Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine
Abstract
:1. Introduction
2. Initiating Endocrine Therapy for PC
3. Nuclear Receptors Associated with Endocrine Therapy for PC
4. Clinicopathological Features of Nuclear Receptors in PC
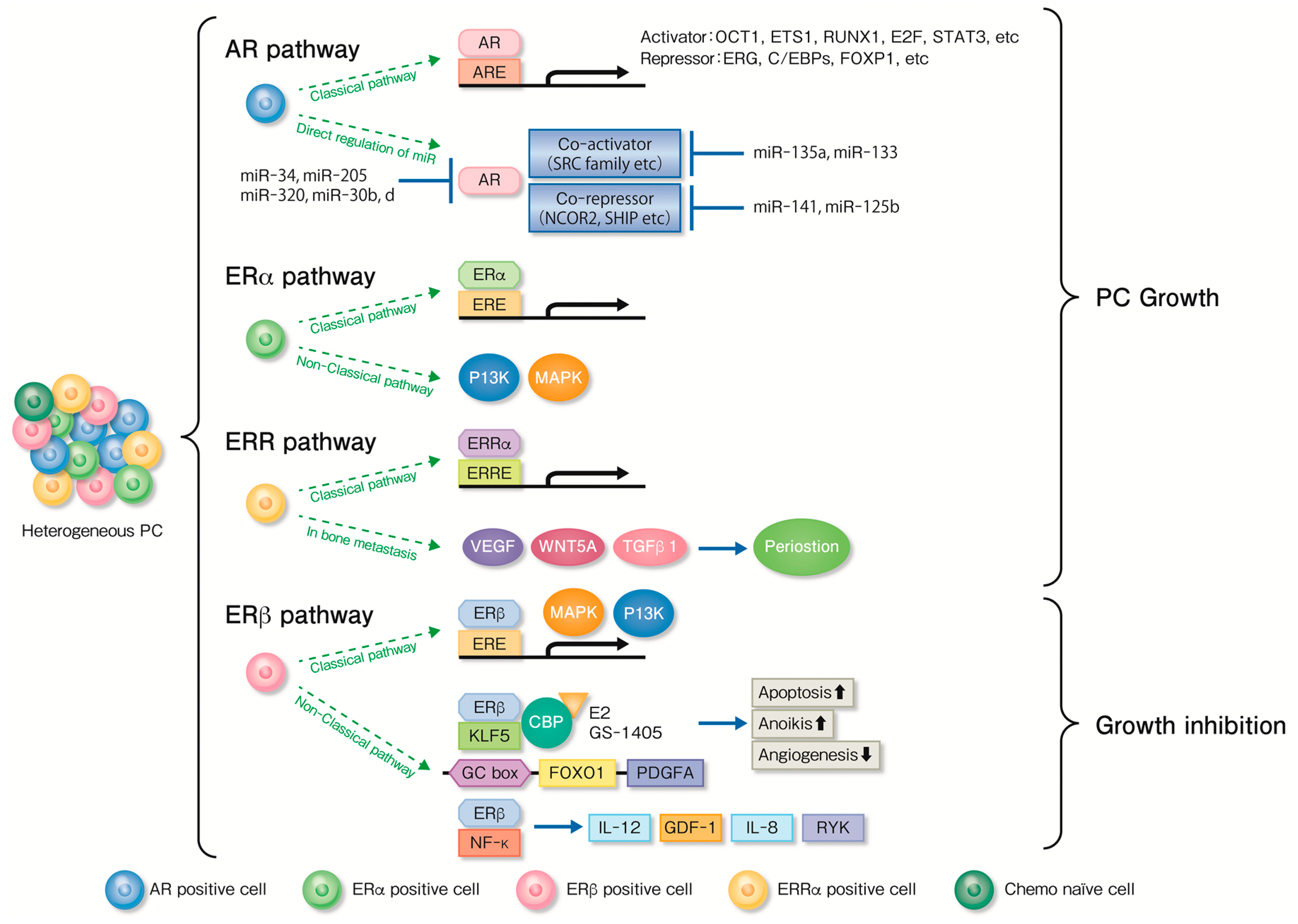
5. Functional Analysis of Nuclear Receptors and Associated Factors for Endocrine Therapy
6. SERMs for PC
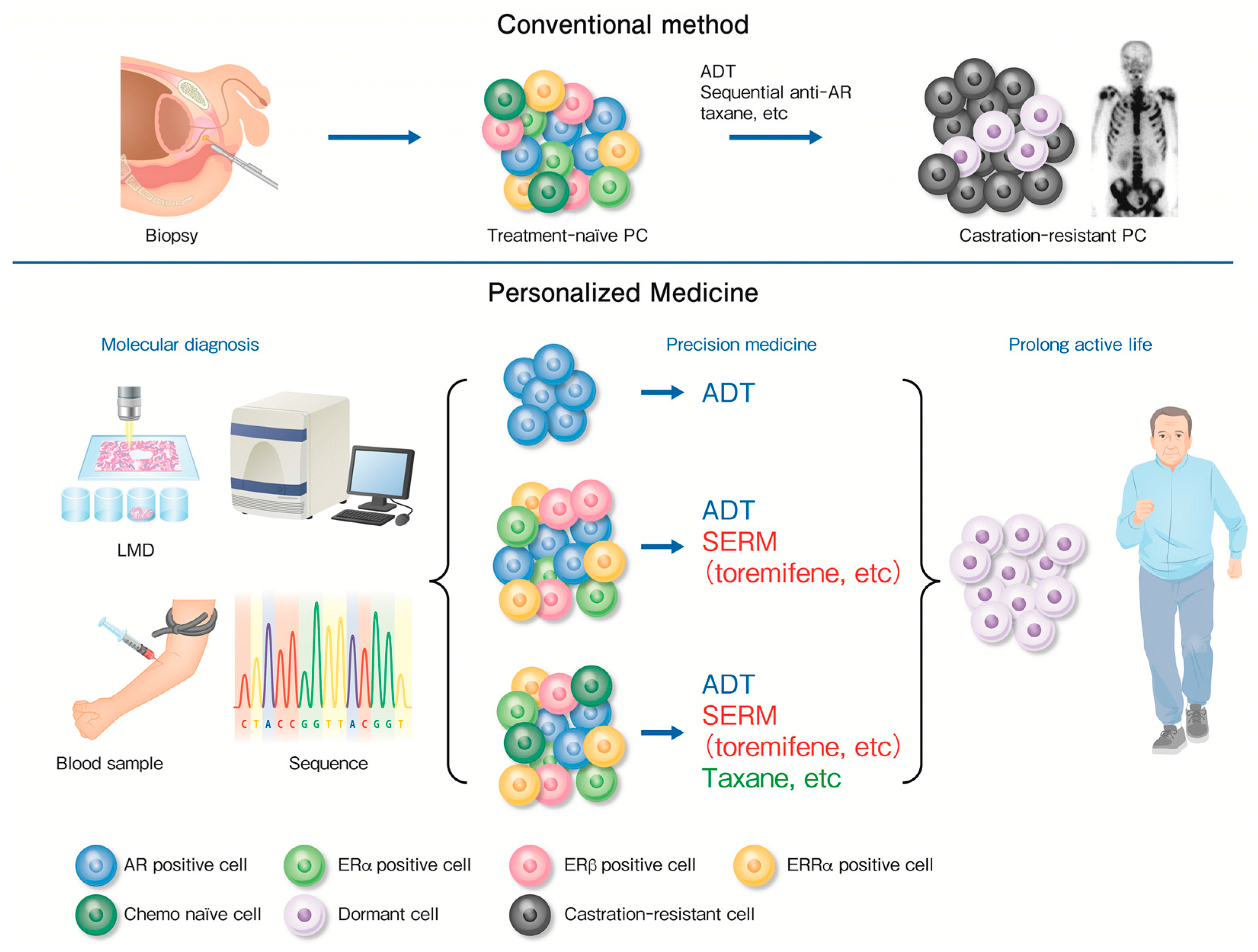
7. Molecular Diagnosis for Precision Medicine
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bossi, A.; Bristow, R.; Carver, B.; Castellano, D.; Chung, B.H.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol. 2017, 73, 178–211. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.I.; Misawa, A.; Inoue, S. Significance of microRNAs in Androgen Signaling and Prostate Cancer Progression. Cancers 2017, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Obinata, D.; Takayama, K.; Takahashi, S.; Inoue, S. Crosstalk of the Androgen Receptor with Transcriptional Collaborators: Potential Therapeutic Targets for Castration-Resistant Prostate Cancer. Cancers 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Ehara, H.; Koji, T.; Deguchi, T.; Yoshii, A.; Nakano, M.; Nakane, P.K.; Kawada, Y. Expression of estrogen receptor in diseased human prostate assessed by non-radioactive in situ hybridization and immunohistochemistry. Prostate 1995, 27, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kirschenbaum, A.; Ren, M.; Erenburg, I.; Schachter, B.; Levine, A.C. Estrogen receptor messenger RNA expression in human benign prostatic hyperplasia: Detection, localization, and modulation with a long-acting gonadotropin-releasing hormone agonist. J. Androl. 1994, 15, 528–533. [Google Scholar] [PubMed]
- Hiramatsu, M.; Maehara, I.; Orikasa, S.; Sasano, H. Immunolocalization of oestrogen and progesterone receptors in prostatic hyperplasia and carcinoma. Histopathology 1996, 28, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Cunha, G.R.; Yonemura, C.U.; Kawamura, J. Temporal and spatial factors in diethylstilbestrol-induced squamous metaplasia of the developing human prostate. Hum. Pathol. 1988, 19, 133–139. [Google Scholar] [CrossRef]
- Triche, T.J.; Harkin, J.C. An ultrastructural study of hormonally induced squamous metaplasia in the coagulating gland of the mouse prostate. Lab. Investig. J. Tech. Methods Pathol. 1971, 25, 596–606. [Google Scholar]
- Levine, A.C.; Kirschenbaum, A.; Droller, M.; Gabrilove, J.L. Effect of the addition of estrogen to medical castration on prostatic size, symptoms, histology and serum prostate specific antigen in 4 men with benign prostatic hypertrophy. J. Urol. 1991, 146, 790–793. [Google Scholar] [CrossRef]
- Noble, R.L. Production of Nb rat carcinoma of the dorsal prostate and response of estrogen-dependent transplants to sex hormones and tamoxifen. Cancer Res. 1980, 40, 3547–3550. [Google Scholar] [PubMed]
- Jensen, E.V.; Jacobson, H.I.; Smith, S.; Jungblut, P.W.; De Sombre, E.R. The use of estrogen antagonists in hormone receptor studies. Gynecol. Investig. 1972, 3, 108–123. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ER β: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Giguere, V.; Yang, N.; Segui, P.; Evans, R.M. Identification of a new class of steroid hormone receptors. Nature 1988, 331, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H.; Fixemer, T.; Hunsicker, I.; Remberger, K. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am. J. Pathol. 1999, 155, 641–647. [Google Scholar] [CrossRef]
- Royuela, M.; de Miguel, M.P.; Bethencourt, F.R.; Sanchez-Chapado, M.; Fraile, B.; Arenas, M.I.; Paniagua, R. Estrogen receptors α and β in the normal, hyperplastic and carcinomatous human prostate. J. Endocrinol. 2001, 168, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Lau, K.M.; Adams, J.Y.; McNeal, J.E.; Taplin, M.E.; Wang, J.; Singh, H.; Ho, S.M. Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am. J. Pathol. 2001, 159, 79–92. [Google Scholar] [CrossRef]
- Pasquali, D.; Rossi, V.; Esposito, D.; Abbondanza, C.; Puca, G.A.; Bellastella, A.; Sinisi, A.A. Loss of estrogen receptor β expression in malignant human prostate cells in primary cultures and in prostate cancer tissues. J. Clin. Endocrinol. Metab. 2001, 86, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, D.; Staibano, S.; Prezioso, D.; Franco, R.; Esposito, D.; Notaro, A.; De Rosa, G.; Bellastella, A.; Sinisi, A.A. Estrogen receptor β expression in human prostate tissue. Mol. Cell. Endocrinol. 2001, 178, 47–50. [Google Scholar] [CrossRef]
- Horvath, L.G.; Henshall, S.M.; Lee, C.S.; Head, D.R.; Quinn, D.I.; Makela, S.; Delprado, W.; Golovsky, D.; Brenner, P.C.; O’Neill, G.; et al. Frequent loss of estrogen receptor-β expression in prostate cancer. Cancer Res. 2001, 61, 5331–5335. [Google Scholar] [PubMed]
- Latil, A.; Bieche, I.; Vidaud, D.; Lidereau, R.; Berthon, P.; Cussenot, O.; Vidaud, M. Evaluation of androgen, estrogen (ER α and ER β), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001, 61, 1919–1926. [Google Scholar] [PubMed]
- Castagnetta, L.A.; Miceli, M.D.; Sorci, C.M.; Pfeffer, U.; Farruggio, R.; Oliveri, G.; Calabro, M.; Carruba, G. Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology 1995, 136, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Hobisch, A.; Hittmair, A.; Daxenbichler, G.; Wille, S.; Radmayr, C.; Hobisch-Hagen, P.; Bartsch, G.; Klocker, H.; Culig, Z. Metastatic lesions from prostate cancer do not express oestrogen and progesterone receptors. J. Pathol. 1997, 182, 356–361. [Google Scholar] [CrossRef]
- Konishi, N.; Nakaoka, S.; Hiasa, Y.; Kitahori, Y.; Ohshima, M.; Samma, S.; Okajima, E. Immunohistochemical evaluation of estrogen receptor status in benign prostatic hypertrophy and in prostate carcinoma and the relationship to efficacy of endocrine therapy. Oncology 1993, 50, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Urano, T.; Ogawa, S.; Ouchi, Y.; Kitamura, T.; Muramatsu, M.; Inoue, S. Differential expression of estrogen receptor β (ERβ) and its C-terminal truncated splice variant ERβcx as prognostic predictors in human prostatic cancer. Biochem. Biophys. Res. Commun. 2001, 289, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Korach, K.S. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 2008, 73, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.A. Estrogen receptor β—A new dimension in estrogen mechanism of action. J. Endocrinol. 1999, 163, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.M.; LaSpina, M.; Long, J.; Ho, S.M. Expression of estrogen receptor (ER)-α and ER-β in normal and malignant prostatic epithelial cells: Regulation by methylation and involvement in growth regulation. Cancer Res. 2000, 60, 3175–3182. [Google Scholar] [PubMed]
- Nelson, A.W.; Groen, A.J.; Miller, J.L.; Warren, A.Y.; Holmes, K.A.; Tarulli, G.A.; Tilley, W.D.; Katzenellenbogen, B.S.; Hawse, J.R.; Gnanapragasam, V.J.; et al. Comprehensive assessment of estrogen receptor β antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol. Cell. Endocrinol. 2017, 440, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.T.; McKee, D.D.; Slentz-Kesler, K.; Moore, L.B.; Jones, S.A.; Horne, E.L.; Su, J.L.; Kliewer, S.A.; Lehmann, J.M.; Willson, T.M. Cloning and characterization of human estrogen receptor β isoforms. Biochem. Biophys. Res. Commun. 1998, 247, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Sundberg, M.; Pristovsek, N.; Ibrahim, A.; Jonsson, P.; Katona, B.; Clausson, C.M.; Zieba, A.; Ramstrom, M.; Soderberg, O.; et al. Insufficient antibody validation challenges oestrogen receptor β research. Nat. Commun. 2017, 8, 15840. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Shigeta, H.; Shi, H.; Teng, C.T. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J. Biol. Chem. 1996, 271, 5795–5804. [Google Scholar] [CrossRef] [PubMed]
- Kraus, R.J.; Ariazi, E.A.; Farrell, M.L.; Mertz, J.E. Estrogen-related receptor α 1 actively antagonizes estrogen receptor-regulated transcription in MCF-7 mammary cells. J. Biol. Chem. 2002, 277, 24826–24834. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.P.; Yu, S.; Wong, K.B.; Chan, L.W.; Lai, F.M.; Wang, X.; Suetsugi, M.; Chen, S.; Chan, F.L. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J. Clin. Endocrinol. Metab. 2005, 90, 1830–1844. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, J.M.; Bonnelye, E.; Chopin-Delannoy, S.; Delmarre, C.; Cavailles, V.; Laudet, V. Transcriptional activities of the orphan nuclear receptor ERR α (estrogen receptor-related receptor-α). Mol. Endocrinol. 1999, 13, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teng, C.T. Estrogen receptor α and estrogen receptor-related receptor α1 compete for binding and coactivator. Mol. Cell. Endocrinol. 2001, 172, 223–233. [Google Scholar] [CrossRef]
- Shi, H.; Shigeta, H.; Yang, N.; Fu, K.; O’Brian, G.; Teng, C.T. Human estrogen receptor-like 1 (ESRL1) gene: Genomic organization, chromosomal localization, and promoter characterization. Genomics 1997, 44, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ariazi, E.A.; Clark, G.M.; Mertz, J.E. Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002, 62, 6510–6518. [Google Scholar] [PubMed]
- Suzuki, T.; Miki, Y.; Moriya, T.; Shimada, N.; Ishida, T.; Hirakawa, H.; Ohuchi, N.; Sasano, H. Estrogen-related receptor α in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004, 64, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Urano, T.; Kumagai, J.; Ogushi, T.; Horie-Inoue, K.; Ouchi, Y.; Kitamura, T.; Muramatsu, M.; Inoue, S. Increased expression of estrogen-related receptor α (ERRα) is a negative prognostic predictor in human prostate cancer. Int. J. Cancer 2007, 120, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Urano, T.; Ijichi, N.; Ikeda, K.; Kumagai, J.; Murata, T.; Takayama, K.; Horie-Inoue, K.; Ouchi, Y.; et al. Differential expression of estrogen-related receptors β and γ (ERRβ and ERRγ) and their clinical significance in human prostate cancer. Cancer Sci. 2010, 101, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Di Masi, A.; De Marinis, E.; Ascenzi, P.; Marino, M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol. Asp. Med. 2009, 30, 297–343. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, B.; Sabbagh, W., Jr.; Juguilon, H.; Bolado, J., Jr.; van Meter, C.M.; Ong, E.S.; Evans, R.M. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998, 12, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T.; McDermott, I.R.; Coffey, D.S. Characterization of two new enzymatic activities of the rat ventral prostate: 5 α-androstane-3 β, 17 β-diol 6 α-hydroxylase and 5 α-androstane-3 β, 17 β-diol 7 α-hydroxylase. Steroids 1979, 33, 675–692. [Google Scholar] [CrossRef]
- Isaacs, J.T.; McDermott, I.R.; Coffey, D.S. The identification and characterization of a new C19O3 steroid metabolite in the rat ventral prostate: 5 α-androstane-3 β, 6 α, 17 β-triol. Steroids 1979, 33, 639–657. [Google Scholar] [CrossRef]
- Waxman, D.J.; Lapenson, D.P.; Aoyama, T.; Gelboin, H.V.; Gonzalez, F.J.; Korzekwa, K. Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch. Biochem. Biophys. 1991, 290, 160–166. [Google Scholar] [CrossRef]
- Fujimura, T.; Takahashi, S.; Urano, T.; Tanaka, T.; Zhang, W.; Azuma, K.; Takayama, K.; Obinata, D.; Murata, T.; Horie-Inoue, K.; et al. Clinical significance of steroid and xenobiotic receptor and its targeted gene CYP3A4 in human prostate cancer. Cancer Sci. 2012, 103, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Urano, T.; Kumagai, J.; Murata, T.; Takayama, K.; Ogushi, T.; Horie-Inoue, K.; Ouchi, Y.; Kitamura, T.; et al. Expression of cytochrome P450 3A4 and its clinical significance in human prostate cancer. Urology 2009, 74, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, J.; Fujimura, T.; Takahashi, S.; Urano, T.; Ogushi, T.; Horie-Inoue, K.; Ouchi, Y.; Kitamura, T.; Muramatsu, M.; Blumberg, B.; et al. Cytochrome P450 2B6 is a growth-inhibitory and prognostic factor for prostate cancer. Prostate 2007, 67, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, Q.; Ou, Z.; Lee, J.H.; Xu, M.; Kochhar, U.; Ren, S.; Huang, M.; Pflug, B.R.; Xie, W. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology 2010, 151, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Schade, G.R.; Holt, S.K.; Zhang, X.; Song, D.; Wright, J.L.; Zhao, S.; Kolb, S.; Lam, H.M.; Levin, L.; Leung, Y.K.; et al. Prostate Cancer Expression Profiles of Cytoplasmic ERβ1 and Nuclear ERβ2 are Associated with Poor Outcomes following Radical Prostatectomy. J. Urol. 2016, 195, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Megas, G.; Chrisofos, M.; Anastasiou, I.; Tsitlidou, A.; Choreftaki, T.; Deliveliotis, C. Estrogen receptor (α and β) but not androgen receptor expression is correlated with recurrence, progression and survival in post prostatectomy T3N0M0 locally advanced prostate cancer in an urban Greek population. Asian J. Androl. 2015, 17, 98–105. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.A.; Glidewell-Kenney, C.; Jimenez, M.A.; Ahearn, P.C.; Weiss, J.; Jameson, J.L.; Levine, J.E. New insights into the classical and non-classical actions of estrogen: Evidence from estrogen receptor knock-out and knock-in mice. Mol. Cell. Endocrinol. 2008, 290, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, I.; Lawrence, M.G.; Balanathan, P.; Rebello, R.; Pearson, H.B.; Garg, E.; Pedersen, J.; Pouliot, N.; Nadon, R.; Watt, M.J.; et al. Estrogen receptor α drives proliferation in PTEN-deficient prostate carcinoma by stimulating survival signaling, MYC expression and altering glucose sensitivity. Oncotarget 2015, 6, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Jonsson, P.; Hartman, J.; Williams, C.; Strom, A.; Gustafsson, J.A. Estrogen receptors β1 and β2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Mol. Endocrinol. 2012, 26, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Akaogi, K.; Suzuki, T.; Osakabe, A.; Yamaguchi, C.; Sunahara, N.; Ishida, J.; Kako, K.; Ogawa, S.; Fujimura, T.; et al. Estrogen regulates tumor growth through a nonclassical pathway that includes the transcription factors ERβ and KLF5. Sci. Signal. 2011, 4, ra22. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Osakabe, A.; Waku, T.; Suzuki, T.; Akaogi, K.; Fujimura, T.; Homma, Y.; Inoue, S.; Yanagisawa, J. Estrogen Exhibits a Biphasic Effect on Prostate Tumor Growth through the Estrogen Receptor β-KLF5 Pathway. Mol. Cell. Biol. 2016, 36, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.K.; Gao, Y.; Lau, K.M.; Zhang, X.; Ho, S.M. ICI 182,780-regulated gene expression in DU145 prostate cancer cells is mediated by estrogen receptor-β/NFκB crosstalk. Neoplasia 2006, 8, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.F.; Maneix, L.; Insunza, J.; Nalvarte, I.; Antonson, P.; Kere, J.; Yu, N.Y.; Tohonen, V.; Katayama, S.; Einarsdottir, E.; et al. Estrogen receptor β, a regulator of androgen receptor signaling in the mouse ventral prostate. Proc. Natl. Acad. Sci. USA 2017, 114, E3816–E3822. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, J.; Kaulfuss, S.; Jarry, H.; Bremmer, F.; Stettner, M.; Burfeind, P.; Thelen, P. Prospects of estrogen receptor β activation in the treatment of castration-resistant prostate cancer. Oncotarget 2017, 8, 34971–34979. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.; Sailland, J.; Vanacker, J.M. ERRs and cancers: Effects on metabolism and on proliferation and migration capacities. J. Steroid Biochem. Mol. Biol. 2012, 130, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.; Lanvin, O.; Tribollet, V.; Macari, C.; North, S.; Vanacker, J.M. Modulating estrogen receptor-related receptor-α activity inhibits cell proliferation. J. Biol. Chem. 2009, 284, 23286–23292. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, G.; Giroux, V.; Lassalle, T.; Carpentier, A.C.; Rivard, N.; Carrier, J.C. ERRα metabolic nuclear receptor controls growth of colon cancer cells. Carcinogenesis 2013, 34, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Chen, Z.J.; Liu, H.; Wei, W.D.; Lu, L.L.; Yang, X.L.; Liang, W.T.; Liu, T.; Liu, H.L.; Du, J.; et al. Inhibition of ERRα suppresses epithelial mesenchymal transition of triple negative breast cancer cells by directly targeting fibronectin. Oncotarget 2015, 6, 25588–25601. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Yu, S.; Xu, Z.; Wu, D.; Ng, C.F.; Yao, X.; Yew, D.T.; Vanacker, J.M.; Chan, F.L. ERRα augments HIF-1 signalling by directly interacting with HIF-1α in normoxic and hypoxic prostate cancer cells. J. Pathol. 2014, 233, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Fradet, A.; Sorel, H.; Bouazza, L.; Goehrig, D.; Depalle, B.; Bellahcene, A.; Castronovo, V.; Follet, H.; Descotes, F.; Aubin, J.E.; et al. Dual function of ERRα in breast cancer and bone metastasis formation: Implication of VEGF and osteoprotegerin. Cancer Res. 2011, 71, 5728–5738. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, M.A.; Joseph, J.D.; Wade, H.E.; Eaton, M.L.; Kunder, R.S.; Kazmin, D.; Chang, C.Y.; McDonnell, D.P. WNT11 expression is induced by estrogen-related receptor α and β-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 2010, 70, 9298–9308. [Google Scholar] [CrossRef] [PubMed]
- Fradet, A.; Bouchet, M.; Delliaux, C.; Gervais, M.; Kan, C.; Benetollo, C.; Pantano, F.; Vargas, G.; Bouazza, L.; Croset, M.; et al. Estrogen related receptor α in castration-resistant prostate cancer cells promotes tumor progression in bone. Oncotarget 2016, 7, 77071–77086. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, A.; Vincent, S.; Decaussin-Petrucci, M.; Meugnier, E.; Viallet, J.; Ruffion, A.; Chalmel, F.; Samarut, J.; Allioli, N. Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene 2015, 34, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Tribollet, V.; Barenton, B.; Kroiss, A.; Vincent, S.; Zhang, L.; Forcet, C.; Cerutti, C.; Perian, S.; Allioli, N.; Samarut, J.; et al. miR-135a Inhibits the Invasion of Cancer Cells via Suppression of ERRα. PLoS ONE 2016, 11, e0156445. [Google Scholar] [CrossRef] [PubMed]
- Lonard, D.M.; Smith, C.L. Molecular perspectives on selective estrogen receptor modulators (SERMs): Progress in understanding their tissue-specific agonist and antagonist actions. Steroids 2002, 67, 15–24. [Google Scholar] [CrossRef]
- Taneja, S.S.; Smith, M.R.; Dalton, J.T.; Raghow, S.; Barnette, G.; Steiner, M.; Veverka, K.A. Toremifene—A promising therapy for the prevention of prostate cancer and complications of androgen deprivation therapy. Expert Opin. Investig. Drugs 2006, 15, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, M.; Smith, C.L. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J. Pharmacol. Exp. Ther. 2000, 295, 431–437. [Google Scholar] [PubMed]
- Raghow, S.; Hooshdaran, M.Z.; Katiyar, S.; Steiner, M.S. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res. 2002, 62, 1370–1376. [Google Scholar] [PubMed]
- Hariri, W.; Sudha, T.; Bharali, D.J.; Cui, H.; Mousa, S.A. Nano-Targeted Delivery of Toremifene, an Estrogen Receptor-α Blocker in Prostate Cancer. Pharm. Res. 2015, 32, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Zoltick, B.; Peacock, T.; Holroyde, C.; Haller, D.; Armstead, B.; Malkowicz, S.B.; Vaughn, D.J. Phase II trial of toremifene in androgen-independent prostate cancer: A Penn cancer clinical trials group trial. Am. J. Clin. Oncol. 2001, 24, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Stein, B.; Sieber, P.; Tutrone, R.; Bailen, J.; Goluboff, E.; Burzon, D.; Bostwick, D.; Steiner, M. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: Results of a double-blind, placebo controlled, phase IIB clinical trial. J. Urol. 2006, 176, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Malkowicz, S.B.; Chu, F.; Forrest, J.; Price, D.; Sieber, P.; Barnette, K.G.; Rodriguez, D.; Steiner, M.S. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of a multicenter phase 3 clinical study. J. Urol. 2008, 179, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.S.; Morton, R.; Barnette, G.; Sieber, P.; Hancock, M.L.; Steiner, M. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. J. Clin. Oncol. 2013, 31, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Malkowicz, S.B.; Chu, F.; Forrest, J.; Sieber, P.; Barnette, K.G.; Rodriquez, D.; Steiner, M.S. Toremifene improves lipid profiles in men receiving androgen-deprivation therapy for prostate cancer: Interim analysis of a multicenter phase III study. J. Clin. Oncol. 2008, 26, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Draper, M.W.; Flowers, D.E.; Huster, W.J.; Neild, J.A.; Harper, K.D.; Arnaud, C. A controlled trial of raloxifene (LY139481) HCl: Impact on bone turnover and serum lipid profile in healthy postmenopausal women. J. Bone Miner. Res. 1996, 11, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Shazer, R.L.; Jain, A.; Galkin, A.V.; Cinman, N.; Nguyen, K.N.; Natale, R.B.; Gross, M.; Green, L.; Bender, L.I.; Holden, S.; et al. Raloxifene, an oestrogen-receptor-β-targeted therapy, inhibits androgen-independent prostate cancer growth: Results from preclinical studies and a pilot phase II clinical trial. BJU Int. 2006, 97, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Nunez-Nateras, R.; Hou, Y.X.; Bryce, A.H.; Northfelt, D.W.; Dueck, A.C.; Wong, B.; Stanton, M.L.; Joseph, R.W.; Castle, E.P. A Study of Combination Bicalutamide and Raloxifene for Patients With Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.K.; Ashraf, U.; Lawrence, D.; Tian, L.; Levine, E.; Silliman, C.; Escott, P.; Payne, V.; Trump, D.L. Phase II study of fulvestrant (Faslodex) in castration resistant prostate cancer. Prostate 2008, 68, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Getzenberg, R.H.; Coss, C.C.; Gittelman, M.M.; Keane, T.; Tutrone, R.; Belkoff, L.; Given, R.; Bass, J.; Chu, F.; et al. Selective estrogen receptor α agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer. Eur. Urol. 2015, 67, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Kume, H.; Urano, T.; Takayama, K.; Yamada, Y.; Suzuki, M.; Fukuhara, H.; Nakagawa, T.; Inoue, S.; et al. Toremifene, a selective estrogen receptor modulator, significantly improved biochemical recurrence in bone metastatic prostate cancer: A randomized controlled phase II a trial. BMC Cancer 2015, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Osborne, C.K.; Morris, C.; Wakeling, A.E. ICI 182,780 (Faslodex): Development of a novel, “pure” antiestrogen. Cancer 2000, 89, 817–825. [Google Scholar] [CrossRef]
- Carlson, R.W.; Allred, D.C.; Anderson, B.O.; Burstein, H.J.; Edge, S.B.; Farrar, W.B.; Forero, A.; Giordano, S.H.; Goldstein, L.J.; Gradishar, W.J.; et al. Metastatic breast cancer, version 1.2012: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2012, 10, 821–829. [Google Scholar] [CrossRef]
- Rothschild, S.I. Targeted Therapies in Non-Small Cell Lung Cancer-Beyond EGFR and ALK. Cancers 2015, 7, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.D.; Eeles, R.; Freedland, S.J.; Isaacs, W.B.; Pomerantz, M.M.; Schalken, J.A.; Tammela, T.L.; Visakorpi, T. The role of genetic markers in the management of prostate cancer. Eur. Urol. 2012, 62, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Markert, E.K.; Mizuno, H.; Vazquez, A.; Levine, A.J. Molecular classification of prostate cancer using curated expression signatures. Proc. Natl. Acad. Sci. USA 2011, 108, 21276–21281. [Google Scholar] [CrossRef] [PubMed]
- Martens-Uzunova, E.S.; Jalava, S.E.; Dits, N.F.; van Leenders, G.J.; Moller, S.; Trapman, J.; Bangma, C.H.; Litman, T.; Visakorpi, T.; Jenster, G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012, 31, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.O.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef]
- Sterbis, J.R.; Gao, C.; Furusato, B.; Chen, Y.; Shaheduzzaman, S.; Ravindranath, L.; Osborn, D.J.; Rosner, I.L.; Dobi, A.; McLeod, D.G.; et al. Higher expression of the androgen-regulated gene PSA/HK3 mRNA in prostate cancer tissues predicts biochemical recurrence-free survival. Clin. Cancer Res. 2008, 14, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Nonn, L.; Vaishnav, A.; Gallagher, L.; Gann, P.H. mRNA and micro-RNA expression analysis in laser-capture microdissected prostate biopsies: Valuable tool for risk assessment and prevention trials. Exp. Mol. Pathol. 2010, 88, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, L.; Darby, S.; Jabbar, T.; Mathers, M.E.; Leung, H.Y.; Robson, C.N.; Sahadevan, K.; O’Toole, K.; Gnanapragasam, V.J. Application of transcript profiling in formalin-fixed paraffin-embedded diagnostic prostate cancer needle biopsies. BJU Int. 2008, 102, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Rubicz, R.; Zhao, S.; Wright, J.L.; Coleman, I.; Grasso, C.; Geybels, M.S.; Leonardson, A.; Kolb, S.; April, C.; Bibikova, M.; et al. Gene expression panel predicts metastatic-lethal prostate cancer outcomes in men diagnosed with clinically localized prostate cancer. Mol. Oncol. 2017, 11, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Inoue, S. Transcriptional network of androgen receptor in prostate cancer progression. Int. J. Urol. 2013, 20, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kaneshiro, K.; Tsutsumi, S.; Horie-Inoue, K.; Ikeda, K.; Urano, T.; Ijichi, N.; Ouchi, Y.; Shirahige, K.; Aburatani, H.; et al. Identification of novel androgen response genes in prostate cancer cells by coupling chromatin immunoprecipitation and genomic microarray analysis. Oncogene 2007, 26, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Tsutsumi, S.; Suzuki, T.; Horie-Inoue, K.; Ikeda, K.; Kaneshiro, K.; Fujimura, T.; Kumagai, J.; Urano, T.; Sakaki, Y.; et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009, 69, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Horie-Inoue, K.; Ikeda, K.; Urano, T.; Murakami, K.; Hayashizaki, Y.; Ouchi, Y.; Inoue, S. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Obinata, D.; Takayama, K.; Urano, T.; Murata, T.; Kumagai, J.; Fujimura, T.; Ikeda, K.; Horie-Inoue, K.; Homma, Y.; Ouchi, Y.; et al. Oct1 regulates cell growth of LNCaP cells and is a prognostic factor for prostate cancer. Int. J. Cancer 2012, 130, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Urano, T.; Takayama, K.; Sugihara, T.; Obinata, D.; Yamada, Y.; Kumagai, J.; Kume, H.; Ouchi, Y.; et al. Expression of androgen and estrogen signaling components and stem cell markers to predict cancer progression and cancer-specific survival in patients with metastatic prostate cancer. Clin. Cancer Res. 2014, 20, 4625–4635. [Google Scholar] [CrossRef] [PubMed]
| Years (References) | Subjective | Objective | Design | Treatments | Number | Results |
|---|---|---|---|---|---|---|
| 2001 [78] | CRPC | Cancer Control | Experimental | ADT+ Toremifene 300–640 mg/m2 | 15 | No cancer inhibitory effect |
| 2006 [79] | HGPIN | Cancer prevention | RCT | Toremifene 20, 40, 60 mg | 514 | Cancer prevention in 20 mg group |
| 2008 [80] | PC | Osteoporosis prevention | RCT | Toremifene 80 mg | 197 | Increased bone density |
| 2008 [82] | PC | Lipid profile improvement | RCT | Toremifene 80 mg | 1389 | Decreased T Cho, LDL, HDL, TG |
| 2013 [81] | HGPIN | Cancer prevention | RCT | Toremifene 20 mg | 1467 | Not significant cancer prevention |
| 2006 [84] | CRPC | Cancer Control | Experimental | Raloxifene 60 mg | 13 | Partial effect (5 of 13 patients) |
| 2017 [85] | CRPC | Cancer Control | Experimental | Raloxifene 60 mg + Bicaltamide 50 mg | 18 | Partial effect (4 of 18 patients) |
| 2008 [86] | CRPC | Cancer Control | Experimental | Fulvestrant 500 mg, 250 mg | 20 | No patients reduced >50% PSA reduction |
| 2015 [87] | Hormone naïve PC | Testosterone reduction | RCT | ADT, ADT+ GTx-758 1000 mg, or ADT+ GTx-758 2000 mg | 164 | Superior testosterone reduction in GTx-756 group |
| 2015 [88] | Hormone naïve PC | Cancer Control | RCT | ADT, ADT+ Toremifene 60 mg, or ADT+ Raloxifene 60 mg | 15 | ADT+ toremifene significantly improved BCR |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimura, T.; Takayama, K.; Takahashi, S.; Inoue, S. Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine. Cancers 2018, 10, 29. https://doi.org/10.3390/cancers10020029
Fujimura T, Takayama K, Takahashi S, Inoue S. Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine. Cancers. 2018; 10(2):29. https://doi.org/10.3390/cancers10020029
Chicago/Turabian StyleFujimura, Tetsuya, Kenichi Takayama, Satoru Takahashi, and Satoshi Inoue. 2018. "Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine" Cancers 10, no. 2: 29. https://doi.org/10.3390/cancers10020029




