Integration Method of Microchannel and Vertical Micromesh Structure for Three-Dimensional Cell Culture Using Inclined Exposure and Inclined Oxygen Ashing
Abstract
:1. Introduction
2. Method
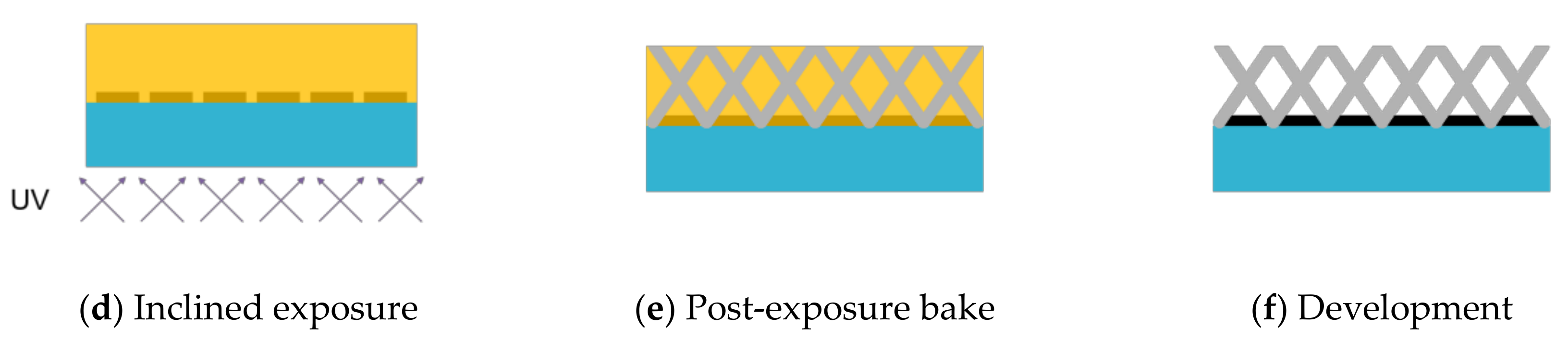
2.1. Principle of Vertical Porous Membrane Integration Method Using an Inclined Exposure Method and Inclined Oxygen Ashing
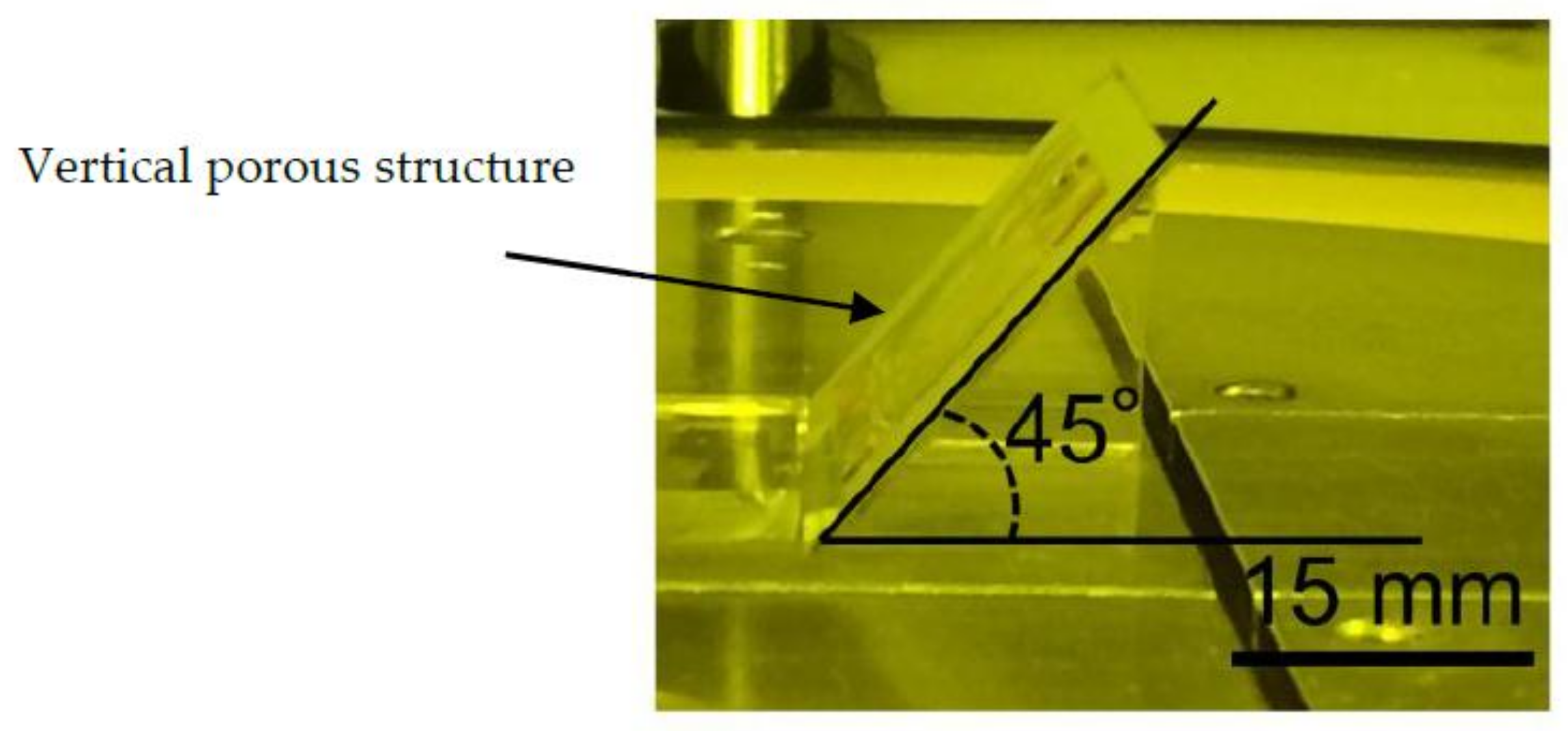
2.2. Inclined Backside Exposure
2.3. Double-Sided Inclined Oxygen Ashing
3. Results
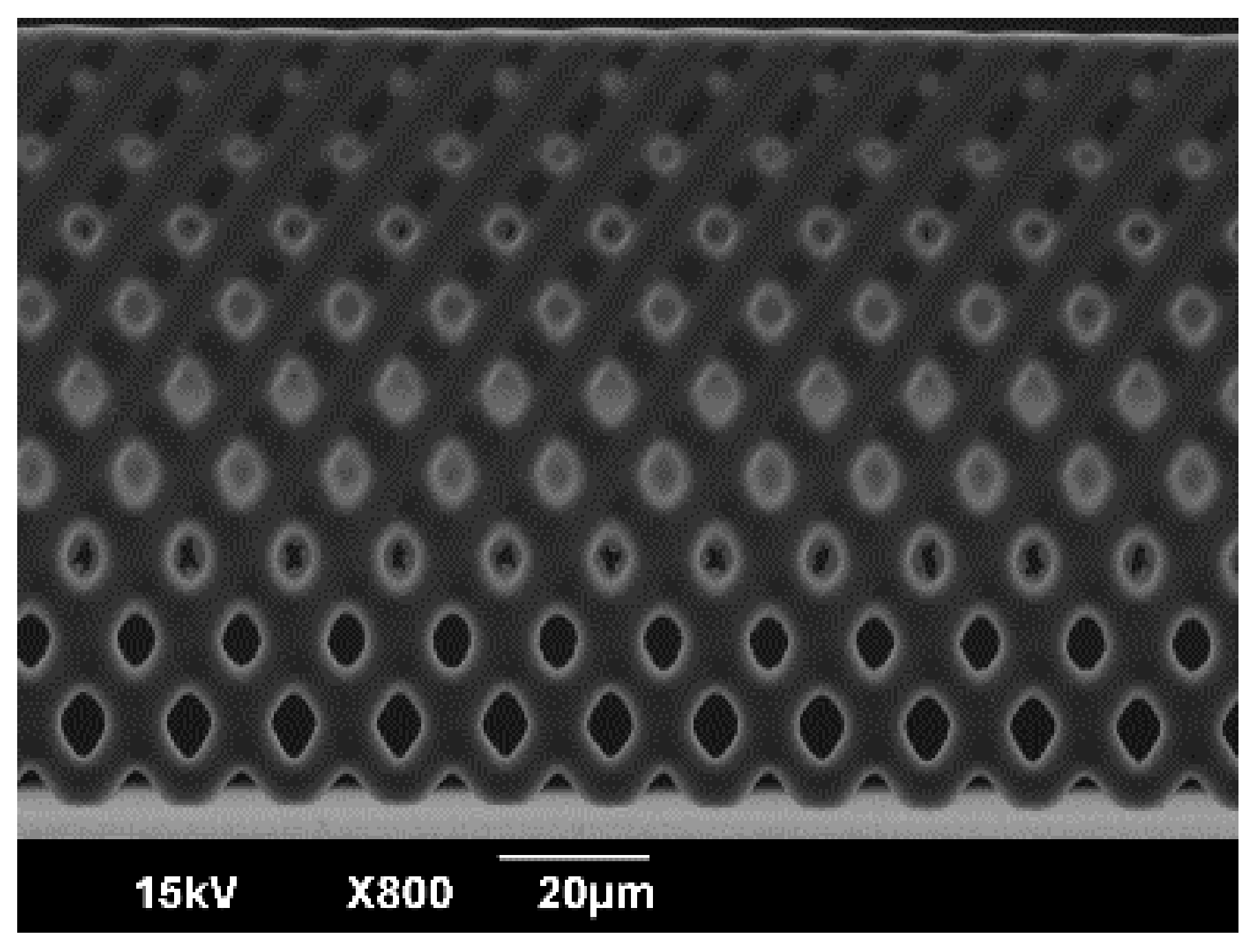
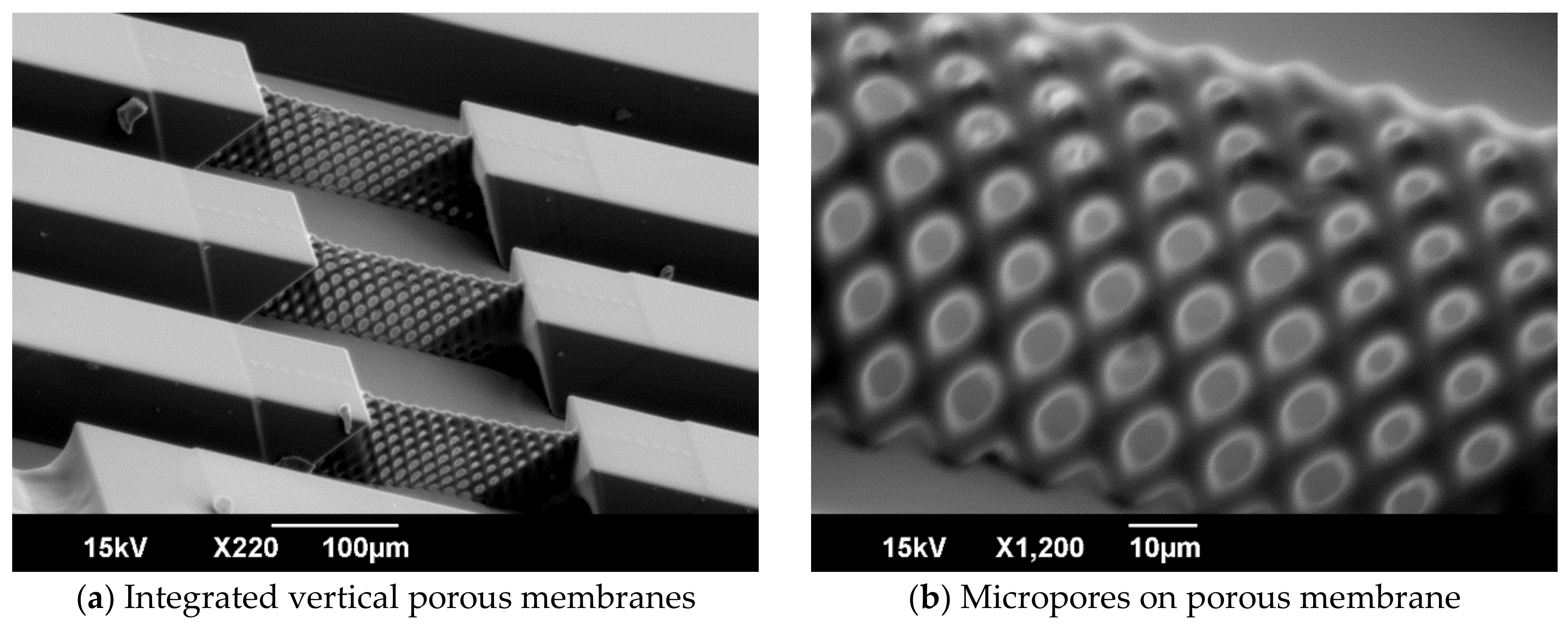
3.1. Fabrication of Vertical Porous Membrane Using Inclined Exposure
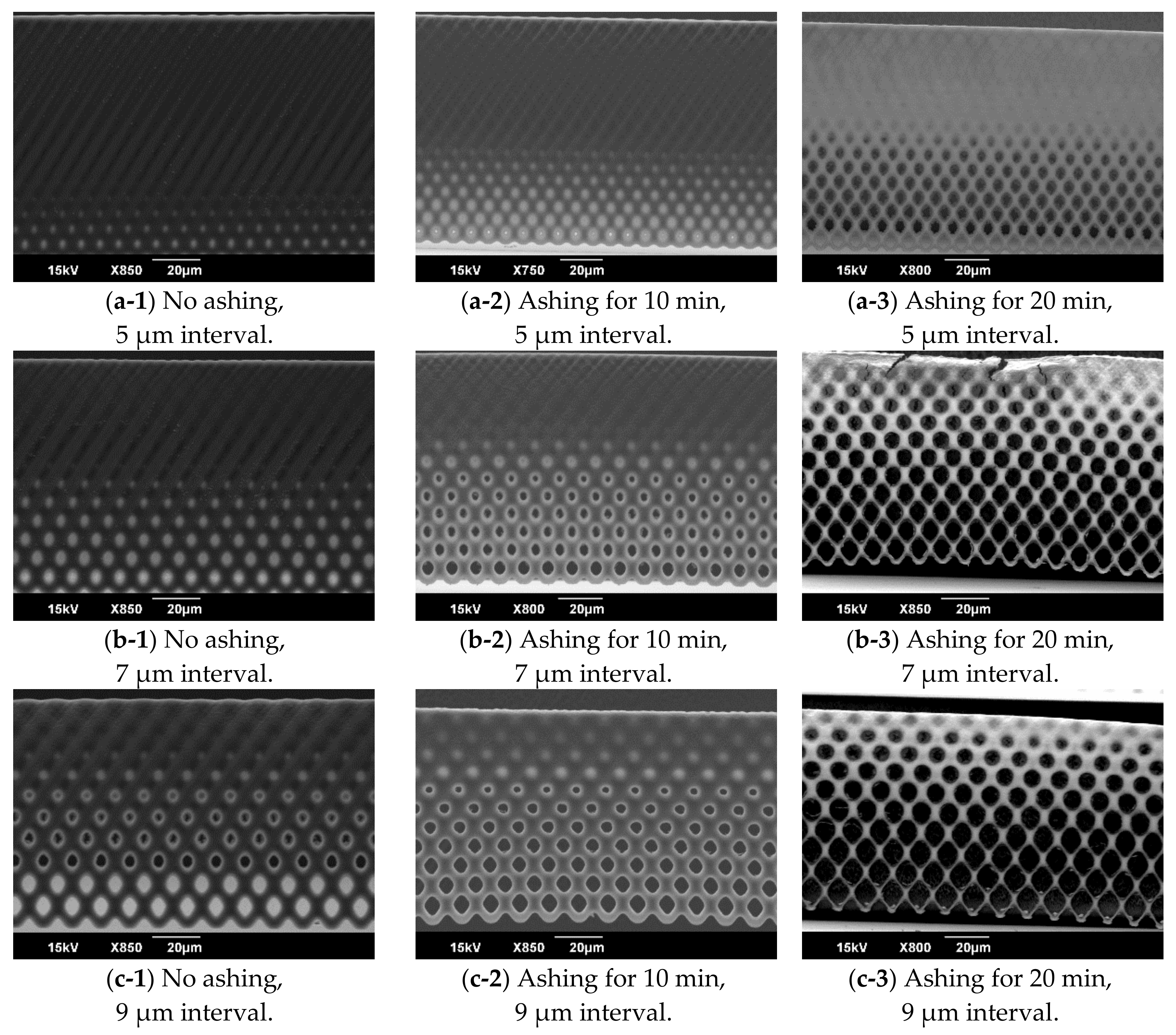
3.2. Evaluation of the Inclined Oxygen Ashing after Inclined Exposure
4. Application for Biomicrofluidics
4.1. Method for Integration of Vertical Porous Membrane in Microchannel
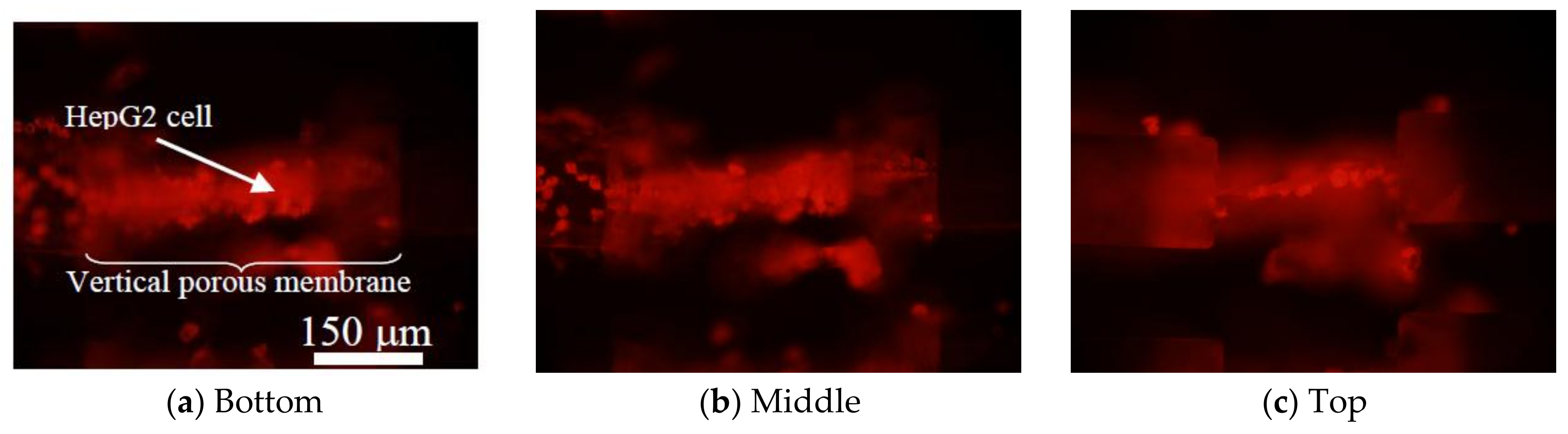
4.2. Cell Culture on Vertical Porous Membranes
5. Discussion
5.1. Fabrication of Vertical Porous Membranes by Inclined Exposure
5.2. Ratio of Open Micropores Obtained by Inclined Oxygen Ashing
5.3. Robustness of Vertical Porous Membrane Fabricated by Inclined Oxygen Ashing
5.4. Cell Culture on Vertical Porous Membrane
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Esch, M.B.; King, T.L.; Shuler, M.L. The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng. 2012, 13, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Mahler, M.J.; Stokol, T.; Shuler, M.L. Body-on-a-chip simulation with GI tract and liver tissue suggests that integrated nanoparticles have the potential to cause liver injury. Lab Chip. 2014, 14, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Viravaidya, K.; Shuler, M.L. Incorporation of 3T3-L1 cells to mimic bioaccumulation in a microscale cell culture analog device for toxicity studies. Biotech. Progr. 2004, 20, 590–597. [Google Scholar]
- Sin, A.; Chin, K.C.; Jamil, M.F.; Kostov, Y.; Rao, G.; Shuler, M.L. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotech. Progr. 2004, 20, 338–345. [Google Scholar]
- Miller, P.G.; Shuler, M.L. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol. Bioeng. 2016, 113, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar]
- Chung, S.; Sudo, R.; Mack, P.J.; Wan, C.; Vickerman, V.; Kamm, R.D. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009, 21, 269–275. [Google Scholar]
- Osaki, T.; Sivathanu, V.; Kamm, R.D. Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci. Rep. 2018, 8, 5168. [Google Scholar] [Green Version]
- Esch, M.B.; Ueno, H.; Applegatec, D.R.; Shuler, M.L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip. 2016, 16, 2719–2729. [Google Scholar] [CrossRef]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamoria, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip. 2018, 18, 115–125. [Google Scholar] [CrossRef]
- Bertsch, A.; Renaud, P. Special Issue: 15 Years of SU-8 as MEMS Material. Micromachines 2015, 6, 790–792. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Huang, Q.A. Comprehensive simulations for ultraviolet lithography process of thick SU-8 Photoresist. Micromachines 2018, 9, 341 (21 pages). [Google Scholar] [CrossRef]
- Tuomikoski, S.; Franssila, S. Free-standing SU-8 microfluidic chips by adhesive bonding and release etching. Sens. Actuator A 2005, 120, 408–415. [Google Scholar] [CrossRef]
- Campo, A.; Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, 81–95. [Google Scholar] [CrossRef]
- Inoue, M.; Okonogi, A.; Terao, K.; Takao, H.; Shimokawa, F.; Oohira, F.; Kotera, H.; Suzuki, T. Cell Culture on MEMS Materials in Micro-Environment Limited by a Physical Condition. Micro Nano Lett. 2012, 7, 725–728. [Google Scholar] [CrossRef]
- Arteaga-Marrero, N.; Auzelyte, V.; Olsson, M.G.; Pallon, J. A SU-8 dish for cell irradiation. Nucl. Instrum. Methods Phys. Res. B 2007, 263, 523–528. [Google Scholar] [CrossRef]
- Nemani, K.V.; Moodie, K.L.; Brennick, J.B.; Su, A.; Gimi, B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mat. Sci. Eng. C 2013, 333, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.B.; Sung, J.H.; Yang, J.; Yu, C.; Yu, J.; March, J.C.; Shuler, M.L. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 2012, 14, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, J.H.; Kang, D.W.; Lee, K.; Kim, C.K. Single- and multi-directional slanted plasma etching of silicon under practical plasma processing conditions. ECS J. Solid State Sci. Technol. 2014, 3, 215–220. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamiltona, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012, 12, 2165–2174. [Google Scholar]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.L.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 159. [Google Scholar]
- Yoon, Y.K.; Park, J.H.; Allen, M.G. Multidirectional UV lithography for complex 3-D MEMS structures. J. Microelectromech. Sys. 2006, 15, 1121–1130. [Google Scholar] [CrossRef]
- Yeon, J.H.; Park, J.K. Cytotoxicity test based on electrochemical impedance measurement of HepG2 cultured in microfabricated cell chip. Anal. Biochem. 2005, 341, 308–315. [Google Scholar]
- Aritomi, K.; Ishitsuka, Y.; Tomishima, Y.; Shimizu, D.; Abe, N.; Shuto, T.; Irikura, M.; Kai, H.; Irie, T. Evaluation of three-dimensional cultured HepG2 cells in a nano culture plate system: an in vitro human model of acetaminophen hepatotoxicity. J. Pharmacol. Sci. 2014, 124, 218–229. [Google Scholar] [CrossRef]
- Sato, H.; Kakinuma, T.; Go, J.S.; Shoji, S. In-channel 3-D micromesh structures using maskless multi-angle exposures and their microfilter application. Sens. Actuator A 2004, 111, 87–92. [Google Scholar] [CrossRef]
- Lee, J.B.; Choi, K.H.; Yoo, K. Innovative SU-8 lithography techniques and their applications. Micromachines 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, H.; Ohoka, M.; Okonogi, A.; Kabata, H.; Kanno, I.; Washizu, M.; Kotera, H. High throughput cell electroporation array fabricated by single-mask inclined UV lithography exposure and oxygen plasma etching. Transducers 2007, 9828569. [Google Scholar]
- Namatsu, H. Supercritical drying for water-rinsed resist systems. Am. Vac. Soc. 2000, 18, 3308–3312. [Google Scholar]
- Walther, F.; Davidovskaya, P.; Zürcher, S.; Kaiser, M.; Herberg, H.; Gigler, A.; Stark, R.W. Stability of the hydrophilic behaviour of oxygen plasma activated SU-8. J. Micromech. Microengin. 2007, 17, 524–531. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, H.; Yamada, K.; Suzuki, T. Integration Method of Microchannel and Vertical Micromesh Structure for Three-Dimensional Cell Culture Using Inclined Exposure and Inclined Oxygen Ashing. Micromachines 2018, 9, 681. https://doi.org/10.3390/mi9120681
Ueno H, Yamada K, Suzuki T. Integration Method of Microchannel and Vertical Micromesh Structure for Three-Dimensional Cell Culture Using Inclined Exposure and Inclined Oxygen Ashing. Micromachines. 2018; 9(12):681. https://doi.org/10.3390/mi9120681
Chicago/Turabian StyleUeno, Hidetaka, Kou Yamada, and Takaaki Suzuki. 2018. "Integration Method of Microchannel and Vertical Micromesh Structure for Three-Dimensional Cell Culture Using Inclined Exposure and Inclined Oxygen Ashing" Micromachines 9, no. 12: 681. https://doi.org/10.3390/mi9120681




