Quantitative Evaluation of Dielectric Breakdown of Silicon Micro- and Nanofluidic Devices for Electrophoretic Transport of a Single DNA Molecule
Abstract
:1. Introduction
2. Materials and Methods
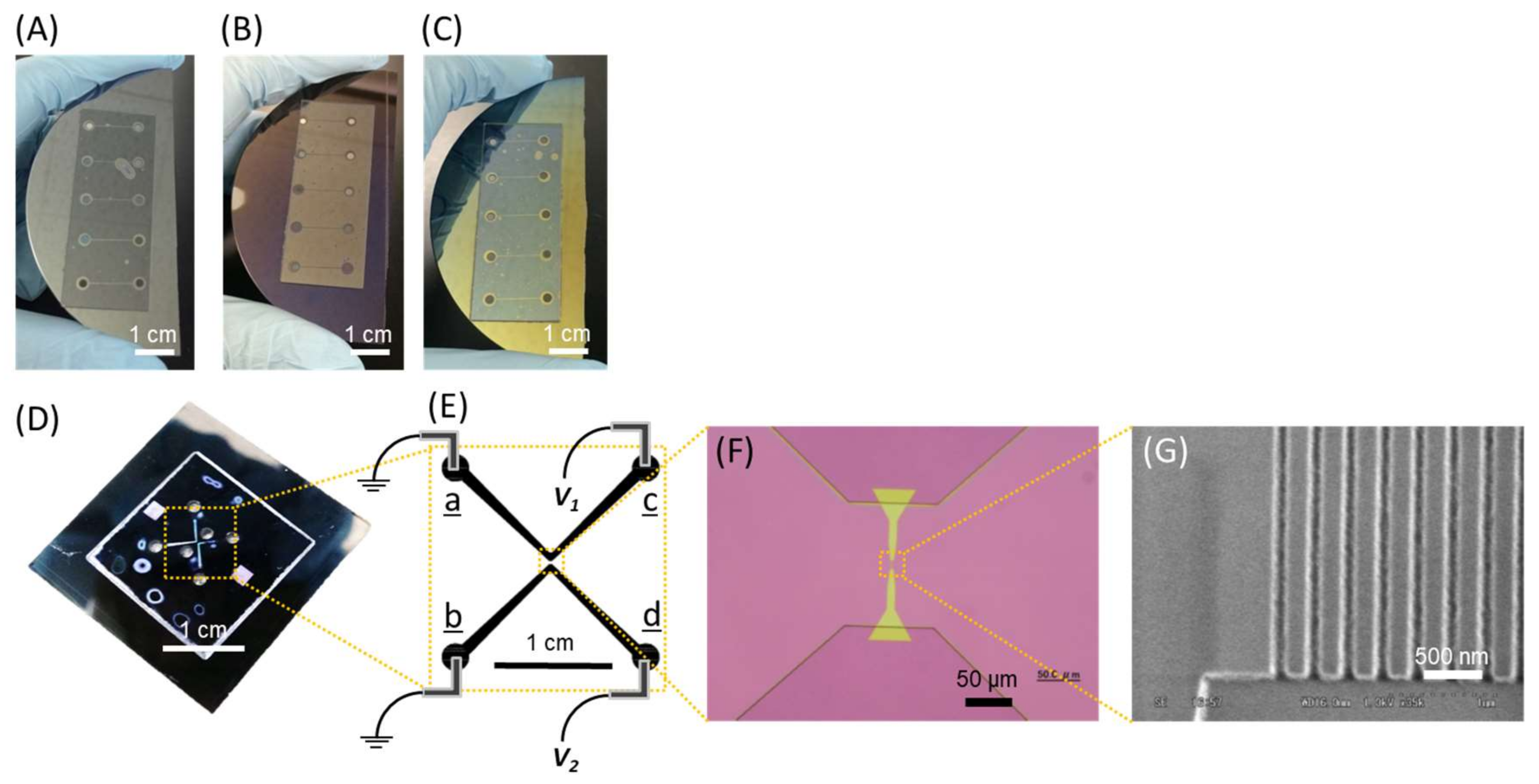
2.1. Device Fabrication
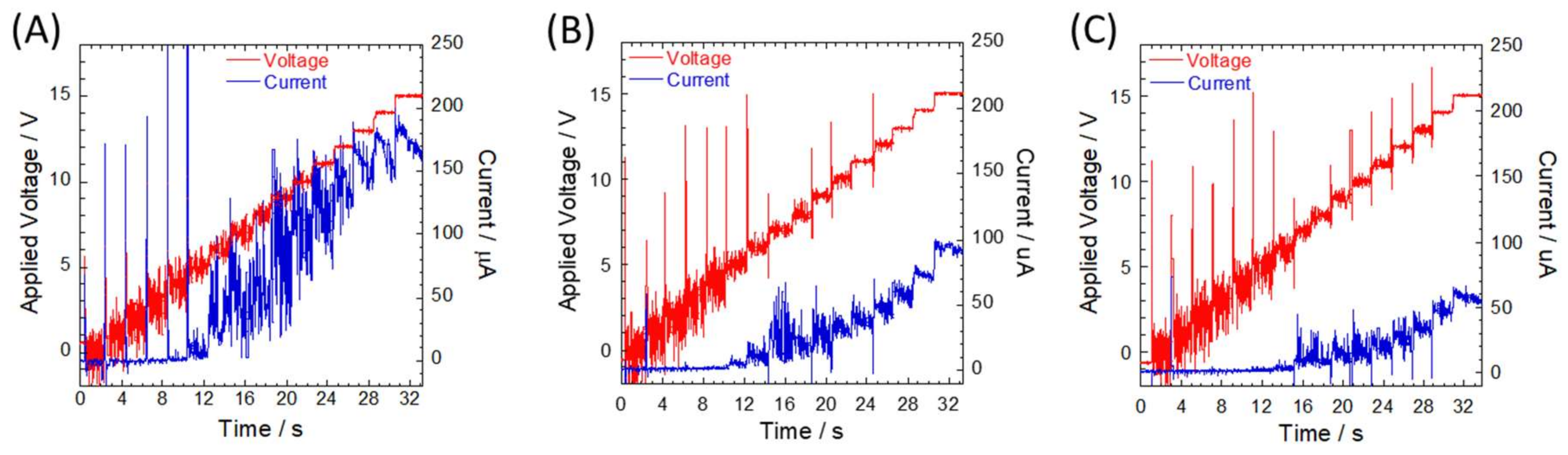
2.2. Current Measurements
2.3. DNA Observation
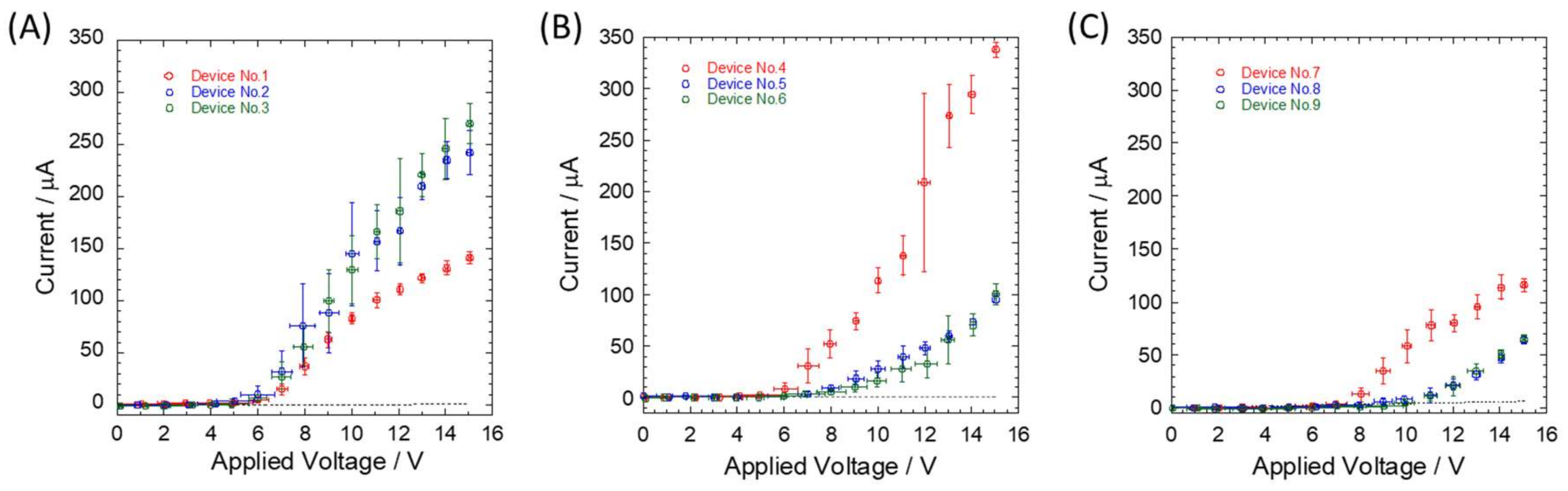
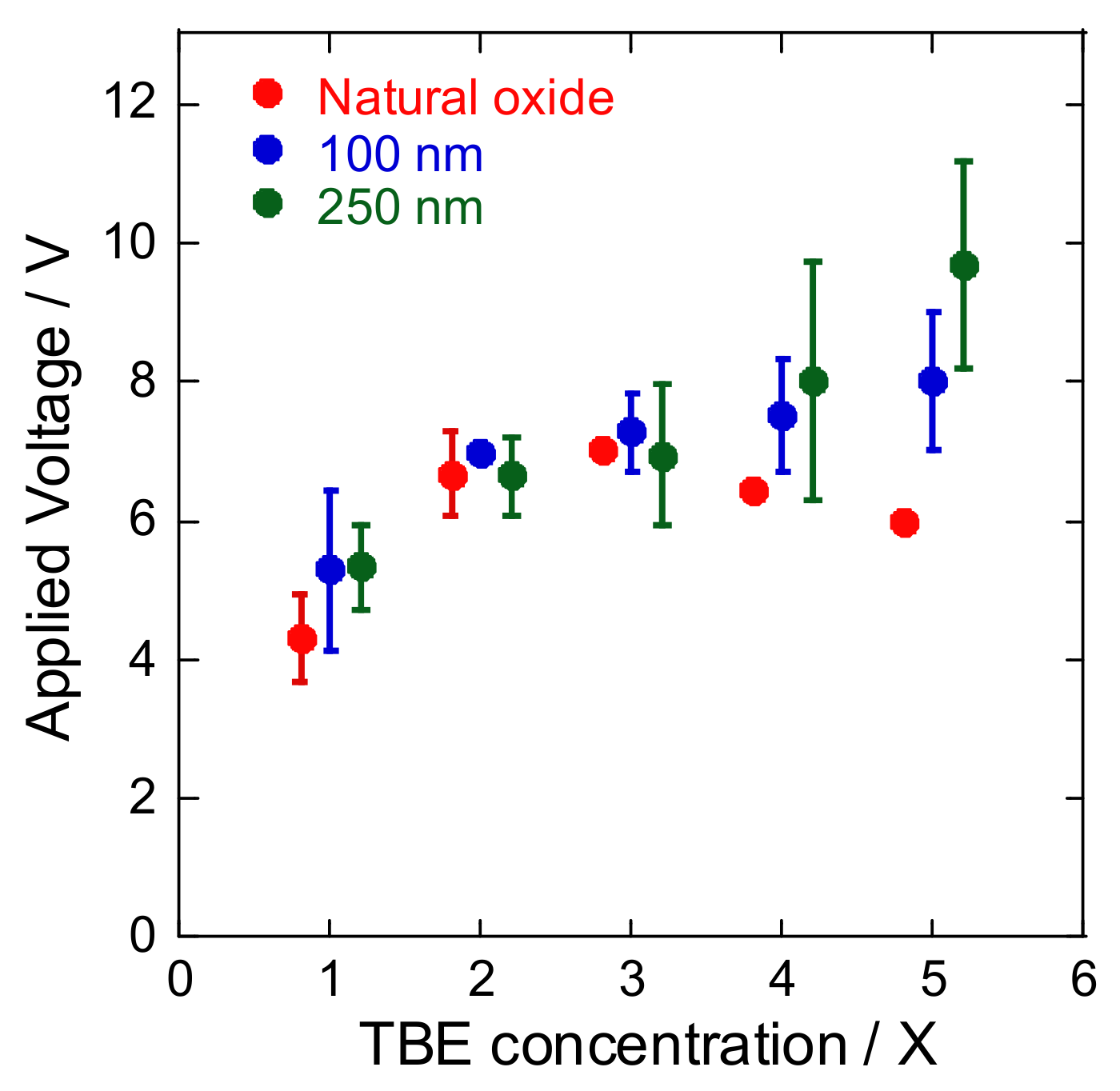
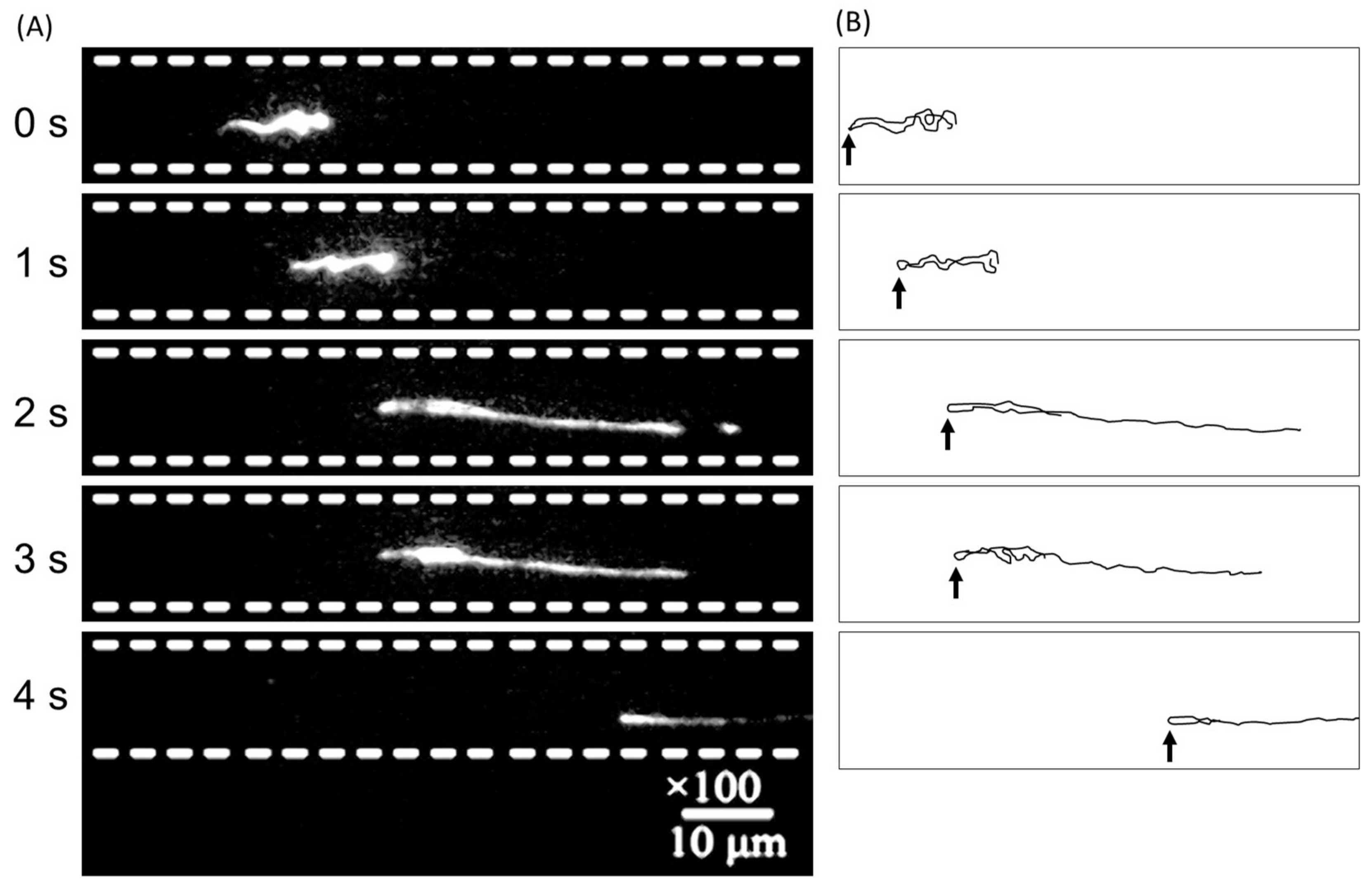
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harrison, D.J.; Glavina, P.G.; Manz, A. Towards miniaturized electrophoresis and chemical-analysis systems on silicon—An alternative to chemical sensors. Sens. Actuators B Chem. 1993, 10, 107–116. [Google Scholar] [CrossRef]
- Xia, Q.; Morton, K.J.; Austin, R.H.; Chou, S.Y. Sub-10 nm self-enclosed self-limited nanofluidic channel arrays. Nano Lett. 2008, 8, 3830–3833. [Google Scholar] [CrossRef] [PubMed]
- Kutchoukov, V.G.; Laugere, F.; van der Vlist, W.; Pakula, L.; Garini, Y.; Bossche, A. Fabrication of nanofluidic devices using glass-to-glass anodic bonding. Sens. Actuators A Phys. 2004, 114, 521–527. [Google Scholar] [CrossRef]
- Fu, J.; Mao, P.; Han, J. Continuous-flow bioseparation using microfabricated anisotropic nanofluidic sieving structures. Nat. Protoc. 2009, 4, 1681–1698. [Google Scholar] [CrossRef] [PubMed]
- Bakajin, O.; Duke, T.A.J.; Tegenfeldt, J.; Chou, C.F.; Chan, S.S.; Austin, R.H.; Cox, E.C. Separation of 100-kilobase DNA molecules in 10 seconds. Anal. Chem. 2001, 73, 6053–6056. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Mao, P.; Han, J. A nanofilter array chip for fast gel-free biomolecule separation. Appl. Phys. Lett. 2005, 87, 263902. [Google Scholar] [CrossRef] [PubMed]
- Hjerten, S.; Wu, B.L. Studies of fish zona pellucida by high-performance ion-exchange chromatography on agarose columns and free zone electrophoresis. J. Chromatogr. 1985, 341, 295–304. [Google Scholar] [CrossRef]
- Han, J.; Craighead, H.G. Entropic trapping and sieving of long DNA molecules in a nanofluidic channel. J. Vac. Sci. Technol. A Vac. Surf. Films 1999, 17, 2142–2147. [Google Scholar] [CrossRef]
- Kaji, N.; Oki, A.; Ogawa, R.; Takamura, Y.; Nishimoto, T.; Nakanishi, H.; Horiike, Y.; Tokeshi, M.; Baba, Y. Influences of electroosmotic flows in nanopillar chips on DNA separation: Experimental results and numerical simulations. Isr. J. Chem. 2007, 47, 161–169. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Di Ventra, M.; Taniguchi, M. Decoding DNA, RNA and peptides with quantum tunnelling. Nat. Nanotechnol. 2016, 11, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.-Q.; Lee, P.W.; Latchford, I.; Narwankar, P.K.; Urdahl, R.; Nickeles, A.S.; Achutharaman, R.; Lewis, C.B. Chemical vapor deposition. In Handbook of Semiconductor Manugacturing Technology, 2nd ed.; Nshi, Y., Doering, R., Eds.; CRC Press: New York, NY, USA, 2007; pp. 309–356. [Google Scholar]
- Gerlach, A.; Maas, D.; Seidel, D.; Bartuch, H.; Schundau, S.; Kaschlik, K. Low-temperature anodic bonding of silicon to silicon wafers by means of intermediate glass layers. Microsyst. Technol. 1999, 5, 144–149. [Google Scholar] [CrossRef]
- Wang, H.Y.; Foote, R.S.; Jacobson, S.C.; Schneibel, J.H.; Ramsey, J.M. Low temperature bonding for microfabrication of chemical analysis devices. Sens. Actuators B Chem. 1997, 45, 199–207. [Google Scholar] [CrossRef]
- Kwok, H.; Briggs, K.; Tabard-Cossa, V. Nanopore fabrication by controlled dielectric breakdown. PLoS ONE 2014, 9, e92880. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, I.; Akahori, R.; Hatano, T.; Takeda, K.-I. Fabricating nanopores with diameters of sub-1 nm to 3 nm using multilevel pulse-voltage injection. Sci. Rep. 2014, 4, 5000. [Google Scholar] [CrossRef] [PubMed]
- Gohma, H.; Hiroyasu, M.; Kaji, N.; Katayama, S.; Sekijima, M.; Yamashita, Y. Effects of methotrexate and indomethacin on hind paw swelling and serum levels of inflammatory cytokines in collagen-induced arthritic rats. J. Pharmacol. Sci. 2007, 103, 252p. [Google Scholar]
- Datta, A.; Gangopadhyay, S.; Temkin, H.; Pu, Q.; Liu, S. Nanofluidic channels by anodic bonding of amorphous silicon to glass to study ion-accumulation and ion-depletion effect. Talanta 2006, 68, 659–665. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, M.; Kaji, N.; Wu, Q.; Naito, T.; Yasui, T.; Taniguchi, M.; Kawai, T.; Baba, Y. Quantitative Evaluation of Dielectric Breakdown of Silicon Micro- and Nanofluidic Devices for Electrophoretic Transport of a Single DNA Molecule. Micromachines 2018, 9, 180. https://doi.org/10.3390/mi9040180
Sano M, Kaji N, Wu Q, Naito T, Yasui T, Taniguchi M, Kawai T, Baba Y. Quantitative Evaluation of Dielectric Breakdown of Silicon Micro- and Nanofluidic Devices for Electrophoretic Transport of a Single DNA Molecule. Micromachines. 2018; 9(4):180. https://doi.org/10.3390/mi9040180
Chicago/Turabian StyleSano, Mamiko, Noritada Kaji, Qiong Wu, Toyohiro Naito, Takao Yasui, Masateru Taniguchi, Tomoji Kawai, and Yoshinobu Baba. 2018. "Quantitative Evaluation of Dielectric Breakdown of Silicon Micro- and Nanofluidic Devices for Electrophoretic Transport of a Single DNA Molecule" Micromachines 9, no. 4: 180. https://doi.org/10.3390/mi9040180




