Development of a Triple-Coaxial Flow Device for Fabricating a Hydrogel Microtube and Its Application to Bioremediation
Abstract
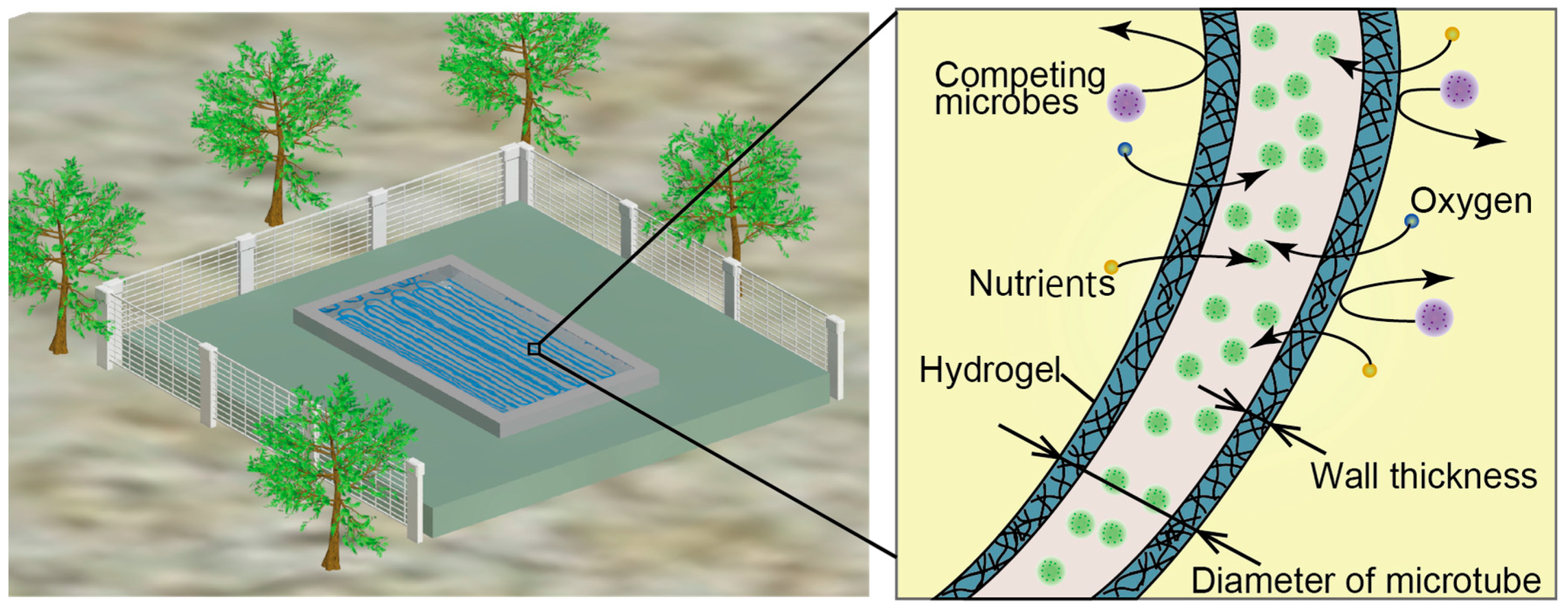
:1. Introduction
2. Materials and Methods
2.1. Materials
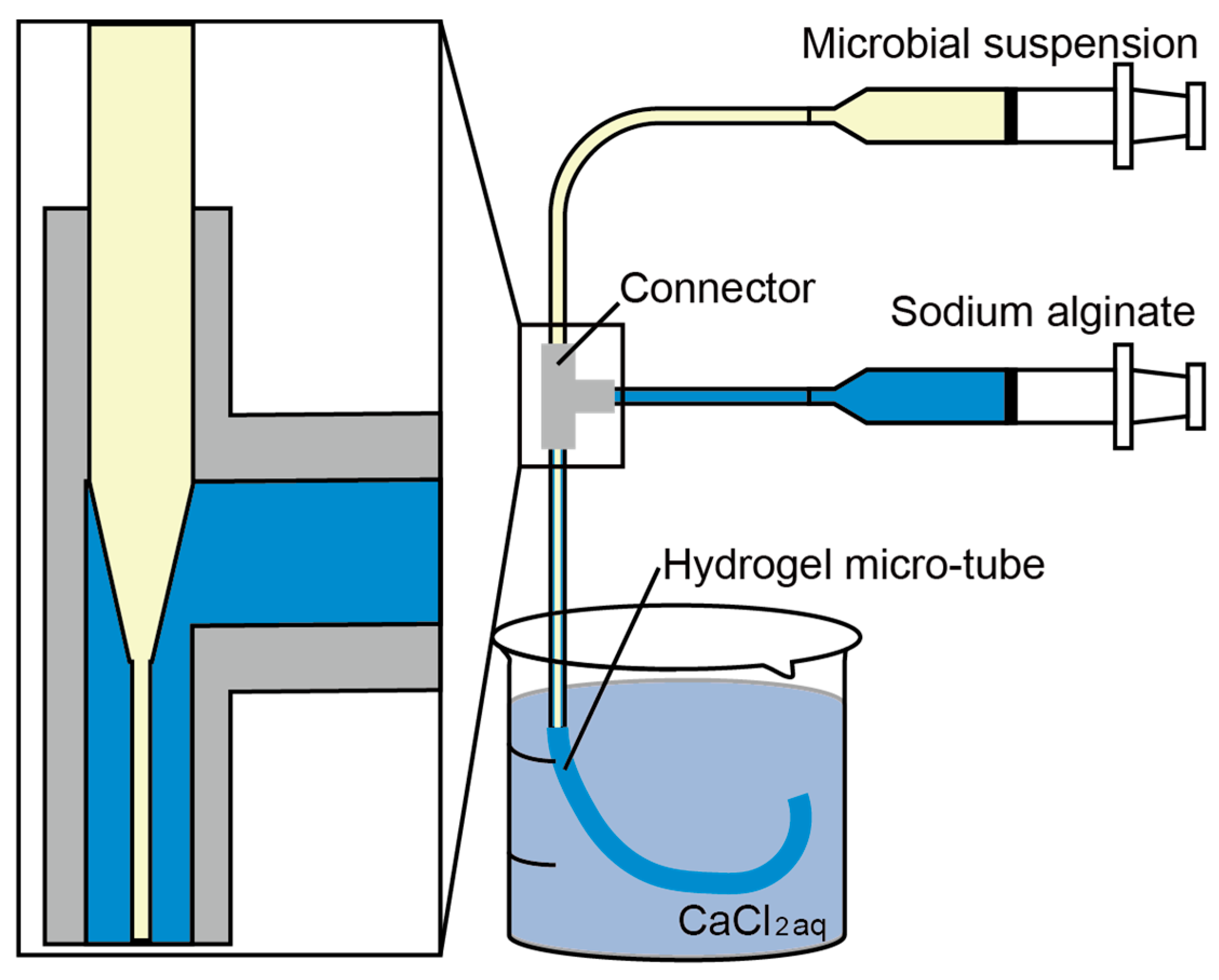
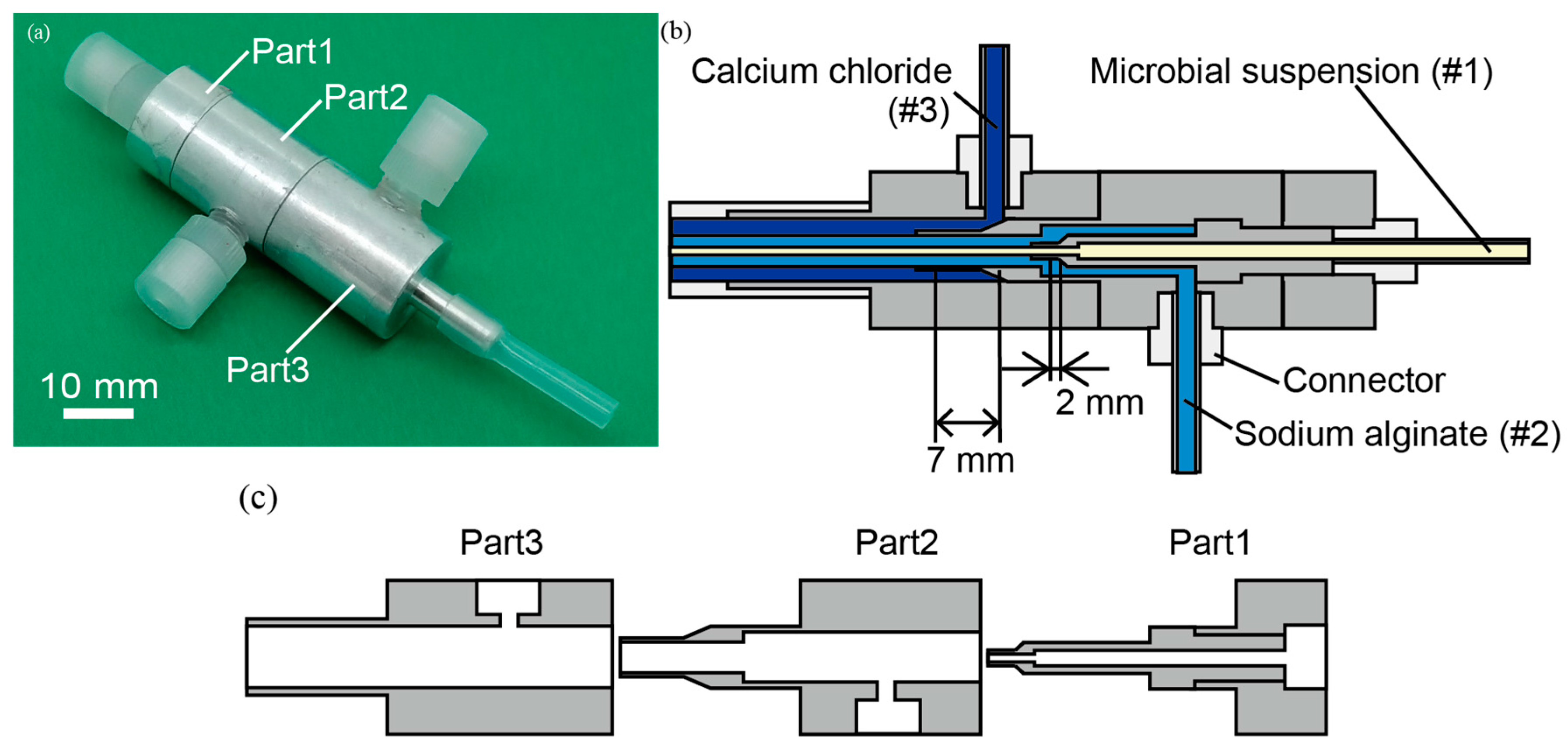
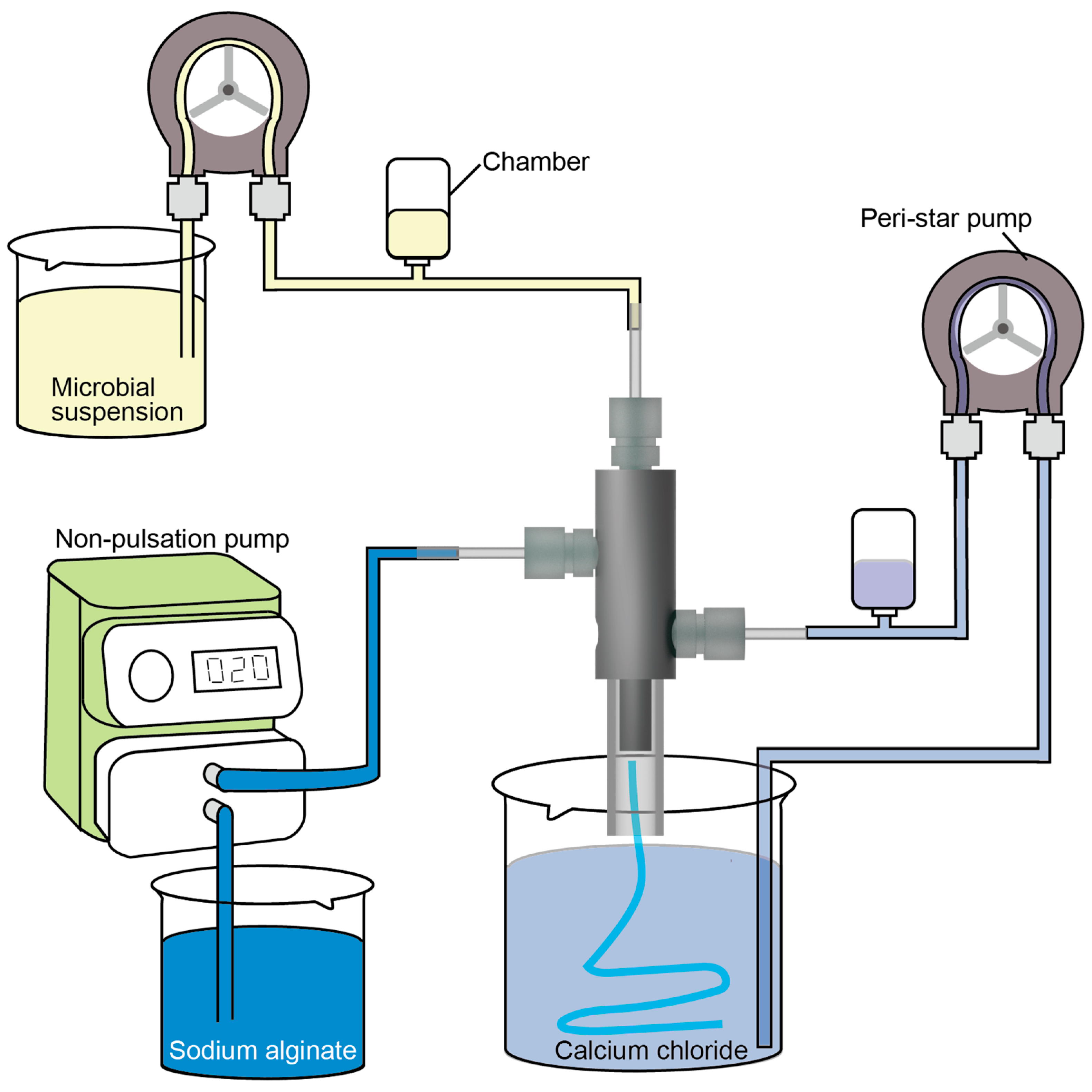
2.2. Triple-Coaxial Flow Device
2.3. Fabrication of a Hollow Hydrogel Microtube
2.4. Bioremediation: Removal of Methylene Blue Dye
3. Results and Discussion
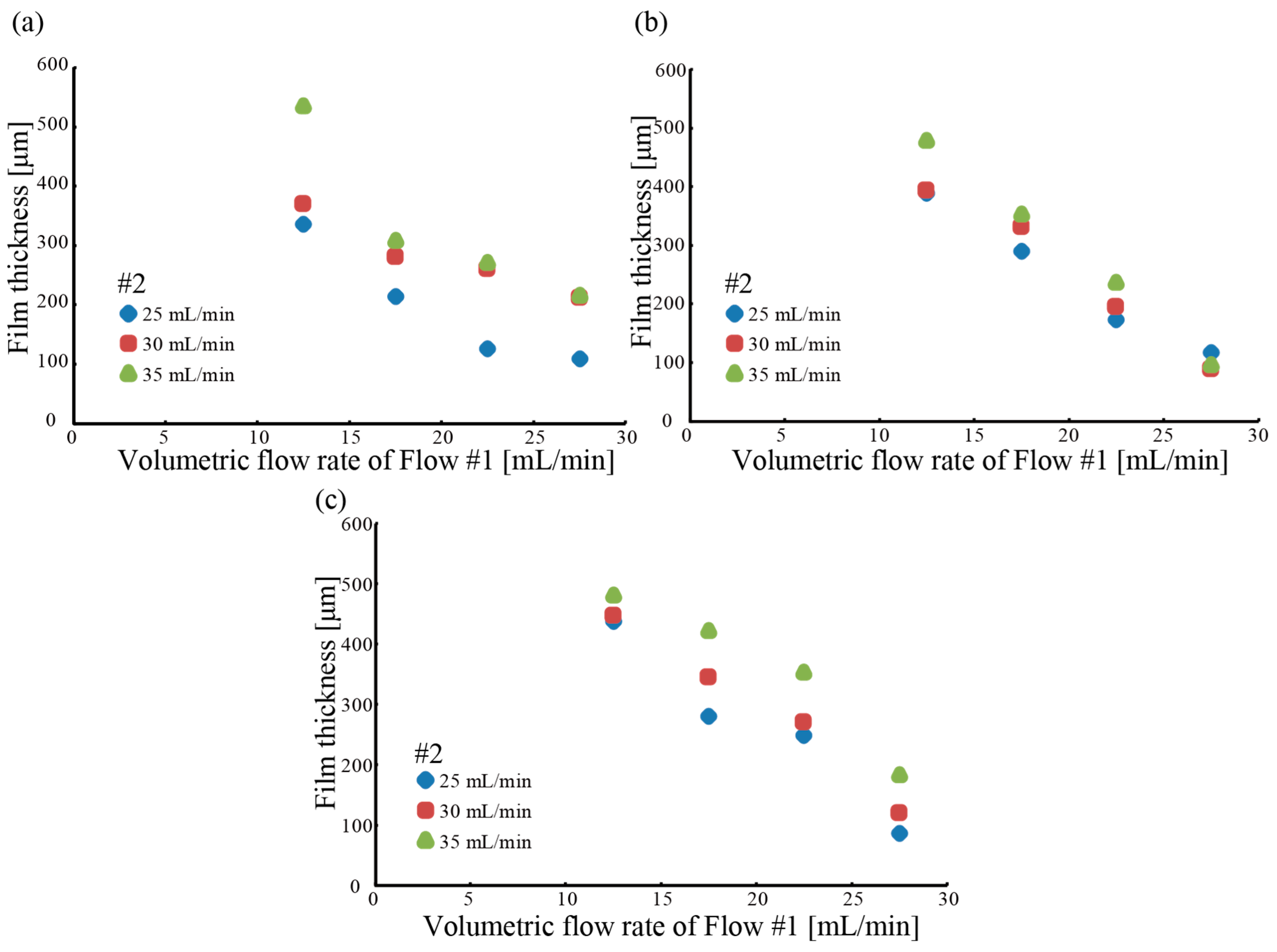
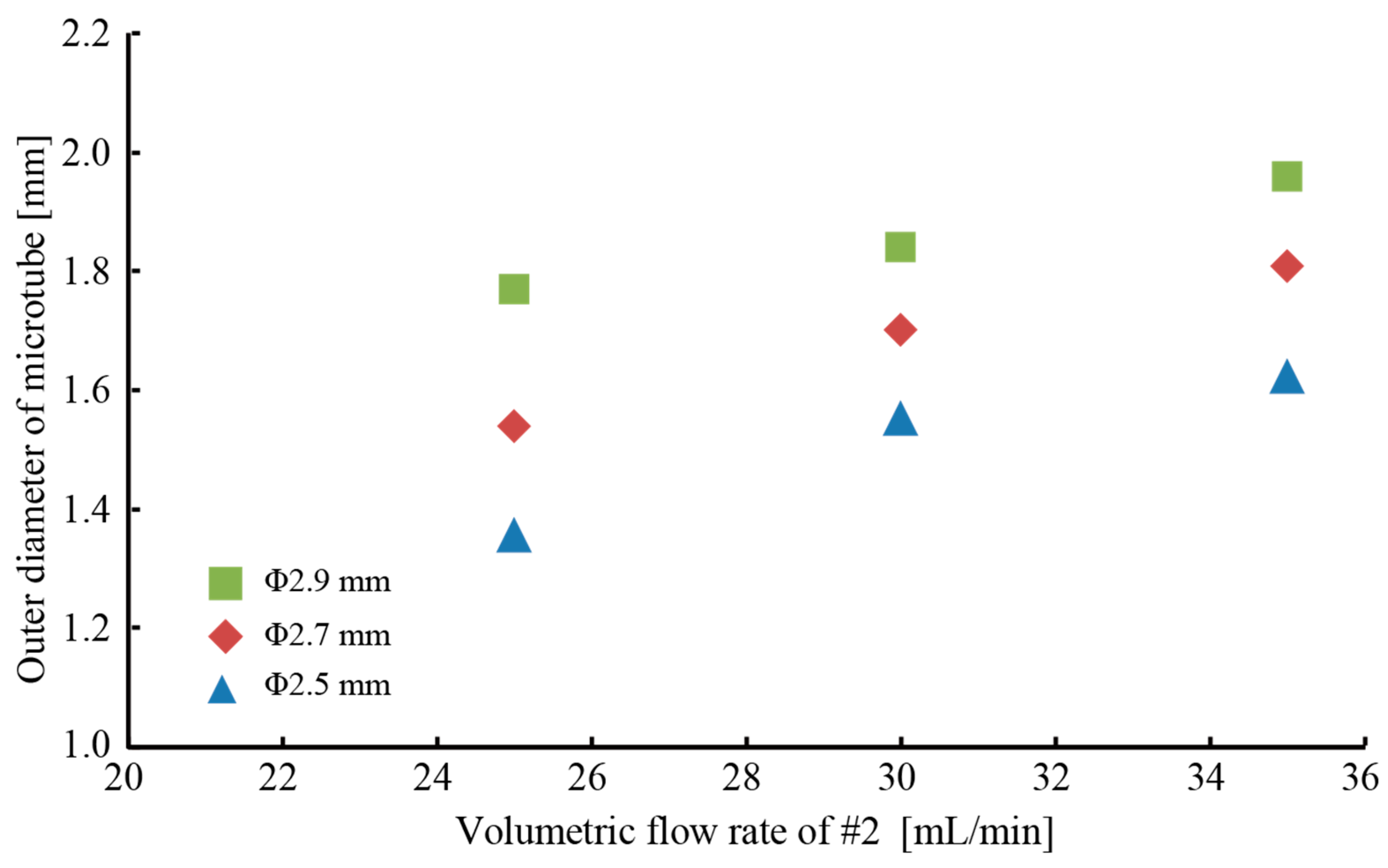
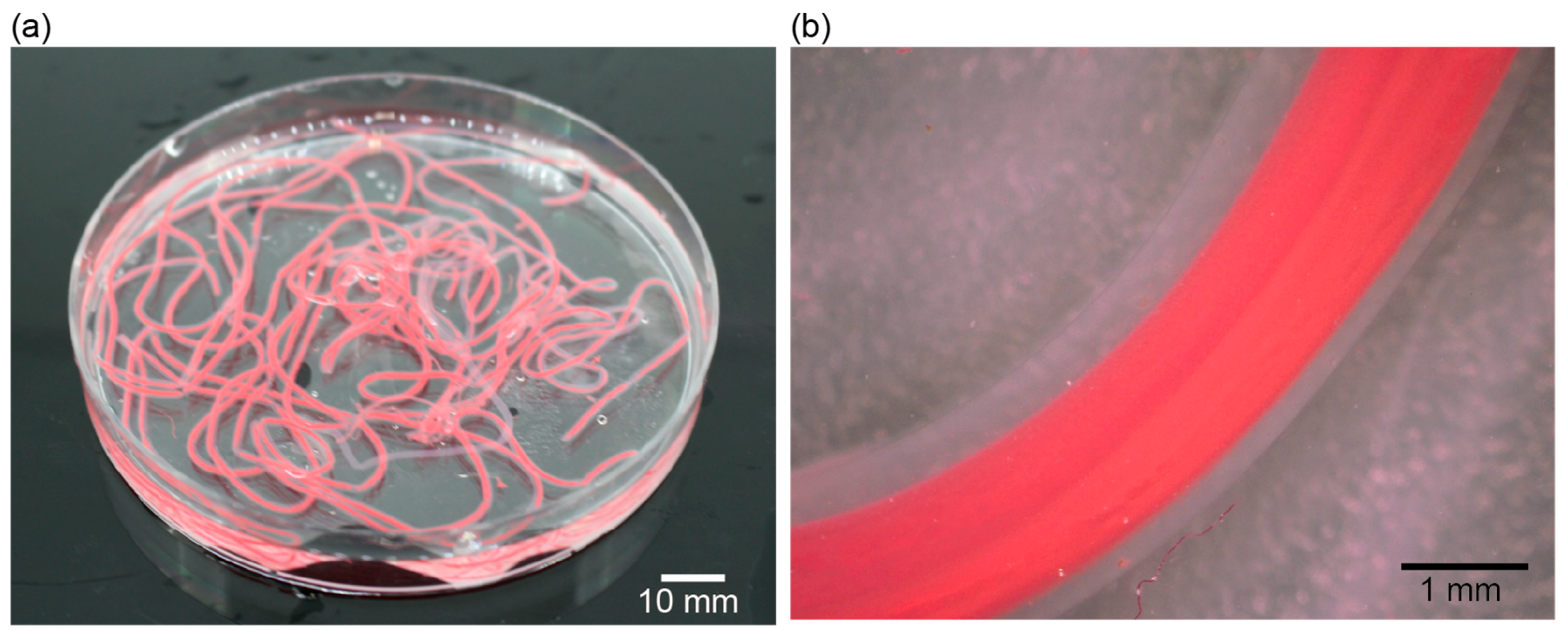
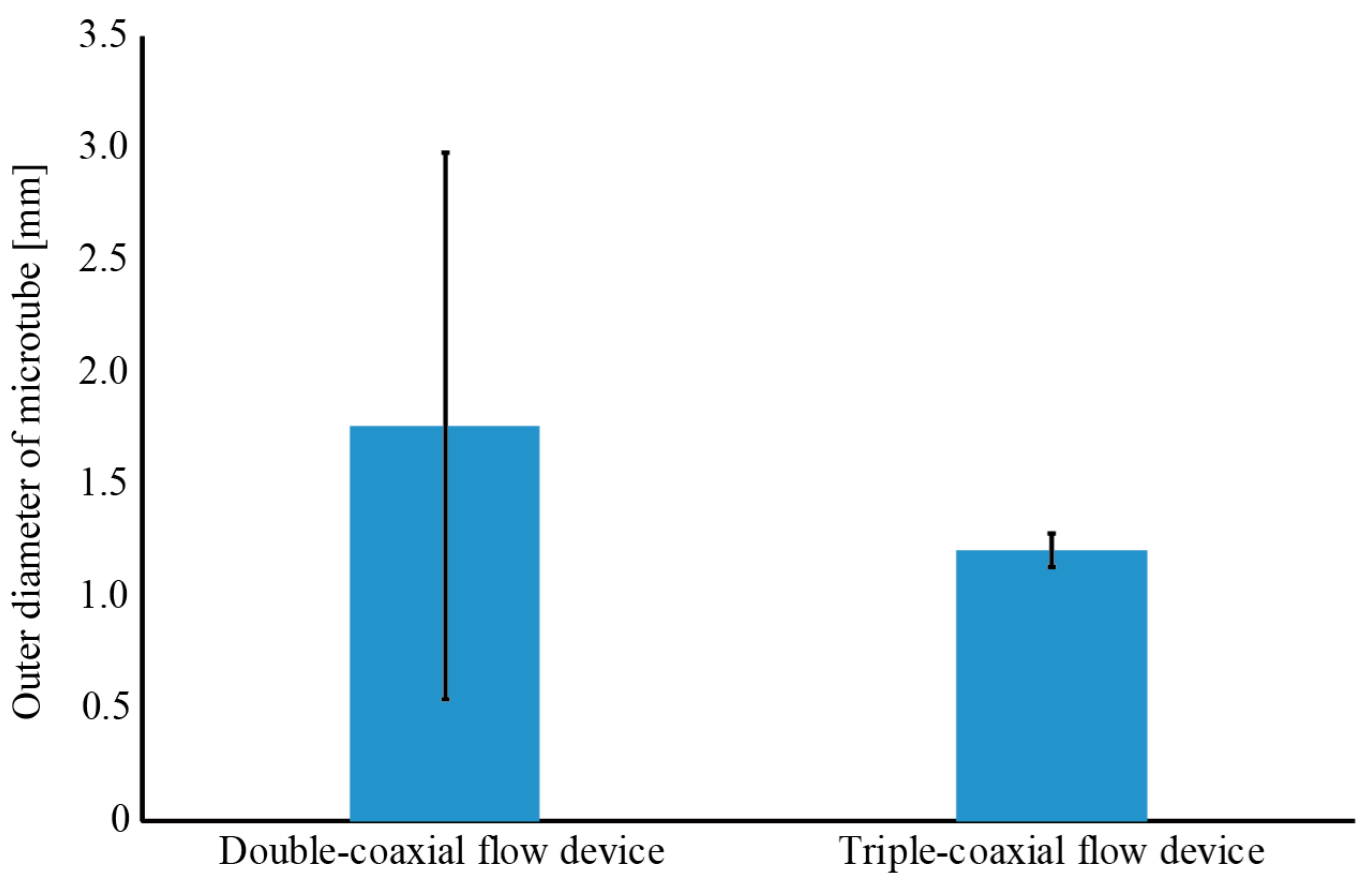
3.1. Fabrication of a Hollow Hydrogel Microtube
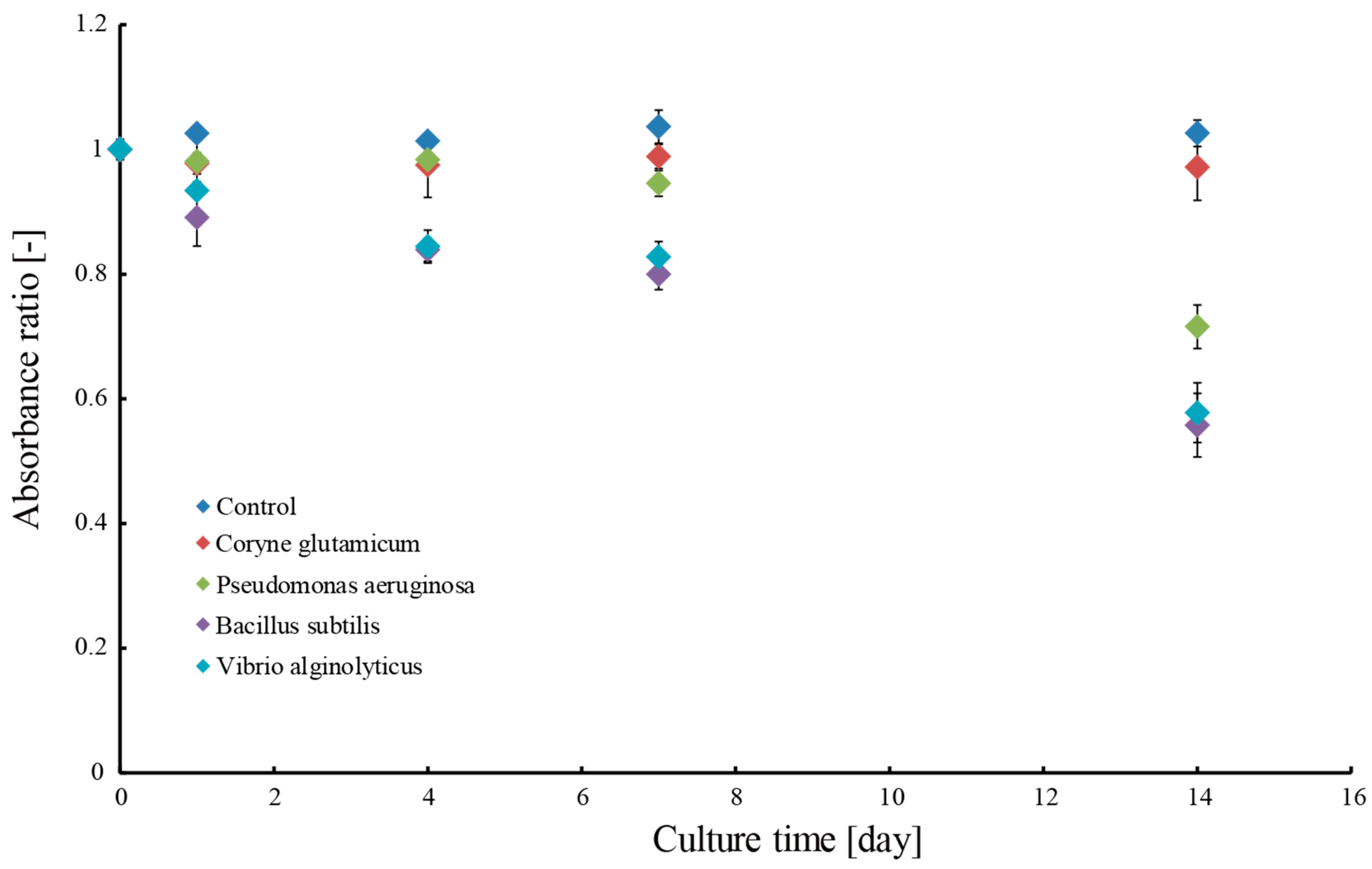
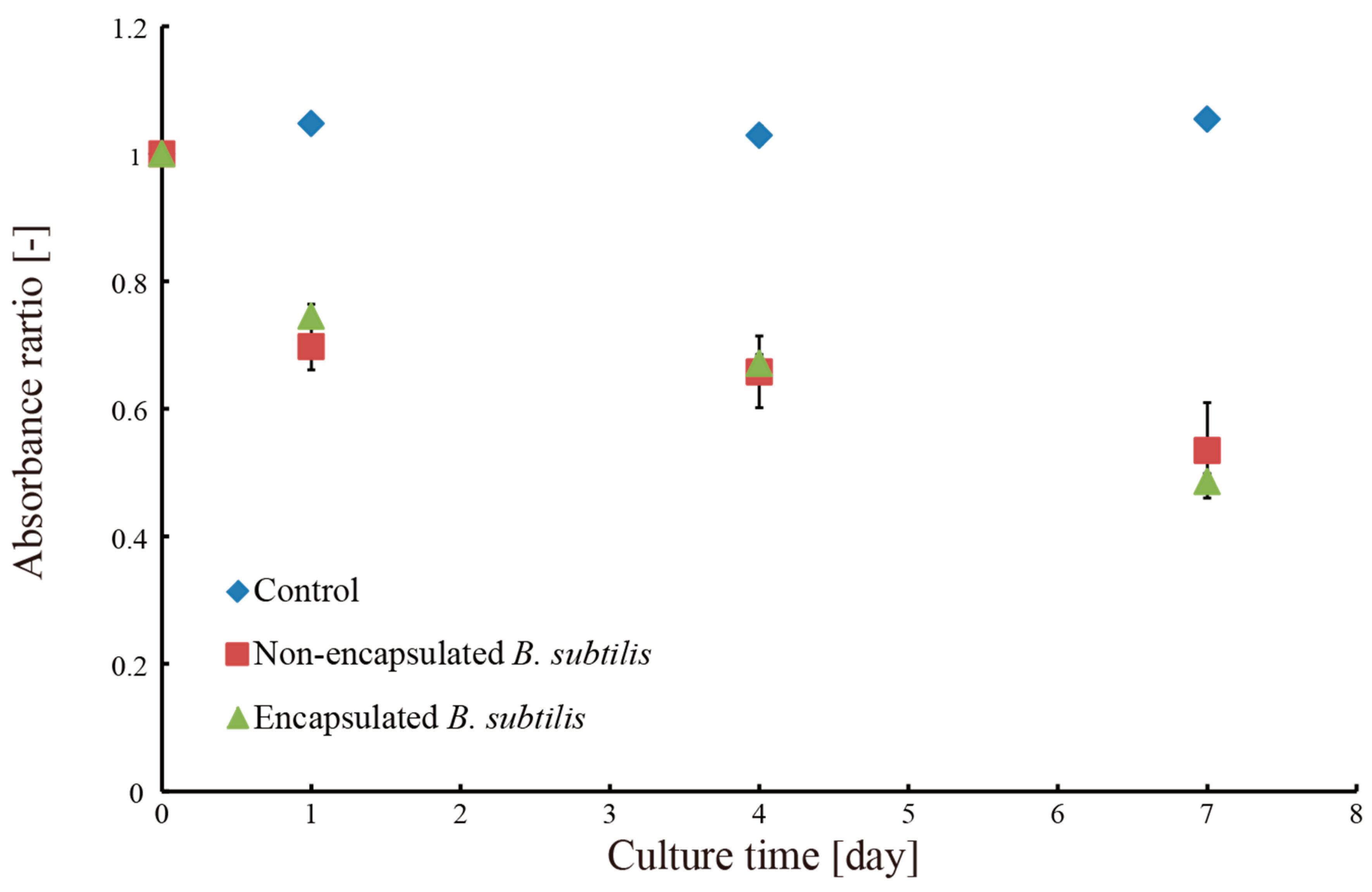
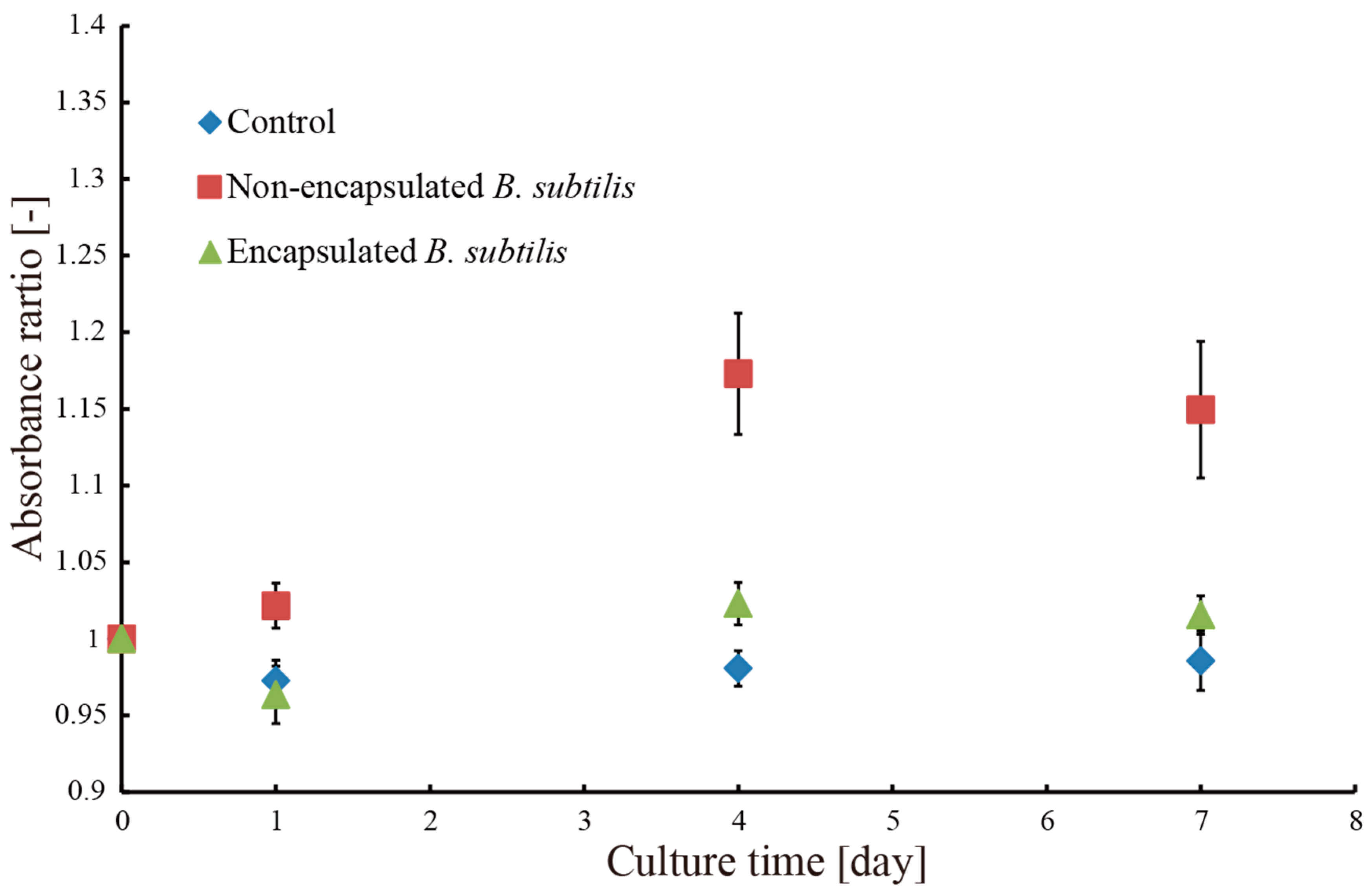
3.2. Removal of Methylene Blue Dye
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ogimoto, K. Basic Knowledge of Microorganisms for Biotechnology: Microorganisms Surrounding Humans; Kodansha: Tokyo, Japan, 2012; ISBN 9784061537309. [Google Scholar]
- Ogawa, T.; Tamori, M.; Kimura, A.; Mine, A.; Sakuyama, H.; Yoshida, E.; Maruta, T.; Suzuki, K.; Ishikawa, T.; Shigeoka, S. Enhancement of photosynthetic capacity in Euglena gracilis by expression of cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase leads to increases in biomass and wax ester production. Biotechnol. Biofuels 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Shimada, R.; Fujita, M.; Yuasa, M.; Sawamura, H.; Watanabe, T.; Nakashima, A.; Suzuki, K. Oral administration of green algae, Euglena gracilis, inhibits hyperglycemia in OLETF rats, a model of spontaneous type 2 diabetes. Food Funct. 2016, 7, 4655–4659. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahnestock, S.R.; Yao, Z.; Bedzyk, L.A. Microbial production of spider silk proteins. J. Biotechnol. 2000, 74, 105–119. [Google Scholar] [CrossRef]
- Heslot, H. Artificial fibrous proteins: A review. Biochimie 1998, 80, 19–31. [Google Scholar] [CrossRef]
- Ogawa, M.; Higashi, K.; Miki, N. Microbial production inside microfabricated hydrogel microtubes. In Proceedings of the 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 1692–1694. [Google Scholar]
- Hirayama, K.; Okitsu, T.; Teramae, H.; Kiriya, D.; Onoe, H.; Takeuchi, S. Cellular building unit integrated with microstrand-shaped bacterial cellulose. Biomaterials 2013, 34, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Boggione, D.M.G.; Batalha, L.S.; Gontijo, M.T.P.; Lopez, M.E.S.; Teixeira, A.V.N.C.; Santos, I.J.B.; Mendonca, R.C.S. Evaluation of microencapsulation of the UFV-AREG1 bacteriophage in alginate-Ca microcapsules using microfluidic devices. Colloids Surf. B Biointerfaces 2017, 158, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Ogawa, M.; Fujimoto, K.; Onoe, H.; Miki, N. Hollow hydrogel microfiber encapsulating microorganisms for mass-cultivation in open systems. Micromachines 2017, 8, 176. [Google Scholar] [CrossRef]
- Ogawa, M.; Higashi, K.; Miki, N. Development of hydrogel microtubes for microbe culture in open environment. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 25–29 August 2015; pp. 5896–5899. [Google Scholar]
- Fujimoto, K.; Higashi, K.; Onoe, H.; Miki, N. Microfluidic mass production system for hydrogel microtubes for microbial culture. Jpn. J. Appl. Phys. 2017, 56. [Google Scholar] [CrossRef]
- Onoe, H.; Takeuchi, S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today 2015, 20, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Kajihara, K. Gel Handbook; NTS Inc.: Tokyo, Japan, 1997; p. 64. ISBN 4860430298. [Google Scholar]
- He, X.; Zhang, R.; Chen, S.; Doolen, G.D. On the three-dimensional Rayleigh–Taylor instability. Phys. Fluids Phys. Fluids A Fluid Dyn. 1999, 11, 1143–1152. [Google Scholar] [CrossRef]
- Wysocki, A.; Royall, C.P.; Winkler, R.G.; Gompper, G.; Tanaka, H.; van Blaaderen, A.; Löwen, H. Direct observation of hydrodynamic instabilities in a driven non-uniform colloidal dispersion. Soft Matter 2009, 5, 1340–1344. [Google Scholar] [CrossRef]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, X.; Zhang, J.D.; Peng, L.; Liu, C.Y. Removal of organic matter and heavy metals of low concentration from wastewater via micellar-enhanced ultrafiltration: An overview. IOP Conf. Ser. Earth Environ. Sci. 2017, 52. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene-carbon nanotube hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Bian, C.; Tong, J.; Fang, Y.; Xia, S. Ultramicroelectrode array modified with magnetically labeled Bacillus subtilis, palladium nanoparticles and reduced carboxy graphene for amperometric determination of biochemical oxygen demand. Microchim. Acta 2017, 184, 763–771. [Google Scholar] [CrossRef]
- Upendar, G.; Dutta, S.; Chakraborty, J.; Bhattacharyya, P. Removal of methylene blue dye using immobilized bacillus subtilis in batch & column reactor. Mater. Today Proc. 2016, 3, 3467–3472. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, K.; Higashi, K.; Onoe, H.; Miki, N. Development of a Triple-Coaxial Flow Device for Fabricating a Hydrogel Microtube and Its Application to Bioremediation. Micromachines 2018, 9, 76. https://doi.org/10.3390/mi9020076
Fujimoto K, Higashi K, Onoe H, Miki N. Development of a Triple-Coaxial Flow Device for Fabricating a Hydrogel Microtube and Its Application to Bioremediation. Micromachines. 2018; 9(2):76. https://doi.org/10.3390/mi9020076
Chicago/Turabian StyleFujimoto, Kazuma, Kazuhiko Higashi, Hiroaki Onoe, and Norihisa Miki. 2018. "Development of a Triple-Coaxial Flow Device for Fabricating a Hydrogel Microtube and Its Application to Bioremediation" Micromachines 9, no. 2: 76. https://doi.org/10.3390/mi9020076




