamab, M.; Emadzadeh, M.; Modiri, A.; Tucker, N. Millifluidic-assisted ionic gelation technique for encapsulation of probiotics in double-layered polysaccharide structure. Food Res. Int. 2022, 160, 111699. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Marquis, M.; Davy, J.; Renard, D. Monodisperse core-shell alginate (micro)-capsules with oil core generated from droplets millifluidic. Food Hydrocoll. 2017, 63, 447–456. [Google Scholar] [CrossRef]
- Chaurasia, A.; Sajjadi, S. Millimetric core–shell drops via buoyancy assisted non-confined microfluidics. Chem. Eng. Sci. 2015, 129, 260–270. [Google Scholar] [CrossRef]
- Shao, T.; Feng, X.; Jin, Y.; Cheng, Y. Controlled production of double emulsions in dual-coaxial capillaries device for millimeter-scale hollow polymer spheres. Chem. Eng. Sci. 2013, 104, 55–63. [Google Scholar] [CrossRef]
- Schmit, A.; Courbin, L.; Marquis, M.; Renard, D.; Panizza, P.A. pendant drop method for the production of calibrated double emulsions and emulsion gels. RSC Adv. 2014, 4, 28504–28510. [Google Scholar] [CrossRef]
- Schoeler, A.; Josephides, D.; Chaurasia, A.; Sajjadi, S.; Mesquida, P. Electrophoretic manipulation of multiple-emulsion droplets. Appl. Phys. Lett. 2014, 104, 074104. [Google Scholar] [CrossRef]
- Abbasi, M.; Song, R.; Cho, S.; Lee, J. Electro-hydrodynamics of emulsion droplets: Physical insights to applications. Micromachines 2020, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.; Zhao, Y.; Chen, Y. Shear-driven two colliding motions of binary double emulsion droplets. Int. J. Heat Mass Transf. 2018, 121, 377–389. [Google Scholar] [CrossRef]
- Kharel, S.; Gautam, A.; Dickescheid, A.; Loo, S. Hollow microparticles as a superior delivery system over solid microparticles for the encapsulation of peptides. Pharm. Res. 2018, 35, 185. [Google Scholar] [CrossRef] [PubMed]
- Poudeh, L.; Okan, B.; Zanjani, J.; Yildiz, M.; Menceloglu, Y. Design and fabrication of hollow and filled graphene-based polymeric spheres via core–shell electrospraying. RSC Adv. 2015, 5, 91147–91157. [Google Scholar] [CrossRef]
- Shao, Q.; Tang, J.; Lin, Y.; Zhang, F.; Yuan, J.; Zhang, H.; Shinya, N.; Qin, L. Synthesis and characterization of graphene hollow spheres for application in supercapacitors. J. Mater. Chem. A 2013, 1, 15423–15428. [Google Scholar] [CrossRef]
- Pestovsky, Y.; Martínez-Antonio, A. The synthesis of alginate microparticles and nanoparticles. Drug Des. Intellect. Prop. Int. J. 2019, 3, 293–327. [Google Scholar] [CrossRef]
- Lin, P.; Chen, Q.; Liu, Y.; Hu, X.; Zhu, Z. Prediction of Newtonian Droplet Breaking Time from a Capillary at Low Weber Numbers. ACS Omega 2022, 7, 23890–23898. [Google Scholar] [CrossRef]
- Wang, N.; Semprebon, C.; Liu, H.; Zhang, C.; Kusumaatmaja, H. Modelling double emulsion formation in planar flow-focusing microchannels. J. Fluid Mech. 2020, 895, A22. [Google Scholar] [CrossRef]
- Vladisavljević, G. Recent advances in the production of controllable multiple emulsions using microfabricated devices. Particuology 2016, 24, 1–17. [Google Scholar] [CrossRef]








| Emulsion | Criteria | Sample |
|---|---|---|
| W/O/O Water/Silicon oil/Sesame oil | 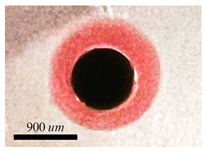 | |
| W/O/W Water/HFE/PVA 1 wt% | 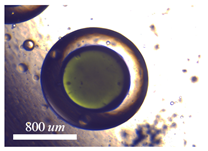 | |
| O/W/O Sesame oil/Water/Silicone oil | & | 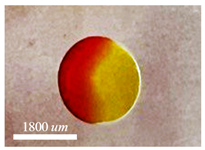 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim Khani, M.M.; Oveysi, M.; Bazargan, V.; Marengo, M. A Simple Non-Embedded Single Capillary Device for On-Demand Complex Emulsion Formation. Micromachines 2024, 15, 239. https://doi.org/10.3390/mi15020239
Karim Khani MM, Oveysi M, Bazargan V, Marengo M. A Simple Non-Embedded Single Capillary Device for On-Demand Complex Emulsion Formation. Micromachines. 2024; 15(2):239. https://doi.org/10.3390/mi15020239
Chicago/Turabian StyleKarim Khani, Mohammad Mahdi, Mehrnaz Oveysi, Vahid Bazargan, and Marco Marengo. 2024. "A Simple Non-Embedded Single Capillary Device for On-Demand Complex Emulsion Formation" Micromachines 15, no. 2: 239. https://doi.org/10.3390/mi15020239





