Core-Shell Structures of Upconversion Nanocrystals Coated with Silica for Near Infrared Light Enabled Optical Imaging of Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. NaYF4:Yb/Er Nanocrystals
2.2.2. UCNC@SiO2 Core-Shell Nanostructures
2.3. Instrumentation
2.4. Cell Viability Assessment
2.5. Cancer Cell Imaging
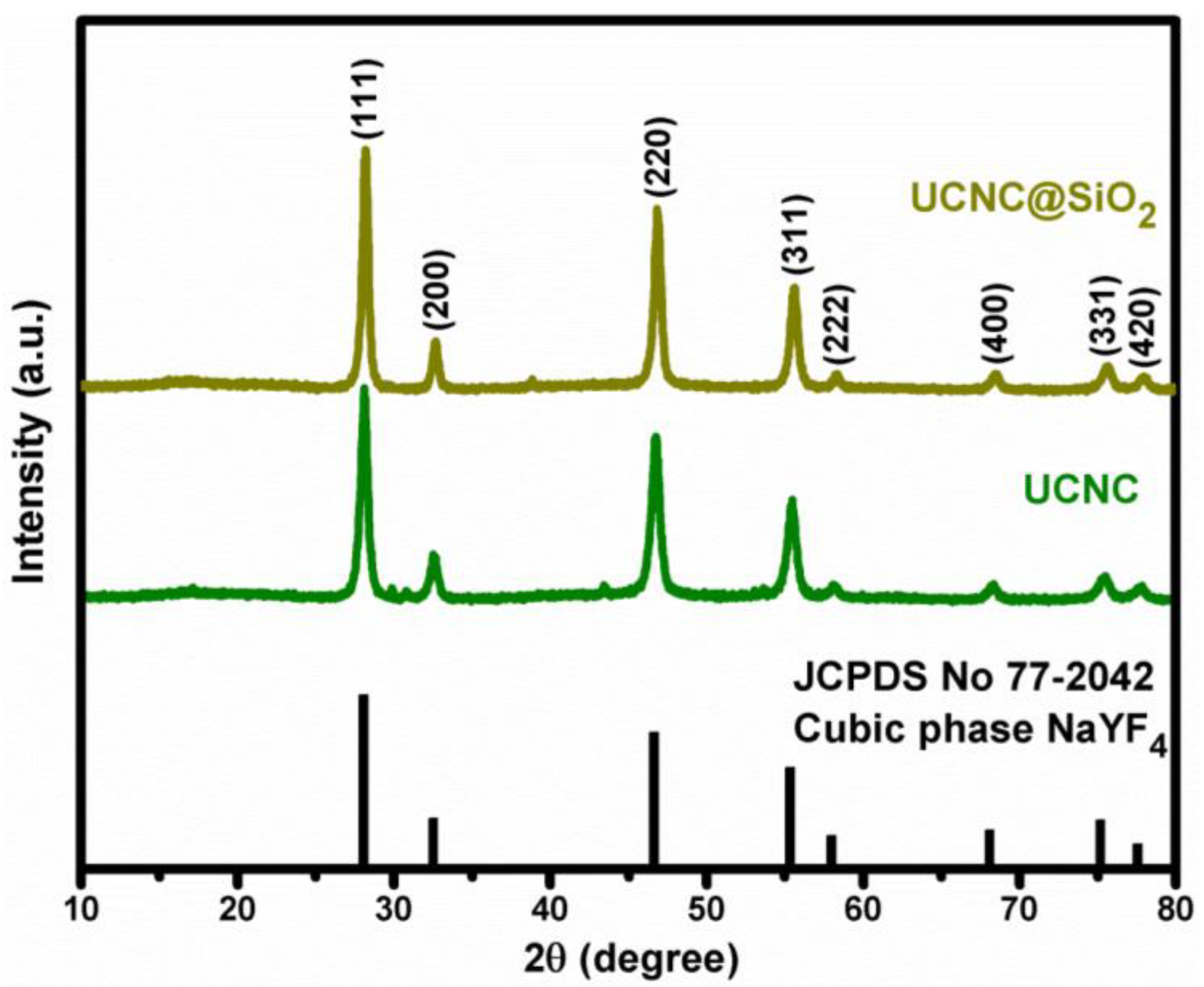
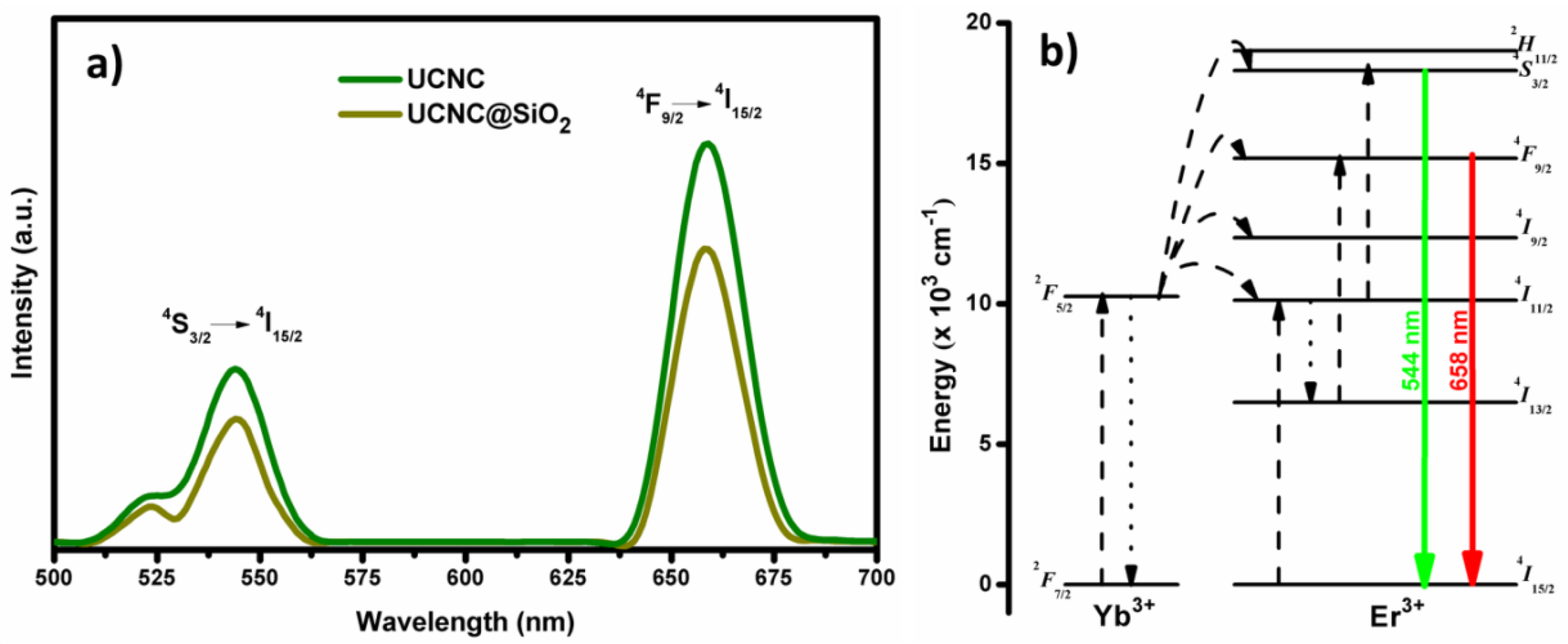
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Jiang, S. Multicolor core/shell-structured upconversion fluorescent nanoparticles. Adv. Mater. 2008, 20, 4765–4769. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin.Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Kobayashi, H.; Hama, Y.; Koyama, Y.; Bernardo, M.; Nagano, T.; Choyke, P.L.; Urano, Y. An enzymatically activated fluorescence probe for targeted tumor imaging. J. Am. Chem. Soc. 2007, 129, 3918–3929. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I. Review: Fluorescent probes for living cells. Histochem. J. 1998, 30, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Dean, K.M.; Palmer, A.E. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat. Chem. Biol. 2014, 10, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Jańczewski, D.; Zhang, Y.; Das, G.K.; Yi, D.K.; Padmanabhan, P.; Bhakoo, K.K.; Tan, T.T.Y.; Selvan, S.T. Bimodal magnetic–fluorescent probes for bioimaging. Microsc. Res. Tech. 2011, 74, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.N.; Pedroni, M.; Piccinelli, F.; Conti, G.; Sbarbati, A.; Ramirez-Hernandez, J.E.; Maestro, L.M.; Iglesias-de la Cruz, M.C.; Sanz-Rodriguez, F.; Juarranz, A.; et al. Nir-to-nir two-photon excited caf2:Tm3+,yb3+ nanoparticles: Multifunctional nanoprobes for highly penetrating fluorescence bio-imaging. ACS Nano 2011, 5, 8665–8671. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, W.; Teoh, C.L.; Han, S.; Kim, B.; Samanta, A.; Er, J.C.; Wang, L.; Yuan, L.; Liu, X.; et al. High-efficiency in vitro and in vivo detection of zn2+ by dye-assembled upconversion nanoparticles. J. Am. Chem. Soc. 2015, 137, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Fiorini, S.; Tambalo, S.; Busquier, H.; Callejas-Fernández, J.; Marzola, P.; Gálvez, N.; Domínguez-Vera, J.M. In vivo long-term magnetic resonance imaging activity of ferritin-based magnetic nanoparticles versus a standard contrast agent. J. Med. Chem. 2014, 57, 5686–5692. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Li, J.; Liu, F.; Padmanabhan, P.; Yeow, E.; Xing, B. Recent advance of biological molecular imaging based on lanthanide-doped upconversion-luminescent nanomaterials. Nanomaterials 2014, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.L.; Rai, M.; Prabhakar, N.; Arppe, R.; Rai, S.B.; Singh, S.K.; Rosenholm, J.M.; Krishnan, V. Controlled synthesis, bioimaging and toxicity assessments in strong red emitting mn2+ doped nayf4:Yb3+/ho3+ nanophosphors. RSC Adv. 2016, 6, 53698–53704. [Google Scholar] [CrossRef]
- Yan, C.; Dadvand, A.; Rosei, F.; Perepichka, D.F. Near-ir photoresponse in new up-converting cdse/nayf4:Yb,er nanoheterostructures. J. Am. Chem. Soc. 2010, 132, 8868–8869. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.-C.; Cuccia, L.A.; Capobianco, J.A. Synthesis of colloidal upconverting nayf4: Er3+/yb3+ and tm3+/yb3+ monodisperse nanocrystals. Nano Lett. 2007, 7, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.-C.; Carling, C.-J.; Gates, B.D.; Branda, N.R. Two-way photoswitching using one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity. J. Am. Chem. Soc. 2010, 132, 15766–15772. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of ligand-free colloidally stable water dispersible brightly luminescent lanthanide-doped upconverting nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Z.; Liu, Z.; Yin, M.; Ren, J.; Qu, X. One-step nucleotide-programmed growth of porous upconversion nanoparticles: Application to cell labeling and drug delivery. Nanoscale 2014, 6, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Chou, Y.-L.; Wang, S.-W.; Hung, S.-T.; Liau, M.-C.; Chao, Y.-J.; Su, C.-H.; Yeh, C.-S. Near-infrared light photocontrolled targeting, bioimaging, and chemotherapy with caged upconversion nanoparticles in vitro and in vivo. ACS Nano 2013, 7, 8516–8528. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Liao, M.-L.; Hong, G.-C.; Chang, W.-W.; Chu, C.-C. Near-infrared-triggered photodynamic therapy toward breast cancer cells using dendrimer-functionalized upconversion nanoparticles. Nanomaterials 2017, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhou, S.; Chen, Z.; Hu, P.; Liu, Y.; Tu, D.; Zhu, H.; Li, R.; Huang, M.; Chen, X. Sub-10 nm lanthanide-doped caf2 nanoprobes for time-resolved luminescent biodetection. Angew. Chem. Int. Ed. 2013, 52, 6671–6676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, M.; Sun, Y.; Zhang, X.; Zhu, X.; Wu, Z.; Wu, D.; Li, F. Fluorine-18-labeled gd3+/yb3+/er3+ co-doped nayf4 nanophosphors for multimodality pet/mr/ucl imaging. Biomaterials 2011, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Sun, L.; Ma, B.; Jin, D.; Dong, L.; Shi, L.; Li, N.; Chen, H.; Huang, W. Simultaneous realization of hg2+ sensing, magnetic resonance imaging and upconversion luminescence in vitro and in vivo bioimaging based on hollow mesoporous silica coated ucnps and ruthenium complex. Nanoscale 2015, 7, 13877–13887. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Dai, Y.; Yang, Q.; Zhang, Y.; Yang, P.; Cheng, Z.; Lian, H.; Li, C.; Hou, Z.; et al. Efficient gene delivery and multimodal imaging by lanthanide-based upconversion nanoparticles. Langmuir 2014, 30, 13042–13051. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lei, P.; Zhang, P.; Wang, Z.; Song, S.; Xu, X.; Liu, X.; Feng, J.; Zhang, H. Growth of lanthanide-doped ligdf4 nanoparticles induced by liluf4 core as tri-modal imaging bioprobes. Biomaterials 2015, 65, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; La, H.; Zhu, R.; El-Banna, G.; Wei, Y.; Han, G. Upconverting nir photons for bioimaging. Nanomaterials 2015, 5, 2148. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal nanostructures for non-enzymatic glucose sensing. Mate. Sci. Eng. C 2017, 70, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.; Zhang, S.Y.; Lim, S.H.; Bosman, M.; Zhang, Z.; Win, K.Y.; Han, M.Y. Ternary cobalt–iron phosphide nanocrystals with controlled compositions, properties, and morphologies from nanorods and nanorice to split nanostructures. Chem. Eur. J. 2011, 17, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.; Tan, H.; Li, S.; Fan, W.Y. Self-organization of spherical, core–shell palladium aggregates by laser-induced and thermal decomposition of [pd (pph3) 4]. Angew. Chem. Int. Ed. 2006, 45, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Gao, H.; Ye, E.; Low, M.; Lim, S.H.; Zhang, S.-Y.; Lieu, X.; Tripathy, S.; Tremel, W.; Han, M.-Y. Graphitically encapsulated cobalt nanocrystal assemblies. Chem. Commun. 2010, 46, 4749–4751. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.; Zhang, S.-Y.; Lim, S.H.; Liu, S.; Han, M.-Y. Morphological tuning, self-assembly and optical properties of indium oxide nanocrystals. Phys. Chem. Chem. Phys. 2010, 12, 11923–11929. [Google Scholar] [CrossRef] [PubMed]
- Lingeshwar Reddy, K.; Balaji, R.; Kumar, A.; Krishnan, V. Lanthanide doped near infrared active upconversion nanophosphors: Fundamental concepts, synthesis strategies, and technological applications. Small 2018, 1801304. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, O.; Thomann, R.; Darbandi, M.; Nann, T. A four-color colloidal multiplexing nanoparticle system. ACS Nano 2008, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Chen, C.-W.; Chan, M.-H.; Chang, Y.-C.; Chang, W.-M.; Chi, L.-H.; Yu, H.-M.; Lin, Y.-F.; Tsai, D.P.; Liu, R.-S.; et al. Mmp2-sensing up-conversion nanoparticle for fluorescence biosensing in head and neck cancer cells. Biosens. Bioelectron. 2016, 80, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase nayf4: Yb, er/tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef] [PubMed]
- Yajuan, S.; Yue, C.; Lijin, T.; Yi, Y.; Xianggui, K.; Junwei, Z.; Hong, Z. Controlled synthesis and morphology dependent upconversion luminescence of nayf 4:Yb, er nanocrystals. Nanotechnology 2007, 18, 275609. [Google Scholar]
- Yi, G.; Lu, H.; Zhao, S.; Ge, Y.; Yang, W.; Chen, D.; Guo, L.-H. Synthesis, characterization, and biological application of size-controlled nanocrystalline nayf4:Yb,er infrared-to-visible up-conversion phosphors. Nano Lett. 2004, 4, 2191–2196. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Z.; Yin, Z.; Song, H.; Xu, W.; Wang, Y.; Zhang, L.; Zhang, H. Self-assembly, highly modified spontaneous emission and energy transfer properties of lapo4:Ce3+, tb3+ inverse opals. Dalton Trans. 2013, 42, 8049–8057. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-x.; Cao, W.-h. Ethanol-assistant solution combustion method to prepare la2o2s:Yb,pr nanometer phosphor. J. Alloys Compd. 2008, 460, 529–534. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Wei, G.; Wang, L.; Zhu, P.; Kim, R.; Zhang, D.; Ding, F.; Zheng, K. Synthesis and upconversion luminescence properties of yf3:Yb3+/tm3+ octahedral nanocrystals. J. Fluorine Chem. 2009, 130, 158–161. [Google Scholar] [CrossRef]
- Dong, B.; Song, H.; Yu, H.; Zhang, H.; Qin, R.; Bai, X.; Pan, G.; Lu, S.; Wang, F.; Fan, L.; et al. Upconversion properties of ln3+ doped nayf4/polymer composite fibers prepared by electrospinning. J. Phys. Chem. C 2008, 112, 1435–1440. [Google Scholar] [CrossRef]
- Qin, X.; Yokomori, T.; Ju, Y. Flame synthesis and characterization of rare-earth (er3+, ho3+, and tm3+) doped upconversion nanophosphors. Appl. Phys. Lett. 2007, 90, 073104. [Google Scholar] [CrossRef]
- Chan, E.M. Combinatorial approaches for developing upconverting nanomaterials: High-throughput screening, modeling, and applications. Chem. Soc. Rev. 2015, 44, 1653–1679. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, L.-D.; Li, Z.-X.; Li, L.-L.; Zhang, J.; Zhang, Y.-W.; Yan, C.-H. Ionic liquid-based route to spherical nayf4 nanoclusters with the assistance of microwave radiation and their multicolor upconversion luminescence. Langmuir 2010, 26, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Nann, T. Monodisperse upconverting nanocrystals by microwave-assisted synthesis. ACS Nano 2009, 3, 3804–3808. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Ying, J.Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Muhr, V.; Wilhelm, S.; Hirsch, T.; Wolfbeis, O.S. Upconversion nanoparticles: From hydrophobic to hydrophilic surfaces. Acc. Chem. Res. 2014, 47, 3481–3493. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Xie, J.; Zhao, B.; Liu, B.; Xu, S.; Ren, N.; Xie, X.; Huang, L.; Huang, W. Rare earth ion-doped upconversion nanocrystals: Synthesis and surface modification. Nanomaterials 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Von Haartman, E.; Jiang, H.; Khomich, A.A.; Zhang, J.; Burikov, S.A.; Dolenko, T.A.; Ruokolainen, J.; Gu, H.; Shenderova, O.A.; Vlasov, I.I.; et al. Core-shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery i: Fabrication. J. Mater. Chem. B 2013, 1, 2358–2366. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. Monodisperse silica-coated polyvinylpyrrolidone/nayf4 nanocrystals with multicolor upconversion fluorescence emission. Angew. Chem. 2006, 118, 7896–7899. [Google Scholar] [CrossRef]
- Liu, J.-N.; Bu, W.-B.; Shi, J.-L. Silica coated upconversion nanoparticles: A versatile platform for the development of efficient theranostics. Acc. Chem. Res. 2015, 48, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Karaman, D.Ş.; Sarparanta, M.P.; Rosenholm, J.M.; Airaksinen, A.J. Multimodality imaging of silica and silicon materials in vivo. Adv. Mater. 2018, 1703651. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.L.; Prabhakar, N.; Arppe, R.; Rosenholm, J.M.; Krishnan, V. Microwave-assisted one-step synthesis of acetate-capped nayf4:Yb/er upconversion nanocrystals and their application in bioimaging. J. Mater. Sci. 2017, 52, 5738–5750. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Jin, S.; Ren, W.; Xing, G.; Li, S.; et al. Mn2+ dopant-controlled synthesis of nayf4:Yb/er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gai, S.; Ma, P.a.; Wang, L.; Zhang, M.; Huang, S.; Yang, P. Highly uniform α-nayf4:Yb/er hollow microspheres and their application as drug carrier. Inorg. Chem. 2013, 52, 9184–9191. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Karaman Didem, S.; Prabhakar, N.; Tadayon, S.; Duchanoy, A.; Toivola Diana, M.; Rajput, S.; Näreoja, T.; Rosenholm Jessica, M. Design considerations for mesoporous silica nanoparticulate systems in facilitating biomedical applications. Open Mater. Sci. 2014, 1, 1. [Google Scholar] [CrossRef]
- Paatero, I.; Casals, E.; Niemi, R.; Özliseli, E.; Rosenholm, J.M.; Sahlgren, C. Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci. Rep. 2017, 7, 8423. [Google Scholar] [CrossRef] [PubMed]
- Gulin-Sarfraz, T.; Sarfraz, J.; Karaman, D.Ş.; Zhang, J.; Oetken-Lindholm, C.; Duchanoy, A.; Rosenholm, J.M.; Abankwa, D. Fret-reporter nanoparticles to monitor redox-induced intracellular delivery of active compounds. RSC Adv. 2014, 4, 16429–16437. [Google Scholar] [CrossRef]
- Svoboda, K.; Yasuda, R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 2006, 50, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingeshwar Reddy, K.; Prabhakar, N.; Rosenholm, J.M.; Krishnan, V. Core-Shell Structures of Upconversion Nanocrystals Coated with Silica for Near Infrared Light Enabled Optical Imaging of Cancer Cells. Micromachines 2018, 9, 400. https://doi.org/10.3390/mi9080400
Lingeshwar Reddy K, Prabhakar N, Rosenholm JM, Krishnan V. Core-Shell Structures of Upconversion Nanocrystals Coated with Silica for Near Infrared Light Enabled Optical Imaging of Cancer Cells. Micromachines. 2018; 9(8):400. https://doi.org/10.3390/mi9080400
Chicago/Turabian StyleLingeshwar Reddy, Kumbam, Neeraj Prabhakar, Jessica M. Rosenholm, and Venkata Krishnan. 2018. "Core-Shell Structures of Upconversion Nanocrystals Coated with Silica for Near Infrared Light Enabled Optical Imaging of Cancer Cells" Micromachines 9, no. 8: 400. https://doi.org/10.3390/mi9080400
APA StyleLingeshwar Reddy, K., Prabhakar, N., Rosenholm, J. M., & Krishnan, V. (2018). Core-Shell Structures of Upconversion Nanocrystals Coated with Silica for Near Infrared Light Enabled Optical Imaging of Cancer Cells. Micromachines, 9(8), 400. https://doi.org/10.3390/mi9080400







