Characterization of Cotton Ball-like Au/ZnO Photocatalyst Synthesized in a Micro-Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedure
2.2. Characterization
2.3. Photocatalytic Test
3. Results and Discussion
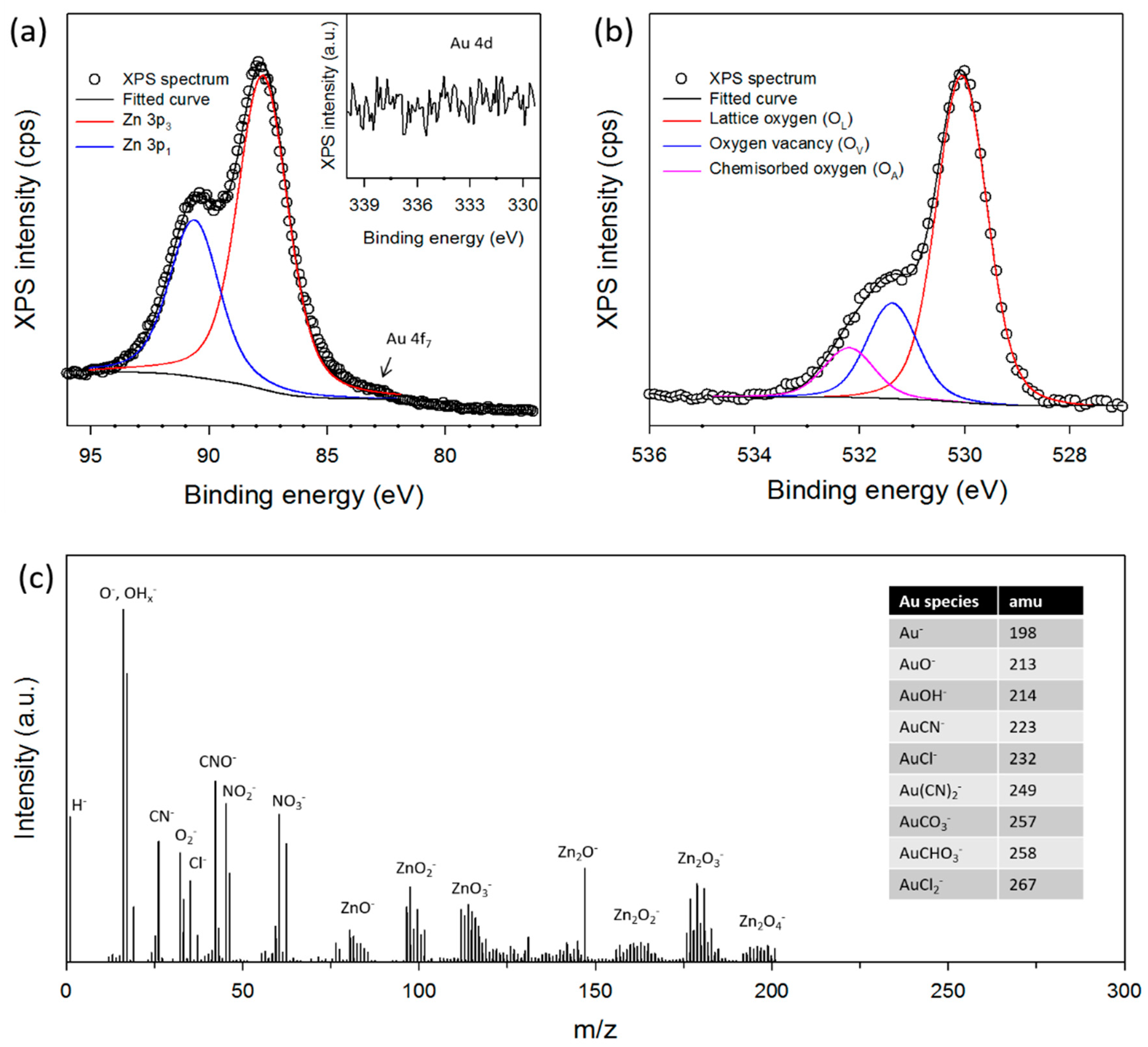
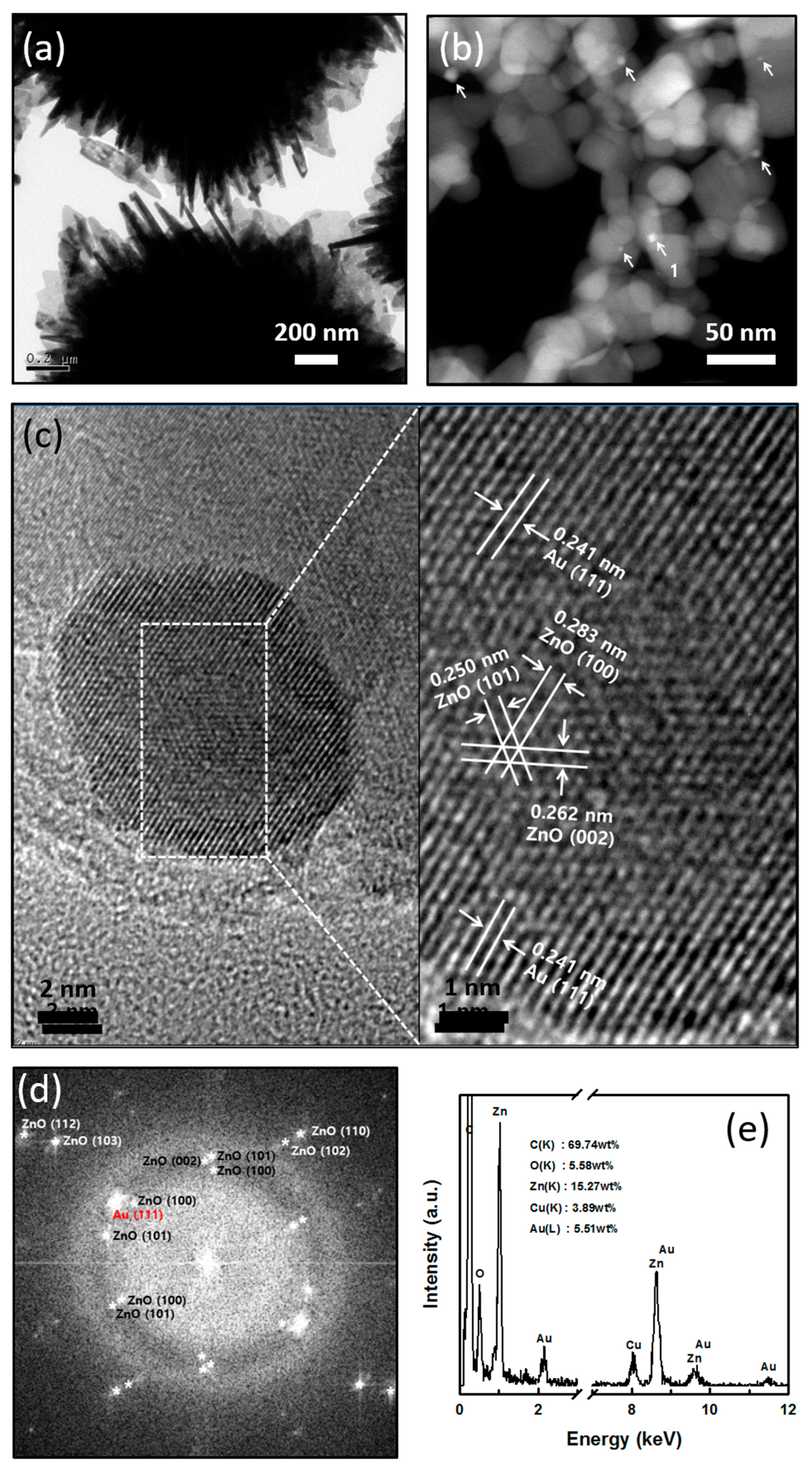
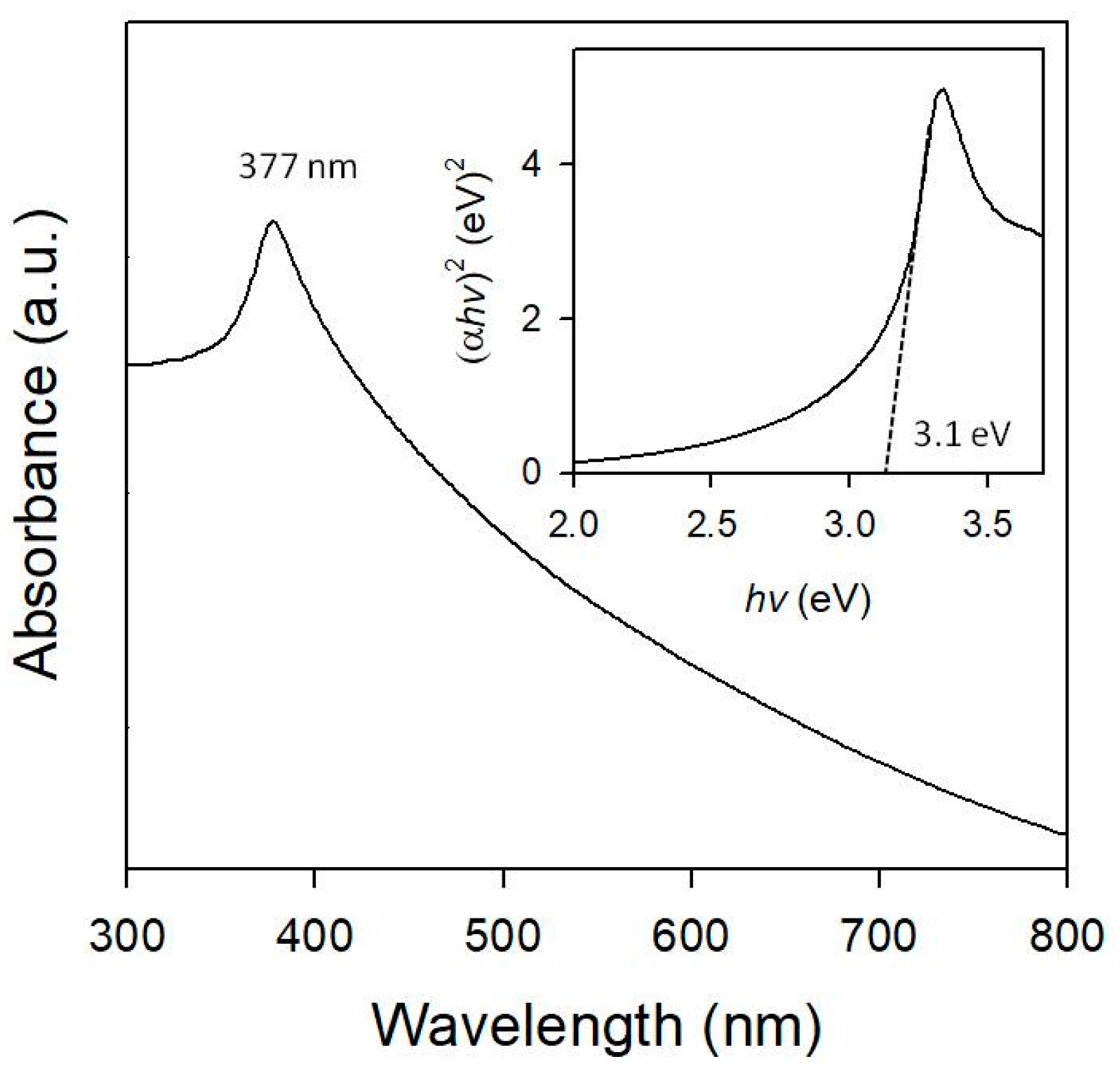
3.1. Characteristics of Au/ZnO
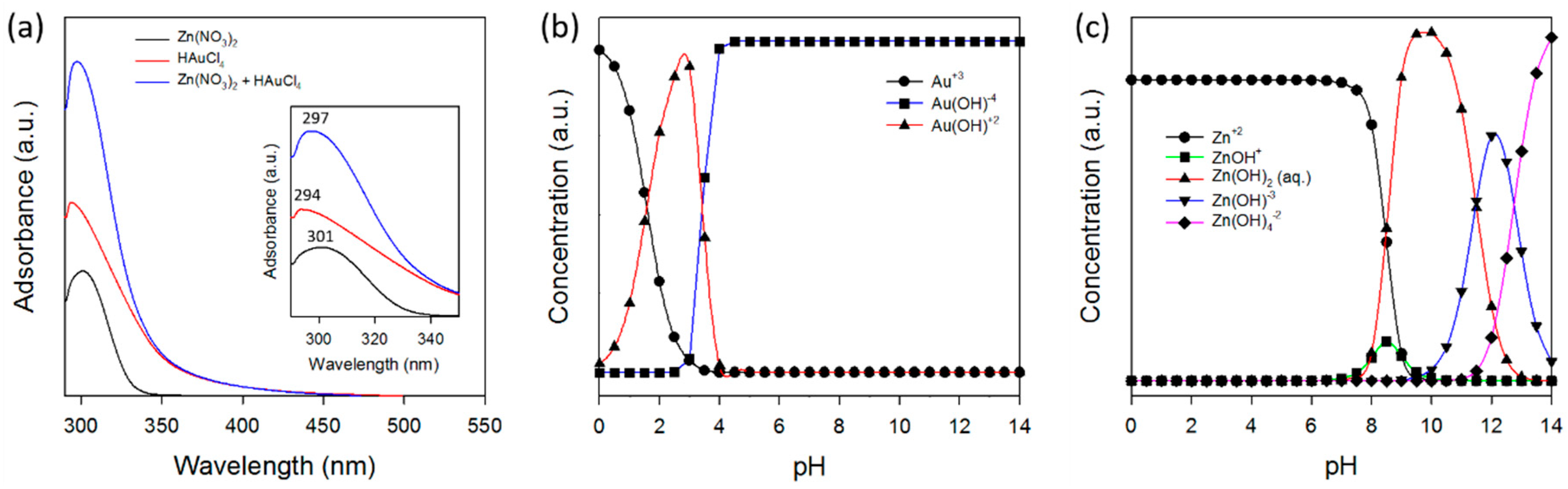
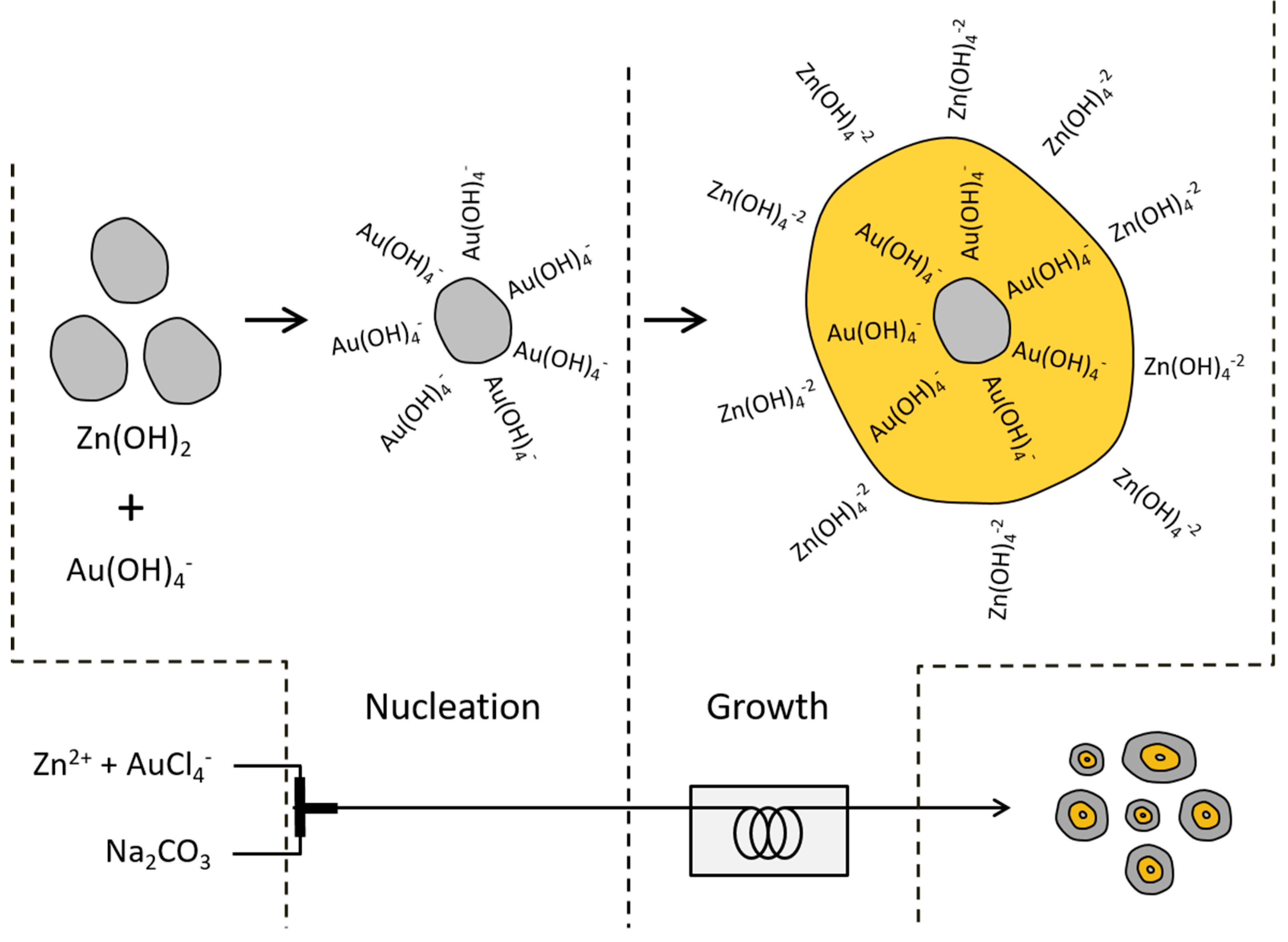
3.2. Formation Pathway of Core-Shell Au/ZnO
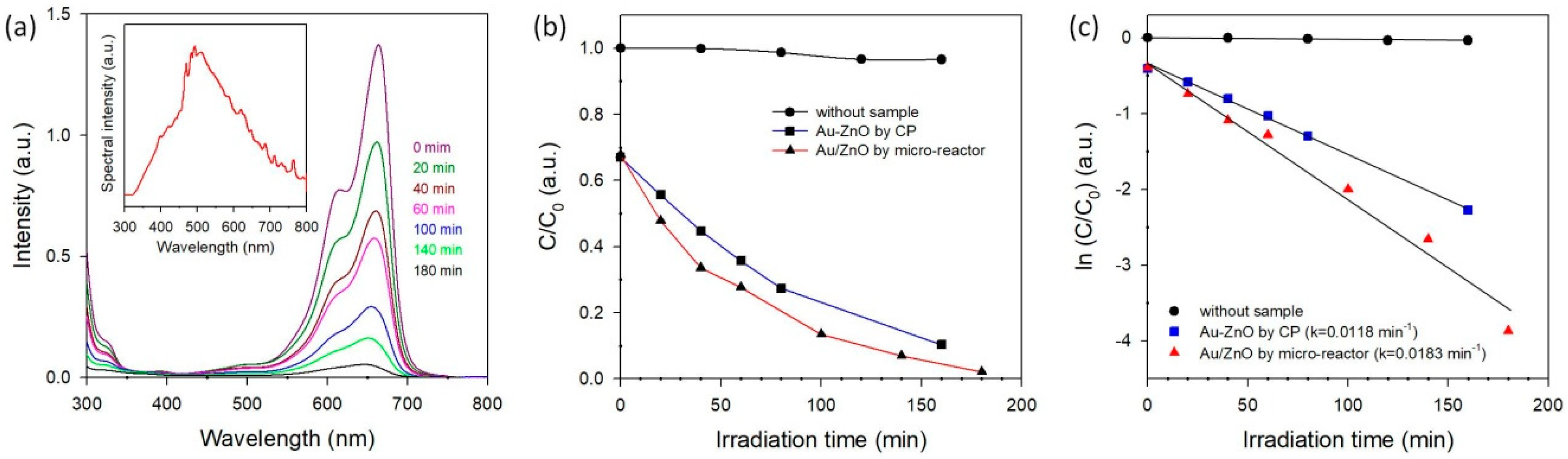
3.3. Photocatalytic Activity of Methylene Blue Degradation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kochuveedu, S.T.; Kim, D.-P.; Kim, D.H. Surface-plasmon-induced visible light photocatalytic activity of TiO2 nanospheres decorated by Au nanoparticles with controlled configuration. J. Phys. Chem. C 2012, 116, 2500–2506. [Google Scholar] [CrossRef]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au−ZnO hybrid nanopyramids and their photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Udawatte, N.; Lee, M.; Kim, J.; Lee, D. Well-defined Au/ZnO nanoparticle composites exhibiting enhanced photocatalytic activities. ACS Appl. Mater. Interfaces 2011, 3, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Pawinrat, P.; Mekasuwandumrong, O.; Panpranot, J. Synthesis of Au–ZnO and Pt–ZnO nanocomposites by one-step flame spray pyrolysis and its application for photocatalytic degradation of dyes. Catal. Commun. 2009, 10, 1380–1385. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, B.; Wang, S. ZnO/Au hybrid nanoarchitectures: Wet-chemical synthesis and structurally enhanced photocatalytic performance. Environ. Sci. Technol. 2009, 43, 8968–8973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Zhang, J.; Chen, F. Enhanced photocatalytic activity of nitrogen-doped titania by deposited with gold. J. Phys. Chem. C 2009, 113, 14689–14695. [Google Scholar] [CrossRef]
- Lee, M.-K.; Tu, H.-F. Au–ZnO and Pt–ZnO films prepared by electrodeposition as photocatalysts. J. Electrochem. Soc. 2008, 155, D758–D762. [Google Scholar] [CrossRef]
- Wu, J.-J.; Tseng, C.-H. Photocatalytic properties of nc-Au/ZnO nanorod composites. Appl. Catal. B Environ. 2006, 66, 51–57. [Google Scholar] [CrossRef]
- Yang, T.-T.; Chen, W.-T.; Hsu, Y.-J.; Wei, K.-H.; Lin, T.-Y.; Lin, T.-W. Interfacial charge carrier dynamics in core−shell Au-CdS nanocrystals. J. Phys. Chem. C 2010, 114, 11414–11420. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Chen, F.; He, D.; Anpo, M. Preparation and photocatalytic properties of Fe3+-doped Ag@TiO2 core–shell nanoparticles. J. Colloid Interface Sci. 2008, 323, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2 core−shell composite clusters under UV−Irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-X.; He, L.; Qiao, S.Z.; Middelberg, A.P.J. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Tao, S.; Yang, M.; Chen, H.; Zhao, S.; Chen, G. Continuous synthesis of Ag/AgCl/ZnO composites using flow chemistry and photocatalytic application. Ind. Eng. Chem. Res. 2018, 57, 3263–3273. [Google Scholar] [CrossRef]
- Tao, S.; Yang, M.; Chen, H.; Ren, M.; Chen, G. Continuous synthesis of hedgehog-like Ag–ZnO nanoparticles in a two-stage microfluidic system. RSC Adv. 2016, 6, 45503–45511. [Google Scholar] [CrossRef]
- Wojnicki, M.; Luty-Blocho, M.; Hessel, V.; Csapo, E.; Ungor, D.; Fitzner, K. Micro droplet formation towards continuous nanoparticles synthesis. Micromachines 2018, 9, 248. [Google Scholar] [CrossRef]
- Lummiss, J.A.M.; Morse, P.D.; Beingessner, R.L.; Jamison, T.F. Towards more efficient, greener syntheses through flow chemistry. Chem. Rec. 2017, 17, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, S.; Khan, S. Droplet-based microfluidic synthesis of anisotropic metal nanocrystals. Small 2009, 5, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, V.S.; Kuhn, S.; Kulkarni, A.; Jensen, K. Size-controlled flow synthesis of gold nanoparticles using a segmented flow microfluidic platform. Langmuir 2012, 28, 7007–7013. [Google Scholar] [CrossRef] [PubMed]
- Hartlieb, K.; Saunders, M.; Jachuck, R.; Raston, C. Continuous flow synthesis of small silver nanoparticles involving hydrogen as the reducing agent. Green Chem. 2010, 12, 1012–1017. [Google Scholar] [CrossRef]
- Ravi Kumar, D.V.; Prasad, B.L.V.; Kulkarni, A.A. Segmented flow synthesis of Ag nanoparticles in spiral microreactor: Role of continuous and dispersed phase. Chem. Eng. J. 2012, 192, 357–368. [Google Scholar] [CrossRef]
- Du Toit, H.; Macdonald, T.J.; Huang, H.; Parkin, I.P.; Gavriilidis, A. Continuous flow synthesis of citrate capped gold nanoparticles using uv induced nucleation. RSC Adv. 2017, 7, 9632–9638. [Google Scholar] [CrossRef]
- Li, S.; Gross, G.A.; Günther, P.M.; Köhler, J.M. Hydrothermal micro continuous-flow synthesis of spherical, cylinder-, star- and flower-like ZnO microparticles. Chem. Eng. J. 2011, 167, 681–687. [Google Scholar] [CrossRef]
- Takagi, M.; Maki, T.; Miyahara, M.; Mae, K. Production of titania nanoparticles by using a new microreactor assembled with same axle dual pipe. Chem. Eng. J. 2004, 101, 269–276. [Google Scholar] [CrossRef]
- Song, Y.; Henry, L. Nearly monodispersion CoSm alloy nanoparticles formed by an in-situ rapid cooling and passivating microfluidic process. Nanoscale Res. Lett. 2009, 4, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kim, K.-J.; Chang, C.-H. Continuous, size and shape-control synthesis of hollow silica nanoparticles enabled by a microreactor-assisted rapid mixing process. Nanotechnology 2017, 28, 235602. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.; Günther, A.; Schmidt, M.; Jensen, K.; Bawendi, M. A microfabricated gas-liquid segmented flow reactor for high-temperature synthesis: The case of CdSe quantum dots. Angew. Chem. 2005, 117, 5583–5587. [Google Scholar] [CrossRef]
- Hostetler, E.B.; Kim, K.-J.; Oleksak, R.P.; Fitzmorris, R.C.; Peterson, D.A.; Chandran, P.; Chang, C.-H.; Paul, B.K.; Schut, D.M.; Herman, G.S. Synthesis of colloidal PbSe nanoparticles using a microwave-assisted segmented flow reactor. Mater. Lett. 2014, 128, 54–59. [Google Scholar] [CrossRef]
- Kim, K.-J.; Oleksak, R.P.; Hostetler, E.B.; Peterson, D.A.; Chandran, P.; Schut, D.M.; Paul, B.K.; Herman, G.S.; Chang, C.-H. Continuous microwave-assisted gas-liquid segmented flow reactor for controlled nucleation and growth of nanocrystals. Cryst. Growth Des. 2014, 14, 5349–5355. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep continuous-flow microsynthesis of magnetic and fluorescent γ-Fe2O3@SiO2 core/shell nanoparticles. Angew. Chem. 2009, 121, 7316–7319. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Yang, C.-H.; Huang, K.-S. Microfluidic assisted preparation of CdSe/ZnS nanocrystals encapsulated into poly(dl-lactide-co-glycolide) microcapsules. Nanotechnology 2007, 18, 305305. [Google Scholar] [CrossRef]
- Chang, C.-H.; Paul, B.K.; Remcho, V.T.; Atre, S.; Hutchison, J.E. Synthesis and post-processing of nanomaterials using microreaction technology. J. Nanopart. Res. 2008, 10, 965–980. [Google Scholar] [CrossRef]
- Kim, K.-J.; Pan, C.; Bansal, S.; Malhotra, R.; Kim, D.-H.; Chang, C.-H. Scalably synthesized environmentally benign, aqueous-based binary nanoparticle inks for Cu2ZnSn(S,Se)4 photovoltaic cells achieving over 9% efficiency. Sustain. Energy Fuels 2017, 1, 267–274. [Google Scholar] [CrossRef]
- Haldar, K.K.; Sen, T.; Patra, A. Au@ZnO core−shell nanoparticles are efficient energy acceptors with organic dye donors. J. Phys. Chem. C 2008, 112, 11650–11656. [Google Scholar] [CrossRef]
- Sun, L.; Wei, G.; Song, Y.; Liu, Z.; Wang, L.; Li, Z. Solution-phase synthesis of Au@ZnO core-shell composites. Mater. Lett. 2006, 60, 1291–1295. [Google Scholar] [CrossRef]
- Han, X.; Qin, W.-J.; Sun, J.; Yang, J.; Niu, K.-Y.; Wang, H.-L.; Du, X.-W. Surfactant-assisted synthesis of ZnO–Au nanostructure with good dispersibility. J. Alloys Compd. 2009, 477, 661–664. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Y.; Li, J.; Ma, J.; Shi, D.; Chen, J.; Yang, J. Fabrication of hierarchical core-shell Au@ZnO heteroarchitectures initiated by heteroseed assembly for photocatalytic applications. J. Colloid Interface Sci. 2014, 418, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, H.-B.; Zheng, Y.-Z.; Ye, R.; Tao, X.; Chen, J.-F. Controllable assembly of well-defined monodisperse Au nanoparticles on hierarchical ZnO microspheres for enhanced visible-light-driven photocatalytic and antibacterial activity. Nanoscale 2015, 7, 19118–19128. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Yingying, S.; Nisar, A.; Sun, H.; Shen, W.; Wei, M.; Zhu, J. Synthesis of hierarchical flower-like ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high performance Li-ion battery anodes. J. Mater. Chem. 2011, 21, 7723–7729. [Google Scholar] [CrossRef]
- Han, Z.; Wei, L.; Zhang, Z.; Zhang, X.; Pan, H.; Chen, J. Visible-light photocatalytic application of hierarchical Au-ZnO flower-rod heterostructures via surface Plasmon resonance. Plasmonics 2013, 8, 1193–1202. [Google Scholar] [CrossRef]
- Qu, X.; Yang, R.; Tong, F.; Zhao, Y.; Wang, M.-H. Hierarchical ZnO microstructures decorated with Au nanoparticles for enhanced gas sensing and photocatalytic properties. Powder Technol. 2018, 330, 259–265. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kreider, P.B.; Choi, C.; Chang, C.-H.; Ahn, H.-G. Visible-light-sensitive Na-doped p-type flower-like ZnO photocatalysts synthesized via a continuous flow microreactor. RSC Adv. 2013, 3, 12702–12710. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kreider, P.B.; Chang, C.-H.; Park, C.-M. Visible-light-sensitive nanoscale Au–ZnO photocatalysts. J. Nanopart. Res. 2013, 15, 1606. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Chen, R.; Zhou, D.; Xiang, L. A review on the fabrication of hierarchical ZnO nanostructures for photocatalysis application. Crystals 2016, 6, 148. [Google Scholar] [CrossRef]
- Qian, K.; Huang, W.; Fang, J.; Lv, S.; He, B.; Jiang, Z.; Wei, S. Low-temperature CO oxidation over Au/ZnO/SiO2 catalysts: Some mechanism insights. J. Catal. 2008, 255, 269–278. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Gu, G.; Wu, L. A Facile method to fabricate ZnO hollow spheres and their photocatalytic property. J. Phys. Chem. B 2008, 112, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.-S.; Wang, C.-M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A Mater. Sci. Process. 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Pandey, B.; Ghosh, S.; Srivastava, P.; Kabiraj, D.; Shripati, T.; Lalla, N.P. Synthesis of nanodimensional ZnO and Ni-doped ZnO thin films by atom beam sputtering and study of their physical properties. Phys. E 2009, 41, 1164–1168. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Zhang, Y.; Ma, S.; Xin, L.; Zhu, L.; Xu, C. Optical properties and photocatalytic performances of Pd modified ZnO samples. J. Phys. Chem. C 2009, 113, 18761–18767. [Google Scholar] [CrossRef]
- Chen, W.-T.; Yang, T.-T.; Hsu, Y.-J. Au-CdS core−shell nanocrystals with controllable shell thickness and photoinduced charge separation property. Chem. Mater. 2008, 20, 7204–7206. [Google Scholar] [CrossRef]
- Wang, X.; Kong, X.; Yu, Y.; Zhang, H. Synthesis and characterization of water-soluble and bifunctional ZnO−Au nanocomposites. J. Phys. Chem. C 2007, 111, 3836–3841. [Google Scholar] [CrossRef]
- Yu, X.; Shavel, A.; An, X.; Luo, Z.; Ibáñez, M.; Cabot, A. Cu2ZnSnS4-Pt and Cu2ZnSnS4-Au heterostructured nanoparticles for photocatalytic water splitting and pollutant degradation. J. Am. Chem. Soc. 2014, 136, 9236–9239. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Weller, H.; Henglein, A. Photochemistry and radiation chemistry of colloidal semiconductors. 23. Electron storage on zinc oxide particles and size quantization. J. Phys. Chem. 1988, 92, 482–487. [Google Scholar] [CrossRef]
- Roig, B.; Theraulaz, F.; Thomas, O. UV recovery of organic pollution parameters: Mineral constituents. In UV-Visible Spectrophotometry of Water and Wastewater, 1st ed.; Thomas, O., Burgess, C., Eds.; Elsevier: Amsterdam, The Netherland, 2007; Volume 27, pp. 115–144. ISBN 9780444530929. [Google Scholar]
- Ivanova, S.; Pitchon, V.; Petit, C.; Herschbach, H.; Dorsselaer, A.V.; Leize, E. Preparation of alumina supported gold catalysts: Gold complexes genesis, identification and speciation by mass spectrometry. Appl. Catal. A Gener. 2006, 298, 203–210. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.-Y.; Zhao, J.-C.; Zheng, Z.-F.; Gao, X.-P. Visible-light-driven oxidation of organic contaminants in air with gold nanoparticle catalysts on oxide supports. Angew. Chem. 2008, 47, 5353–5356. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Corma, A.; García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R. The use of gold nanoparticles in diagnostics and detection. Chem. Soc. Rev. 2008, 37, 2028–2045. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-J.; Kreider, P.B.; Ahn, H.-G.; Chang, C.-H. Characterization of Cotton Ball-like Au/ZnO Photocatalyst Synthesized in a Micro-Reactor. Micromachines 2018, 9, 322. https://doi.org/10.3390/mi9070322
Kim K-J, Kreider PB, Ahn H-G, Chang C-H. Characterization of Cotton Ball-like Au/ZnO Photocatalyst Synthesized in a Micro-Reactor. Micromachines. 2018; 9(7):322. https://doi.org/10.3390/mi9070322
Chicago/Turabian StyleKim, Ki-Joong, Peter B. Kreider, Ho-Geun Ahn, and Chih-Hung Chang. 2018. "Characterization of Cotton Ball-like Au/ZnO Photocatalyst Synthesized in a Micro-Reactor" Micromachines 9, no. 7: 322. https://doi.org/10.3390/mi9070322




