Acoustic Actuators for the Manipulation of Micro/Nanorobots: State-of-the-Art and Future Outlooks
Abstract
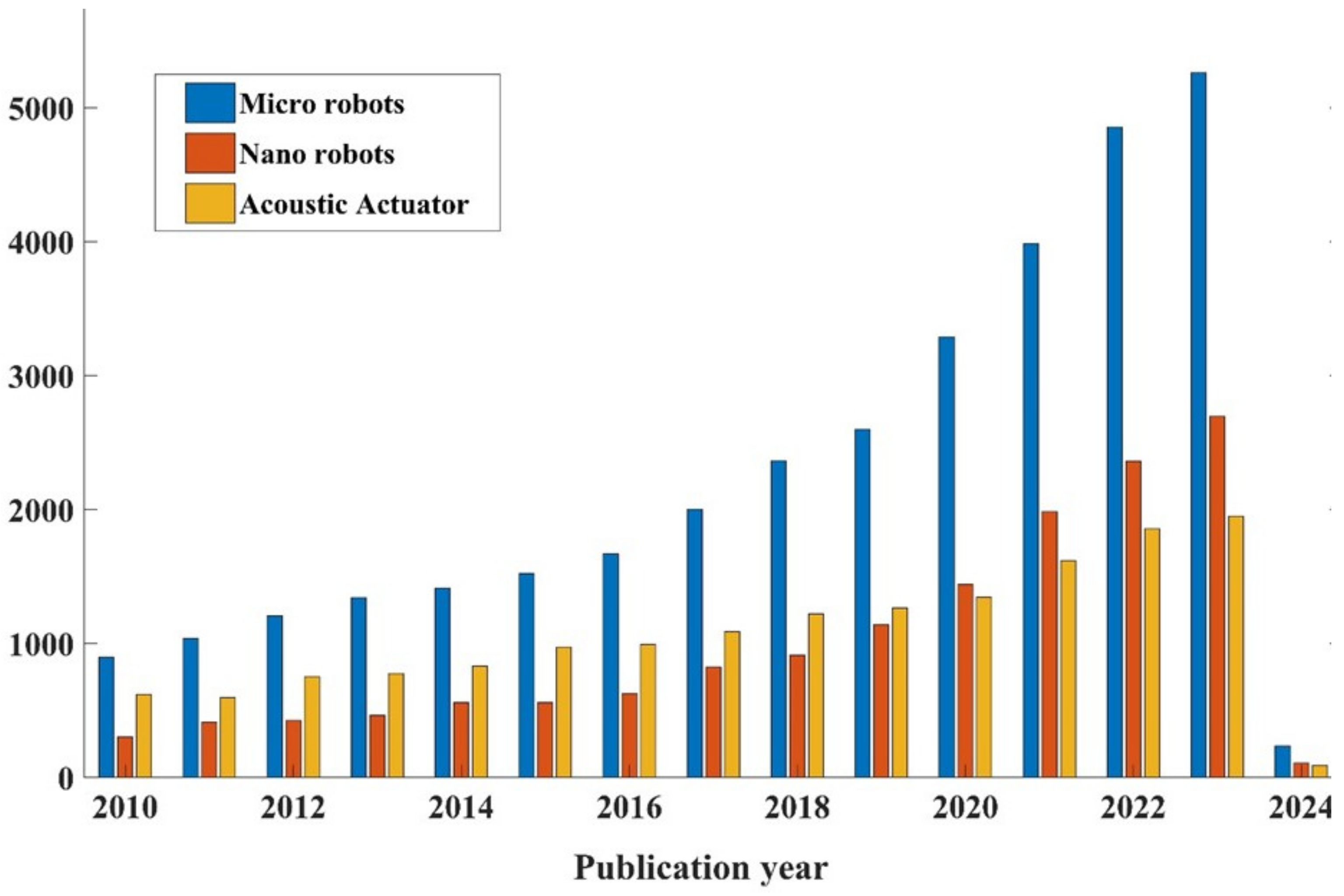
1. Introduction
2. Principle of Acoustic Manipulation
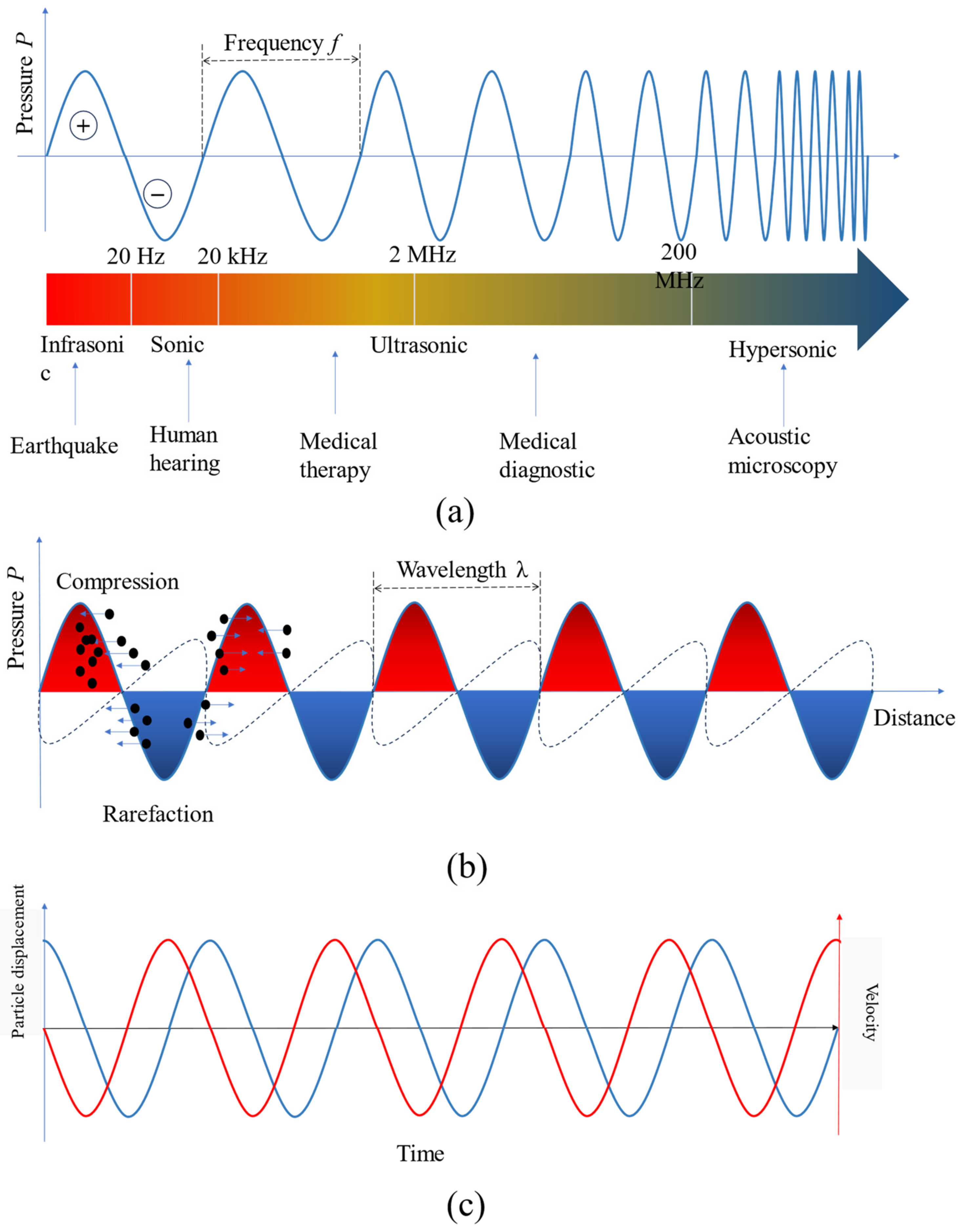
2.1. Acoustic Fundamentals
- Infrasonic: These are acoustic waves with frequencies less than 20 Hz. They are typically used in applications such as seismic monitoring and for studying low-frequency sound phenomena.
- Sonic: This category includes acoustic waves with frequencies ranging from 20 Hz to 20 kHz, which is the range of human hearing. Sonic waves are widely used in various fields, including music, communication, and environmental noise analysis.
- Ultrasonic: Acoustic waves in the ultrasonic range have frequencies from 20 kHz up to 200 MHz. They find extensive use in medical applications, such as ultrasound imaging, non-destructive testing, and cleaning processes.
- Hypersonic: Acoustic waves with frequencies higher than 200 MHz fall into the hypersonic category. These waves are primarily used in acoustic microscopy, which enables high-resolution imaging of small-scale structures.
2.2. Acoustic Radiation Force
2.3. Acoustically Actuated Micro/Nanorobot Strategy
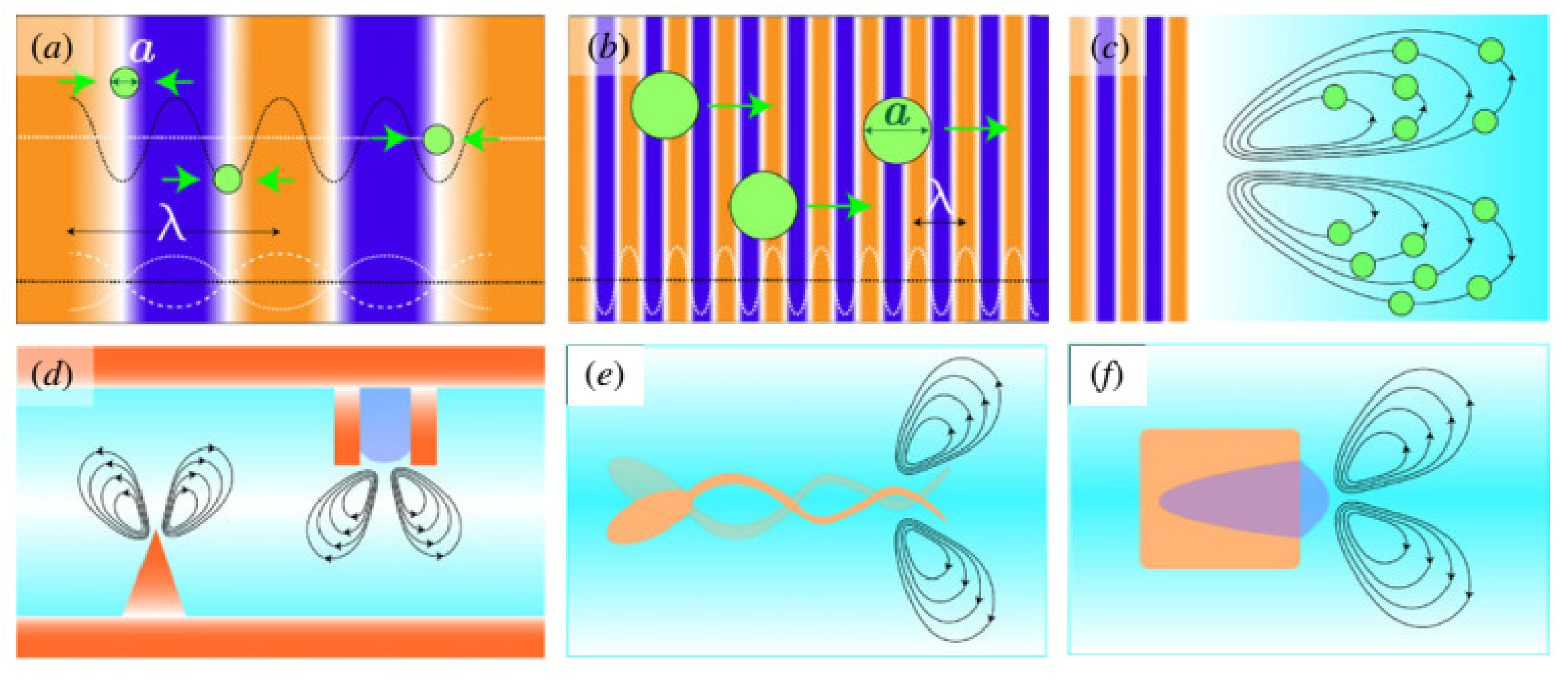
2.3.1. Acoustic Tweezer Propulsion
- Standing-wave tweezers
- Traveling-wave tweezers
2.3.2. Streaming-Driven Acoustic
3. Acoustic Manipulation of Micro/Nanorobots
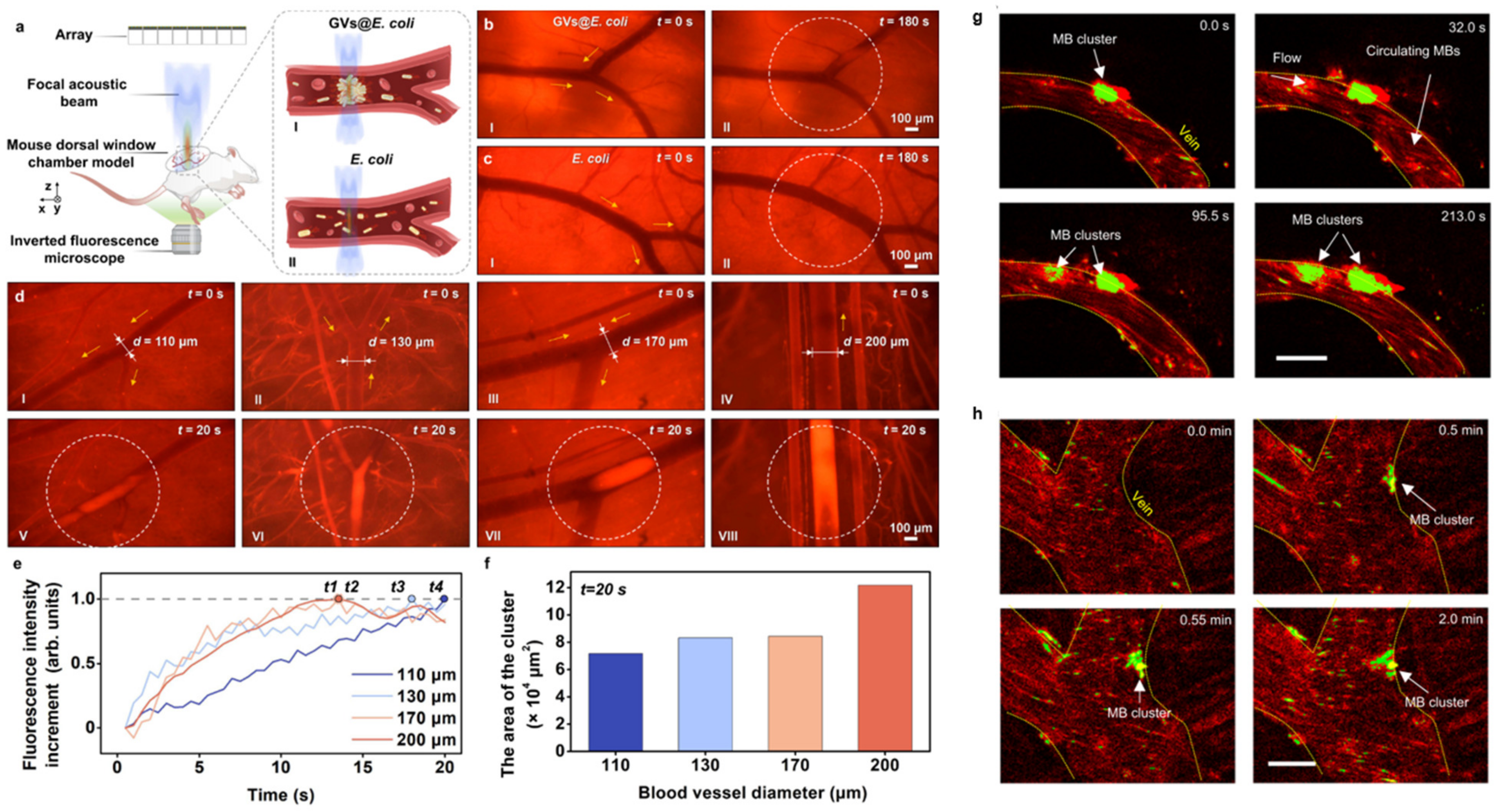
3.1. Bubble-Based Microrobots
3.2. Bubble-Free Microrobots
3.3. Biohybrid Microrobots
3.4. Nanorobots
4. Challenges and Future Outlooks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Huang, H.; Stratton, Z.; Huang, Y.; Huang, T.J. Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, M.; Brismar, H.; Önfelt, B. Acoustofluidics 18: Microscopy for acoustofluidic micro-devices. Lab Chip 2012, 12, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Trimmer, W.S.N. Microrobots and micromechanical systems. Sens. Actuators 1989, 19, 267–287. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, C.; Dong, L.; Zhao, J. A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo. Micromachines 2021, 12, 1249. [Google Scholar] [CrossRef]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Mi-crobubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.H.; Wu, B.; Das, S. Multistimuli-responsive microrobots: A comprehensive review. Front. Robot. AI 2022, 9, 1027415. [Google Scholar] [CrossRef]
- Kong, L.; Guan, J.; Pumera, M. Micro- and nanorobots based sensing and biosensing. Curr. Opin. Electrochem. 2018, 10, 174–182. [Google Scholar] [CrossRef]
- Lu, X.; Bao, J.; Wei, Y.; Zhang, S.; Liu, W.; Wu, J. Emerging Roles of Microrobots for Enhancing the Sensitivity of Biosensors. Nanomaterials 2023, 13, 2902. [Google Scholar] [CrossRef]
- Soto, F.; Karshalev, E.; Zhang, F.; de Avila, B.E.F.; Nourhani, A.; Wang, J. Smart Materials for Microrobots. Chem. Rev. 2022, 122, 5365–5403. [Google Scholar] [CrossRef] [PubMed]
- Gotovtsev, P. Microbial Cells as a Microrobots: From Drug Delivery to Advanced Biosensors. Biomimetics 2023, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Hoang, M.C.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Medical Microrobot—A Drug Delivery Capsule Endoscope with Active Locomotion and Drug Release Mechanism: Proof of Concept. Int. J. Control Autom. Syst. 2020, 18, 65–75. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Go, G.; Zhen, J.; Hoang, M.C.; Kang, B.; Choi, E.; Park, J.-O.; Kim, C.-S. Locomotion and disaggregation control of paramagnetic nanoclusters using wireless electromagnetic fields for enhanced targeted drug delivery. Sci. Rep. 2021, 11, 15122. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Go, G.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Medical Microrobot—Wireless Manipulation of a Drug Delivery Carrier through an External Ultrasonic Actuation: Preliminary Results. Int. J. Control Autom. Syst. 2020, 18, 175–185. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Le, V.H.; Kim, C.S.; Han, J.; Park, J.O.; Choi, E. A Novel Macrophage-Based Microrobot Bearing Multiple Smart Nanotherapeutics for Targeting and Drug Delivery to Solid Tumors. In Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, Seoul, Republic of Korea, 21–24 August 2018; pp. 55–60. [Google Scholar] [CrossRef]
- Cao, H.X.; Jung, D.; Lee, H.-S.; Du Nguyen, V.; Choi, E.; Kang, B.; Park, J.-O.; Kim, C.-S. Holographic Acoustic Tweezers for 5-DoF Manipulation of Nanocarrier Clusters toward Targeted Drug Delivery. Pharmaceutics 2022, 14, 1490. [Google Scholar] [CrossRef] [PubMed]
- Sundvik, M.; Nieminen, H.J.; Salmi, A.; Panula, P.; Hæggström, E. Effects of acoustic levitation on the development of zebrafish, Danio rerio, embryos. Sci. Rep. 2015, 5, 13596. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.; Baggio, A.L. Designing single-beam multitrapping acoustical tweezers. Ultrasonics 2015, 56, 449–455. [Google Scholar] [CrossRef]
- Baudoin, M.; Thomas, J.-L.; Al Sahely, R.; Gerbedoen, J.-C.; Gong, Z.; Sivery, A.; Matar, O.B.; Smagin, N.; Favreau, P.; Vlandas, A. Spatially selective manipulation of cells with single-beam acoustical tweezers. Nat. Commun. 2020, 11, 4244. [Google Scholar] [CrossRef]
- Ozcelik, A.; Rufo, J.; Guo, F.; Gu, Y.; Li, P.; Lata, J.; Huang, T.J. Acoustic tweezers for the life sciences. Nat. Methods 2018, 15, 1021–1028. [Google Scholar] [CrossRef]
- Li, J.; Crivoi, A.; Peng, X.; Shen, L.; Pu, Y.; Fan, Z.; Cummer, S.A. Three dimensional acoustic tweezers with vortex streaming. Commun. Phys. 2021, 4, 113. [Google Scholar] [CrossRef]
- Tran, S.B.Q.; Marmottant, P.; Thibault, P. Fast acoustic tweezers for the two-dimensional manipulation of individual particles in microfluidic channels. Appl. Phys. Lett. 2012, 101, 114103. [Google Scholar] [CrossRef]
- Wang, T.; Yin, Q.; Huang, H.Y.; Wang, Z.; Song, H.; Luo, X. Probiotic Escherichia coli Nissle 1917 propelled micro-robot with pH sensitivity for hypoxia targeted intestinal tumor therapy. Colloids Surf. B Biointerfaces 2023, 225, 113277. [Google Scholar] [CrossRef] [PubMed]
- Villa, K.; Viktorova, J.; Plutnar, J.; Ruml, T.; Hoang, L.; Pumera, M. Chemical Microrobots as Self-Propelled Microbrushes against Dental Biofilm. Cell Rep. Phys. Sci. 2020, 1, 100181. [Google Scholar] [CrossRef]
- Qiao, S.; Ouyang, H.; Zheng, X.; Qi, C.; Ma, L. Magnetically actuated hydrogel-based capsule microrobots for intravascular targeted drug delivery. J. Mater. Chem. B 2023, 11, 6095–6105. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, H.; Zhang, J.; Xu, J.; Song, Z.; Zhan, G.; Bai, X.; Feng, L. Cell-Based Micro/Nano-Robots for Biomedical Applications: A Review. Small 2023, 20, 2304607. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Park, J.-O.; Choi, E. Macrophage-Based Microrobots for Anticancer Therapy: Recent Progress and Future Perspectives. Biomimetics 2023, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Bogue, R. Miniature and microrobots: A review of recent developments. Ind. Robot. Int. J. 2015, 42, 98–102. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, Z.; Ferreira, A.; Zhang, L. Control and Autonomy of Microrobots: Recent Progress and Perspective. Adv. Intell. Syst. 2022, 4, 2100279. [Google Scholar] [CrossRef]
- Kim, H.; Ali, J.; Cheang, U.K.; Jeong, J.; Kim, J.S.; Kim, M.J. Micro Manipulation Using Magnetic Microrobots. J. Bionic. Eng. 2016, 13, 515–524. [Google Scholar] [CrossRef]
- Bunea, A.; Martella, D.; Nocentini, S.; Parmeggiani, C.; Taboryski, R.; Wiersma, D.S. Light-Powered Microrobots: Challenges and Opportunities for Hard and Soft Responsive Microswimmers. Adv. Intell. Syst. 2021, 3, 2000256. [Google Scholar] [CrossRef]
- Sitti, M.; Wiersma, D.S.; Sitti, M.; Wiersma, D.S. Pros and Cons: Magnetic versus Optical Microrobots. Adv. Mater. 2020, 32, 1906766. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, S.; Mair, L.; Ahmed, S.; Huang, T.J.; Mallouk, T.E. Acoustic Propulsion of Nanorod Motors Inside Living Cells. Angew. Chem. Int. Ed. Engl. 2014, 53, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Moemer, W.E. Method for trapping and manipulating nanoscale objects in solution. Appl. Phys. Lett. 2005, 86, 093109. [Google Scholar] [CrossRef]
- Mohanty, S.; Khalil, I.S.M.; Misra, S. Contactless acoustic micro/nano manipulation: A paradigm for next generation applications in life sciences. Proc. R. Soc. A 2020, 476, 20200621. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, T.; Beer, D.; Küller, J.; Fischer, G.; Zhykhar, A.; Fiedler, M. MEMS Acoustical Actuators: Principles, Challenges and Perspectives. Mech. Mach. Sci. 2021, 96, 125–136. [Google Scholar] [CrossRef]
- Wu, J. Acoustical tweezers. J. Acoust. Soc. Am. 1991, 89, 2140–2143. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani, A.; Yasa, O.; Wrede, P.; Sitti, M. Acoustically powered surface-slipping mobile micro-robots. Proc. Natl. Acad. Sci. USA 2020, 117, 3469–3477. [Google Scholar] [CrossRef]
- Rufo, J.; Zhang, P.; Zhong, R.; Lee, L.P.; Huang, T.J. A sound approach to advancing healthcare systems: The future of biomedical acoustics. Nat. Commun. 2022, 13, 3459. [Google Scholar] [CrossRef]
- Andrade, M.A.B.; Pérez, N.; Adamowski, J.C. Review of Progress in Acoustic Levitation. Braz. J. Phys. 2017, 48, 190–213. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of Ultrasound in Medicine. Med. Rev.|AIM 2011, 19, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kinsler, L.E.; Frey, A.R.; Coppens, A.B.; Sanders, J.V. Fundamentals of Acoustics, 4th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1999; Volume 560, Available online: https://www.wiley.com/en-us/Fundamentals+of+Acoustics%2C+4th+Edition-p-9780471847892 (accessed on 19 November 2023).
- Ing, L.V.K. On the acoustic radiation pressure on spheres. Proc. R. Soc. Lond. A Math. Phys. Sci. 1934, 147, 212–240. [Google Scholar] [CrossRef]
- Gor’kov, L.P. On the Forces Acting on a Small Particle in an Acoustical Field in an Ideal Fluid. SPhD 1962, 6, 773. Available online: https://ui.adsabs.harvard.edu/abs/1962SPhD....6..773G/abstract (accessed on 30 October 2022).
- Bruus, H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012, 12, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.B.; Barnkob, R.; Jensen, M.J.H.; Bruus, H. A numerical study of microparticle acoustophoresis driven by acoustic radiation forces and streaming-induced drag forces. Lab Chip 2012, 12, 4617–4627. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, B.W. Dynamic-field devices for the ultrasonic manipulation of microparticles. Lab Chip 2016, 16, 2360–2375. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, C.; Mo, R.; Hu, J.; Li, S. Interaction between particles and bubbles driven by ultrasound: Acoustic radiation force on an elastic particle immersed in the ideal fluid near a bubble. Ultrason. Sonochem. 2020, 67, 105166. [Google Scholar] [CrossRef]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating bubbles: A versatile tool for lab on a chip applications. Lab Chip 2012, 12, 4216–4227. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Fu, C.; Ren, X.; Xu, Z.; Liu, X. Precise micro-particle and bubble manipulation by tunable ultrasonic bottle beams. Ultrason. Sonochem. 2021, 75, 105602. [Google Scholar] [CrossRef]
- Sadhal, S.S. Acoustofluidics 16: Acoustics streaming near liquid-gas interfaces: Drops and bubbles. Lab Chip 2012, 12, 2771–2781. [Google Scholar] [CrossRef]
- Dijkink, R.J.; Van Der Dennen, J.P.; Ohl, C.D.; Prosperetti, A. The ‘acoustic scallop’: A bubble-powered actuator. J. Micromech. Microeng. 2006, 16, 1653. [Google Scholar] [CrossRef]
- Guo, F.; Mao, Z.; Chen, Y.; Xie, Z.; Lata, J.P.; Li, P.; Ren, L.; Liu, J.; Yang, J.; Dao, M.; et al. Three-dimensional manipulation of single cells using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2016, 113, 1522–1527. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Chen, C.; Hartman, J.H.; Huang, P.H.; Wang, L.; Tian, Z.; Zhang, P.; Faulkenberry, D.; Meyer, J.N.; et al. Surface acoustic waves enable rotational manipulation of Caenorhabditis elegans. Lab Chip 2019, 19, 984–992. [Google Scholar] [CrossRef]
- Gedge, M.; Hill, M. Acoustofluidics 17: Theory and applications of surface acoustic wave devices for particle manipulation. Lab Chip 2012, 12, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, S.-C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.-K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Cho, H.; Ha, B.H.; Jung, J.H.; Park, J.; Sung, H.J. Acoustofluidic particle manipulation inside a sessile droplet: Four distinct regimes of particle concentration. Lab Chip 2016, 16, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.S.; Bruus, H. Bulk-driven acoustic streaming at resonance in closed microcavities. Phys. Rev. E 2019, 100, 023104. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.Y.; Yin, J.F.; Zhai, W.; Yan, N.; Wang, W.L.; Zhang, J.; Drinkwater, B.W. Dynamics of levitated objects in acoustic vortex fields. Sci. Rep. 2017, 7, 7093. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.; Seah, S.A.; Drinkwater, B.W.; Sahoo, D.R.; Long, B.; Subramanian, S. Holographic acoustic elements for manipulation of levitated objects. Nat. Commun. 2015, 6, 8661. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.X.; Jung, D.; Lee, H.-S.; Go, G.; Nan, M.; Choi, E.; Kim, C.-S.; Park, J.-O.; Kang, B. Micromotor manipulation using ultrasonic active traveling waves. Micromachines 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Phys, J.A. Focused acoustic vortex generated by a circular array of planar sector transducers using an acoustic lens, and its application in object manipulation Focused acoustic vortex generated by a circular array of planar sector transducers using an acoustic len. J. Appl. Phys. 2020, 128, 084901. [Google Scholar] [CrossRef]
- Melde, K.; Mark, A.G.; Qiu, T.; Fischer, P. Holograms for acoustics. Nature 2016, 537, 518–522. [Google Scholar] [CrossRef]
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7, 11085. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ou, H.; Wei, Y.; Ding, X.; Wang, X.; Zhao, C.; Bao, J.; Liu, W. Superfast fuel-free tubular hydrophobic micromotors powered by ultrasound. Sens. Actuators B Chem. 2022, 372, 132667. [Google Scholar] [CrossRef]
- Huang, P.-H.; Nama, N.; Mao, Z.; Li, P.; Rufo, J.; Chen, Y.; Xie, Y.; Wei, C.-H.; Wang, L.; Huang, T.J. A reliable and programmable acoustofluidic pump powered by oscillating sharp-edge structures. Lab Chip 2014, 14, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Marmottant, P.; Hilgenfeldt, S. A bubble-driven microfluidic transport element for bioengineering. Proc. Natl. Acad. Sci. USA 2004, 101, 9523–9527. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani, A.; Pena-Francesch, A.; Bozuyuk, U.; Cetin, H.; Wrede, P.; Sitti, M. High shear rate propulsion of acoustic microrobots in complex biological fluids. Sci. Adv. 2022, 8, 5126. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Lu, M.; Nourhani, A.; Lammert, P.E.; Stratton, Z.; Muddana, H.S.; Crespi, V.H.; Huang, T.J. Selectively manipulable acoustic-powered microswimmers. Sci. Rep. 2015, 5, 9744. [Google Scholar] [CrossRef] [PubMed]
- Kaynak, M.; Ozcelik, A.; Nourhani, A.; Lammert, P.E.; Crespi, V.H.; Huang, T.J. Acoustic actuation of bioinspired microswimmers. Lab Chip 2017, 17, 395–400. [Google Scholar] [CrossRef]
- Ahmed, D.; Baasch, T.; Jang, B.; Pane, S.; Dual, J.; Nelson, B.J. Artificial Swimmers Propelled by Acoustically Activated Flagella. Nano Lett. 2016, 16, 4968–4974. [Google Scholar] [CrossRef]
- Li, C.; Qin, B.; Gopinath, A.; Arratia, P.E.; Thomases, B.; Guy, R.D. Flagellar swimming in viscoelastic fluids: Role of fluid elastic stress revealed by simulations based on experimental data. J. R. Soc. Interface 2017, 14, 20170289. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dejous, C.; Hallil, H. Trends and Applications of Surface and Bulk Acoustic Wave Devices: A Review. Micromachines 2022, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Baudoin, M. Single Beam Acoustical Tweezers Based on Focused Beams: A Numerical Analysis of 2D and 3D Trapping Capabilities. Available online: http://films-lab.univ-lille1.fr/michael (accessed on 29 May 2022).
- Thomas, J.-L.; Baresch, D.; Marchiano, R. Single-beam acoustical tweezers. Complex. Light. Opt. Forces XI 2017, 10120, 101200Y. [Google Scholar] [CrossRef]
- Jia, Y.R.; Wu, D.J.; Yao, J.; Wei, Q.; Xu, Z.; Liu, X.J. Acoustic tweezing for both Rayleigh and Mie particles based on acoustic focused petal beams. Appl. Phys. Lett. 2020, 116, 263504. [Google Scholar] [CrossRef]
- Rufo, J.; Cai, F.; Friend, J.; Wiklund, M.; Huang, T.J. Acoustofluidics for biomedical applications. Nat. Rev. Methods Primers 2022, 2, 30. [Google Scholar] [CrossRef]
- Simon, G.; Busch, C.; Andrade, M.A.B.; Reboud, J.; Cooper, J.M.; Desmulliez, M.P.Y.; Riehle, M.O.; Bernassau, A.L. Bandpass sorting of heterogeneous cells using a single surface acoustic wave transducer pair. Biomicrofluidics 2021, 15, 14105. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Hammarström, B.; Wiklund, M. Ultrasonic Based Tissue Modelling and Engineering. Micromachines 2018, 9, 594. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, J.; Tian, J.; Wei, J.; Hu, Y. Surface-Acoustic-Wave (SAW)-Driven Device for Three-Dimensional Cell Manipulation. IEEE Trans. Biomed. Eng. 2023, 70, 780–788. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, M.; Wang, J.; Wen, B.; Lu, L.; Ren, H. Acoustofluidics for cell patterning and tissue engineering. Eng. Regen. 2022, 3, 397–406. [Google Scholar] [CrossRef]
- Démoré, C.E.M.; Dahl, P.M.; Yang, Z.; Glynne-Jones, P.; Melzer, A.; Cochran, S.; MacDonald, M.P.; Spalding, G.C. Acoustic Tractor Beam. Phys. Rev. Lett. 2014, 112, 174302. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.A.; Maxwell, A.D.; Dalecki, D.; Sapozhnikov, O.A.; Bailey, M.R. Phase holograms for the three-dimensional patterning of unconstrained microparticles. Sci. Rep. 2023, 13, 9160. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Ochiai, Y.; Rekimoto, J. Three-dimensional noncontact manipulation by opposite ultrasonic phased arrays. Jpn. J. Appl. Phys. 2014, 53, 07KE07. [Google Scholar] [CrossRef]
- Franklin, A.; Marzo, A.; Malkin, R.; Drinkwater, B.W. Three-dimensional ultrasonic trapping of micro-particles in water with a simple and compact two-element transducer. Appl. Phys. Lett. 2017, 111, 94101. [Google Scholar] [CrossRef]
- Prisbrey, M.; Raeymaekers, B. Ultrasound Noncontact Particle Manipulation of Three-dimensional Dynamic User-specified Patterns of Particles in Air. Phys. Rev. Appl. 2018, 10, 034066. [Google Scholar] [CrossRef]
- Marzo, A.; Drinkwater, B.W. Holographic acoustic tweezers. Proc. Natl. Acad. Sci. USA 2019, 116, 84–89. [Google Scholar] [CrossRef]
- Fan, X.D.; Zhang, L. Trapping Force of Acoustical Bessel Beams on a Sphere and Stable Tractor Beams. Phys. Rev. Appl. 2019, 11, 014055. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, T.; Li, S.; Zhang, Q.; Huang, J.; Liu, Y.; Zhuang, J.; Li, Y.; Du, X.; Niu, L.; et al. Self-Navigated 3D Acoustic Tweezers in Complex Media Based on Time Reversal. Research 2021, 2021, 9781394. [Google Scholar] [CrossRef]
- Memoli, G.; Caleap, M.; Asakawa, M.; Sahoo, D.R.; Drinkwater, B.W.; Subramanian, S. Metamaterial bricks and quantization of meta-surfaces. Nat. Commun. 2017, 8, 14608. Available online: https://www.nature.com/articles/ncomms14608 (accessed on 20 November 2023). [CrossRef]
- Cao, H.X.; Jung, D.; Lee, H.-S.; Du Nguyen, V.; Choi, E.; Kim, C.-S.; Park, J.-O.; Kang, B. Fabrication, Acoustic Characterization and Phase Reference-Based Calibration Method for a Single-Sided Multi-Channel Ultrasonic Actuator. Micromachines 2022, 13, 2182. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Maxwell, A.D.; Wang, Y.-N.; Cunitz, B.W.; Khokhlova, V.A.; Sapozhnikov, O.A.; Bailey, M.R. Noninvasive acoustic manipulation of objects in a living body. Proc. Natl. Acad. Sci. USA 2020, 117, 02001779. [Google Scholar] [CrossRef]
- Ovchinnikov, M.; Zhou, J.; Yalamanchili, S. Acoustic streaming of a sharp edge. J. Acoust. Soc. Am. 2014, 136, 22–29. [Google Scholar] [CrossRef]
- Lo, W.-C.; Fan, C.-H.; Ho, Y.-J.; Lin, C.-W.; Yeh, C.-K. Tornado-inspired acoustic vortex tweezer for trapping and manipulating microbubbles. Proc. Natl. Acad. Sci. USA 2021, 118, e2023188118. [Google Scholar] [CrossRef]
- Mohanty, S.; de Cumis, U.S.; Solsona, M.; Misra, S. Bi-directional transportation of micro-agents induced by symmetry-broken acoustic streaming. AIP Adv. 2019, 9, 035352. [Google Scholar] [CrossRef]
- Ohlin, M. Ultrasonic Fluid and Cell Manipulation; Engineering Sciences, KTH Royal Institute of Technology: Stockholm, Sweden, 2015. [Google Scholar]
- Armstrong, J.P.K.; Puetzer, J.L.; Serio, A.; Guex, A.G.; Kapnisi, M.; Breant, A.; Zong, Y.; Assal, V.; Skaalure, S.C.; King, O.; et al. Engineering Anisotropic Muscle Tissue using Acoustic Cell Patterning. Adv. Mater. 2018, 30, 1802649. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, M. Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators. Lab Chip 2012, 12, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Pang, W.; Yang, Y.; Li, T.; Duan, X. Theoretical and experimental characterizations of gigahertz acoustic streaming in microscale fluids. Nanotechnol. Precis. Eng. 2019, 2, 15–22. [Google Scholar] [CrossRef]
- Wu, M.; Ozcelik, A.; Rufo, J.; Wang, Z.; Fang, R.; Huang, T.J. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, H.; Deng, W.; Huang, X.; Li, F.; Lu, J.; Liu, Z. Acoustic tweezers and motor for living cells. Appl. Phys. Lett. 2020, 116, 123503. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.-W.; Yu, P.-P.; Zhang, S.-H.; Zhou, J.-H.; Li, Y.-M.; Gong, L. Trapping and Manipulation of Single Cells in Crowded Environments. Front. Bioeng. Biotechnol. 2020, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.; Sturgis, J.; Ragheb, K.; Sherman, D.; Burkholder, K.; Robinson, J.P.; Bhunia, A.K.; Mohammed, S.; Bashir, R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007, 2, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, T.; Zhang, Q.; Huang, J.; Hu, Q.; Li, Y.; Wang, C.; Zheng, H. 3D Acoustic Manipulation of Living Cells and Organisms Based on 2D Array. IEEE Trans. Biomed. Eng. 2022, 69, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lu, I.-L.; Chiang, W.-H.; Lin, Y.-W.; Tsai, Y.-C.; Chen, H.-H.; Chang, C.-W.; Chiang, C.-S.; Chiu, H.-C. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J. Control. Release 2017, 254, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bezer, J.H. Ultrasound-Driven Microbubble Dynamics in Microvessels. Ph.D. Thesis, Imperial College London, London, UK, 2022. [Google Scholar]
- Wu, H.; Tang, Z.; You, R.; Pan, S.; Liu, W.; Zhang, H.; Li, T.; Yang, Y.; Sun, C.; Pang, W.; et al. Manipulations of micro/nanoparticles using gigahertz acoustic streaming tweezers. Nano-Technol. Precis. Eng. 2022, 5, 23001. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, H.; Wang, L.; Jin, J.; Zhao, C. A novel mode-switching-based piezoelectric acoustic tweezer for transporting, positioning, and sorting ICF microspheres. Sens. Actuators A Phys. 2023, 360, 114537. [Google Scholar] [CrossRef]
- Hu, Q.; Ma, T.; Zhang, Q.; Wang, J.; Yang, Y.; Cai, F.; Zheng, H. 3-D Acoustic Tweezers Using a 2-D Matrix Array with Time-Multiplexed Traps. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3646–3653. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, M.; Thomas, J.-L.; Thomas, J.-L. Acoustic Tweezers for Particle and Fluid Micromanipulation. An-nual Review of Fluid Mechanics. Annu. Rev. 2019, 52, 10. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Dao, M.; Suresh, S.; Huang, T.J.; Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.H.; Truica, C.I. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Liu, D.; Wang, Y.; Lu, M.; Zhang, Q.; Huang, J.; Li, Y.; Ma, T.; Yan, F.; et al. In-vivo programmable acoustic manipulation of genetically engineered bacteria. Nat. Commun. 2023, 14, 3297. [Google Scholar] [CrossRef]
- Fonseca, A.D.C.; Glück, C.; Droux, J.; Ferry, Y.; Frei, C.; Wegener, S.; Weber, B.; El Amki, M.; Ahmed, D. Ultrasound trapping and navigation of microrobots in the mouse brain vasculature. Nat. Commun. 2023, 14, 5889. [Google Scholar] [CrossRef] [PubMed]
- Mahkam, N.; Aghakhani, A.; Sheehan, D.; Gardi, G.; Katzschmann, R.; Sitti, M. Acoustic Streaming-Induced Multimodal Locomotion of Bubble-Based Microrobots. Adv. Sci. 2023, 10, 2304233. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Lin, Y.H.; Paul, A.; van den Broek, M.R.P.; Segers, T.; Misra, S. Acoustically Actuated Flow in Microrobots Powered by Axisymmetric Resonant Bubbles. Adv. Intell. Syst. 2023, 2023, 2300465. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, J.; Pan, Y.; Zhuang, L.; Wang, P. Surface acoustic wave (SAW) techniques in tissue engineering. Cell Tissue Res. 2021, 386, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cui, H.; Lu, G.; Jiang, L.; Hensleigh, R.; Zeng, Y.; Rayes, A.; Panduranga, M.K.; Acharya, M.; Wang, Z.; et al. 3D Printing and processing of miniaturized transducers with near-pristine piezoelectric ceramics for localized cavitation. Nat. Commun. 2023, 14, 1–11. [Google Scholar] [CrossRef]
- Lee, J.G.; Raj, R.R.; Thome, C.P.; Day, N.B.; Martinez, P.; Bottenus, N.; Gupta, A.; Shields, C.W. Bubble-Based Microrobots with Rapid Circular Motions for Epithelial Pinning and Drug Delivery. Small 2023, 19, 2300409. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Paskert, A.; Zhang, Z.; Wittkowski, R.; Ahmed, D. An acoustically controlled helical microrobot. Sci. Adv. 2023, 9, eadh5260. [Google Scholar] [CrossRef]
- Kaynak, M.; Dirix, P.; Sakar, M.S. Addressable Acoustic Actuation of 3D Printed Soft Robotic Microsystems. Adv. Sci. 2020, 7, 2001120. [Google Scholar] [CrossRef]
- Wrede, P.; Aghakhani, A.; Bozuyuk, U.; Yildiz, E.; Sitti, M. Acoustic Trapping and Manipulation of Hollow Microparticles under Fluid Flow Using a Single-Lens Focused Ultrasound Transducer. ACS Appl. Mater. Interfaces 2023, 15, 52224–52236. [Google Scholar] [CrossRef]
- Han, J.; Zhen, J.; Du Nguyen, V.; Go, G.; Choi, Y.; Ko, S.Y.; Park, J.-O.; Park, S. Hybrid-Actuating Macrophage-Based Microrobots for Active Cancer Therapy. Sci. Rep. 2016, 6, 28717. [Google Scholar] [CrossRef] [PubMed]
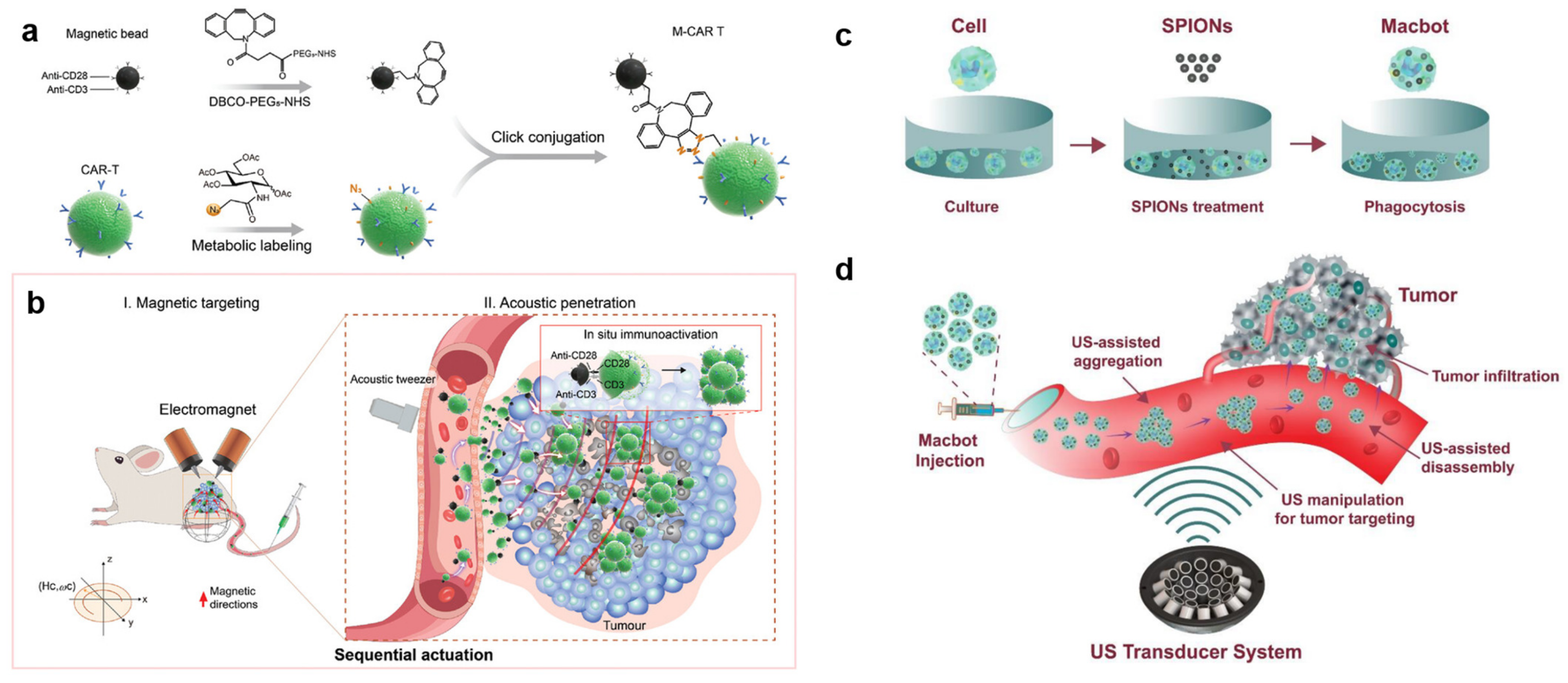
- Tang, X.; Yang, Y.; Zheng, M.; Yin, T.; Huang, G.; Lai, Z.; Zhang, B.; Chen, Z.; Xu, T.; Ma, T.; et al. Magnetic–Acoustic Sequentially Actuated CAR T Cell Microrobots for Precision Navigation and In Situ Antitumor Immunoactivation. Adv. Mater. 2023, 35, 2211509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 2021, 6, 9519. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zhang, Y.; Bao, M.; Xin, C.; Wei, Z.; Lin, G.; Wang, Z. A Multifunctional Magnetic Red Blood Cell-Mimetic Micromotor for Drug Delivery and Image-Guided Therapy. ACS Appl. Mater. Interfaces 2022, 14, 3825–3837. [Google Scholar] [CrossRef] [PubMed]
- Gwisai, T.; Mirkhani, N.; Christiansen, M.G.; Nguyen, T.T.; Ling, V.; Schuerle, S. Magnetic torque–driven living microrobots for increased tumor infiltration. Sci. Robot. 2022, 7, eabo0665. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Le, V.H.; Zheng, S.; Han, J.; Park, J.-O. Preparation of tumor targeting cell-based microrobots carrying NIR light sensitive therapeutics manipulated by electromagnetic actuating system and Chemotaxis. J. Microb. Robot. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhang, Y.; Xu, Y.; Li, Y.; Xie, Z.; Wang, H.; Lin, Y.; Lin, Q.; Gong, T.; et al. Live Macrophage-Delivered Doxorubicin-Loaded Liposomes Effectively Treat Triple-Negative Breast Cancer. ACS Nano 2022, 16, 9799–9809. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Li, J.; Gao, W.; Xu, T.; Christianson, C.; Gao, W.; Galarnyk, M.; He, Q.; Zhang, L.; et al. Turning erythrocytes into functional micromotors. ACS Nano 2014, 8, 12041–12048. [Google Scholar] [CrossRef]
- Cao, H.X.; Du Nguyen, V.; Jung, D.; Choi, E.; Kim, C.-S.; Park, J.-O.; Kang, B. Acoustically Driven Cell-Based Microrobots for Targeted Tumor Therapy. Pharmaceutics 2022, 14, 2143. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Xu, T.; Kiristi, M.; Liu, W.; Wu, Z.; Wang, J. Magneto–Acoustic Hybrid Nanomotor. Nano Lett. 2015, 15, 4814–4821. [Google Scholar] [CrossRef]
- Liu, F.W.; Cho, S.K. 3-D swimming microdrone powered by acoustic bubbles. Lab Chip 2021, 21, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, T.; Luo, S.T.; Luoab, T. Biologically inspired micro-robotic swimmers remotely controlled by ultrasound waves. Lab Chip 2021, 21, 4095–4103. [Google Scholar] [CrossRef]
- Dillinger, C.; Knipper, J.; Nama, N.; Ahmed, D. Steerable acoustically powered starfish-inspired microrobot. Nanoscale 2024, 16, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Dillinger, C.; Nama, N.; Ahmed, D. Ultrasound-activated ciliary bands for microrobotic systems inspired by starfish. Nat. Commun. 2021, 12, 6455. [Google Scholar] [CrossRef] [PubMed]
- Janiak, J.; Li, Y.; Ferry, Y.; Doinikov, A.A.; Ahmed, D. Acoustic microbubble propulsion, train-like assembly and cargo transport. Nat. Commun. 2023, 14, 4705. [Google Scholar] [CrossRef] [PubMed]
- Jooss, V.M.; Bolten, J.S.; Huwyler, J.; Ahmed, D. In vivo acoustic manipulation of microparticles in zebrafish embryos. Sci. Adv. 2022, 8, 2785. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Nama, N.; McNeill, J.M.; Soto, F.; Yan, Z.; Liu, W.; Wang, W.; Wang, J.; Mallouk, T.E. 3D steerable, acoustically powered microswimmers for single-particle manipulation. Sci. Adv. 2019, 5, eaax3084. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, L.; Jiao, N. Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly. Micromachines 2022, 13, 1068. [Google Scholar] [CrossRef]


| Acoustic Actuation Strategy | Propelled Mechanism | Acoustic Control Type | Application |
|---|---|---|---|
| Acoustic tweezer | Standing-wave tweezers | Surface acoustic wave [4,39,54,55,56,57] Bulk acoustic wave [58,59] | Nanorobots manipulation cell delivery cell separation or patterning Levitation of cells and submillimeter organisms |
| Traveling-wave tweezers | Acoustic phase modulation [60,61,62] Acoustic lens [63,64] | 3D translation and rotation of MNRs high-resolution ultrasonic imaging bioprinting and tissue engineering Targeted drug delivery | |
| Streaming-driven acoustic | Bubble oscillation | Tubular shape [50,65,66,67] Cup shaped [68,69] | 3D translation and rotation of MNRs Targeted drug delivery |
| Geometric design | Axis symmetric shape [70,71] Flagellar swimmer [72,73] | Cell separation or patterning Targeted drug delivery Sensing |
| No. | Acoustic Actuator Type, Operating Frequency | Robot Type | Applications | Ref. |
|---|---|---|---|---|
| 1 | 30 ultrasound transducer array, active traveling waves, 1 MHz | Nanorobot | 3D manipulation | [17] |
| 2 | Piezoelectric disk transducer, 237 kHz | Bubble-based microrobot | 3D manipulation | [39] |
| 3 | Ultrasonic 16-transducer array, active traveling wave, 1 MHz | Bubble-free microrobot | 3D manipulation, targeted drug delivery | [62] |
| 4 | Piezoelectric transducer, 4.6 kHz | Bubble-free microrobot | Remote actuation | [71] |
| 5 | 64-element array acoustic tweezer, 3 MHz | Bubble-based microrobot | In vivo tumor targeting | [116] |
| 6 | Piezo transducer, 490 kHz | Bubble-based microrobot | In vivo manipulation | [117] |
| 7 | Piezoelectric transducer, 70–270 kHz | Bubble-based microrobot | Multiple DoF locomotion, cancer cell lysing | [118] |
| 8 | PA1951 transducer, 50–120 kHz | Bubble-based microrobot | Debris clearance, cell collection | [119] |
| 9 | Ceramic piezoelectric transducer, 320 kHz | Bubble-based microrobot | Epithelial pinning and drug delivery | [122] |
| 10 | Piezo transducer, 12−19 kHz | Bubble-free microrobot | 2D, 3D manipulation | [123] |
| 11 | Piezo transducer, 5–270 kHz | Bubble-free microrobot | Biomanipulation, targeted therapy | [124] |
| 12 | Focused US transducer, 500 kHz and 2 MHz | Bubble-free microrobot | Active cell tagging, navigation, and US imaging | [125] |
| 13 | 64-element array acoustic tweezer, 3 MHz, | Biohybrid microrobot | In vivo manipulation, anticancer therapy | [127] |
| 14 | Piezoelectric transducer, 2.93 MHz | Biohybrid microrobot | Therapeutic transport | [133] |
| 15 | 30 ultrasound transducer array, active traveling waves, 1 MHz | Biohybrid microrobot | 3D manipulation, targeted drug delivery | [134] |
| 16 | Piezoelectric transducer, 618 kHz, 2.66 MHz | Nanorobot | Autonomous reconfiguring operation | [135] |
| 17 | Piezo-actuator, 4.6 kHz | Bubble-based microrobot | 3D maneuverability | [136] |
| 18 | Immersion ultrasound transducer, 234 kHz, 301 kHz | Bubble-based microrobot | Targeted drug delivery, remote microsurgery | [137] |
| 19 | Piezoelectric transducer, 28.0 kHz | Bubble-free microrobot | Acousto-magnetic manipulation | [138] |
| 20 | Piezo transducer, 20–100 kHz | Bubble-free microrobot | Analogous microparticle trap | [139] |
| 21 | Piezo transducer, 22.3−23 kHz | Bubble-based microrobot | Train-like assembly and cargo transport | [140] |
| 22 | Piezo transducer, 4.25 MHz | Bubble-based microrobot | In vivo manipulation | [141] |
| 23 | Piezo ceramic transducer, 1 to 3 MHz | Bubble-based microrobot | Single-particle manipulation | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.X.; Nguyen, V.D.; Park, J.-O.; Choi, E.; Kang, B. Acoustic Actuators for the Manipulation of Micro/Nanorobots: State-of-the-Art and Future Outlooks. Micromachines 2024, 15, 186. https://doi.org/10.3390/mi15020186
Cao HX, Nguyen VD, Park J-O, Choi E, Kang B. Acoustic Actuators for the Manipulation of Micro/Nanorobots: State-of-the-Art and Future Outlooks. Micromachines. 2024; 15(2):186. https://doi.org/10.3390/mi15020186
Chicago/Turabian StyleCao, Hiep Xuan, Van Du Nguyen, Jong-Oh Park, Eunpyo Choi, and Byungjeon Kang. 2024. "Acoustic Actuators for the Manipulation of Micro/Nanorobots: State-of-the-Art and Future Outlooks" Micromachines 15, no. 2: 186. https://doi.org/10.3390/mi15020186
APA StyleCao, H. X., Nguyen, V. D., Park, J.-O., Choi, E., & Kang, B. (2024). Acoustic Actuators for the Manipulation of Micro/Nanorobots: State-of-the-Art and Future Outlooks. Micromachines, 15(2), 186. https://doi.org/10.3390/mi15020186







