Selectivity Dependence of 1,1-Difluoro-1-Chloroethane Dehydrohalogenation on the Metal–Support Interaction over SrF2 Catalyst
Abstract
:1. Introduction
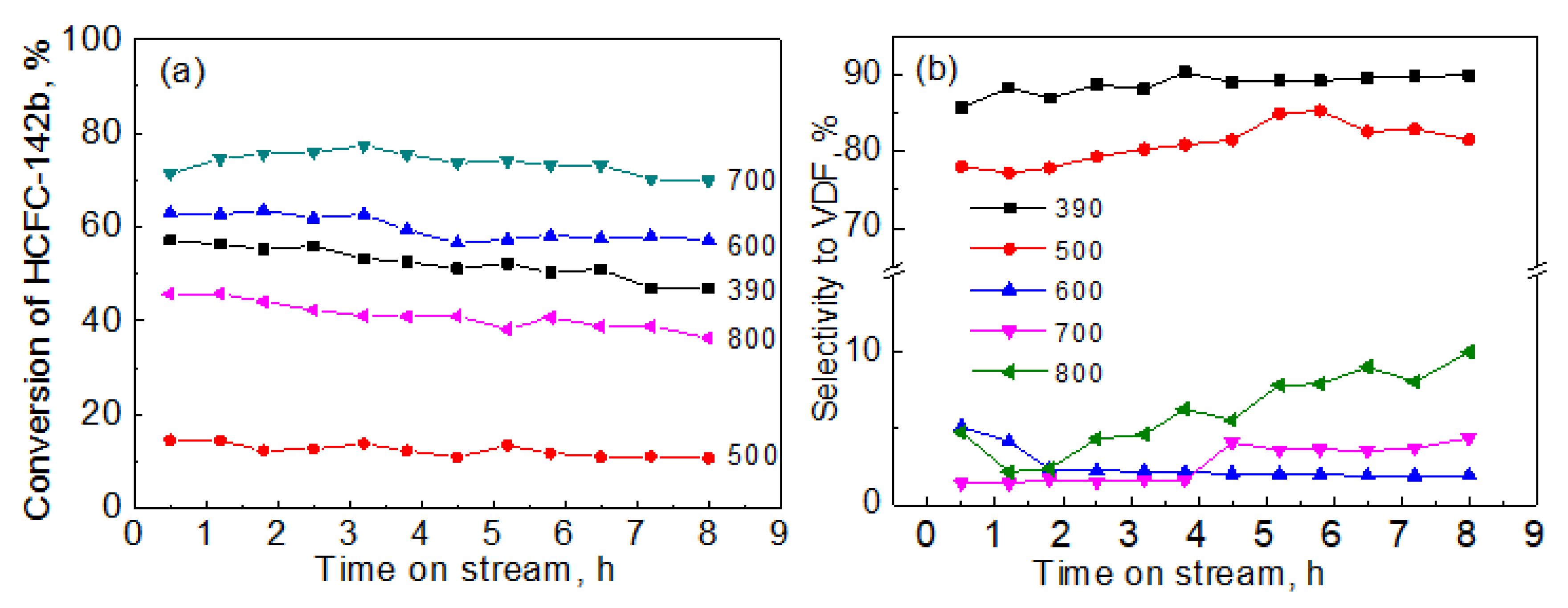
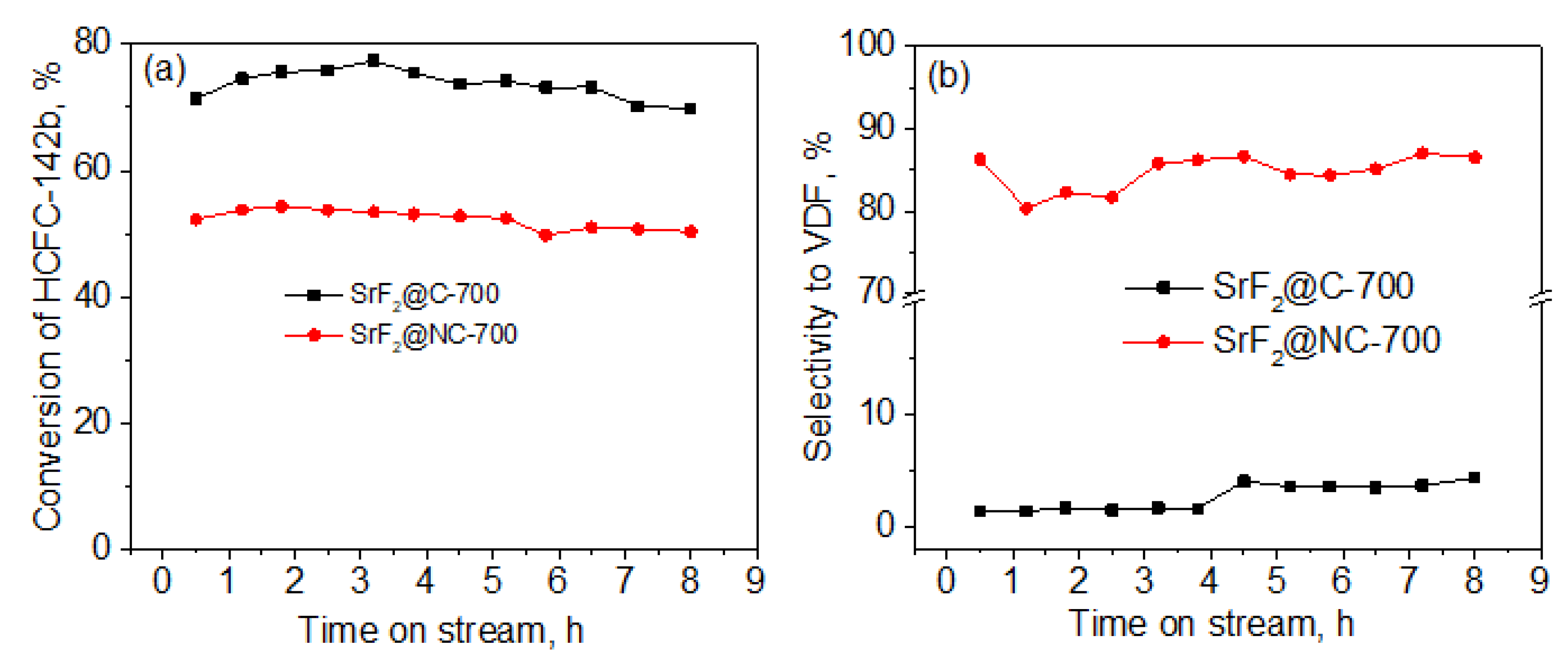
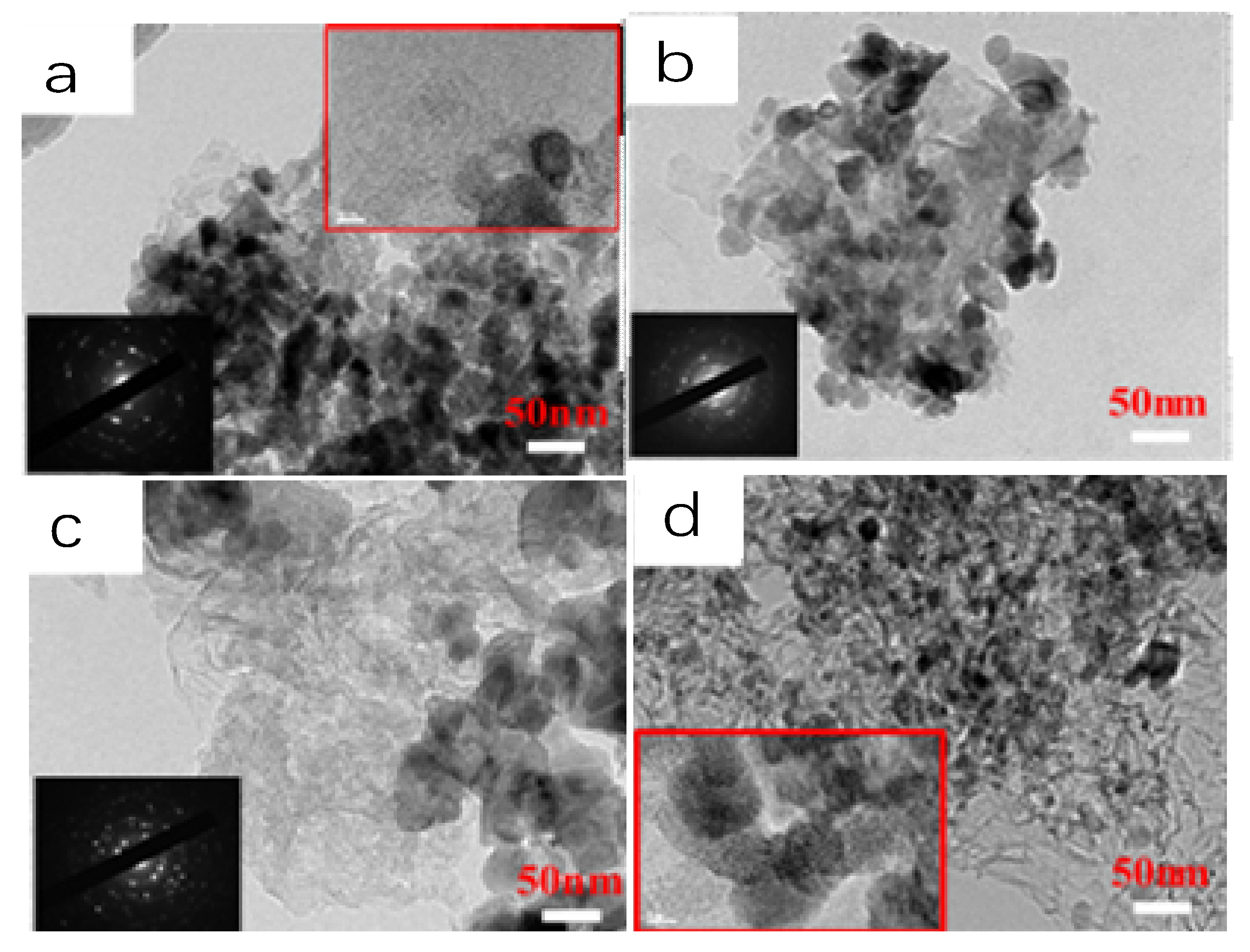
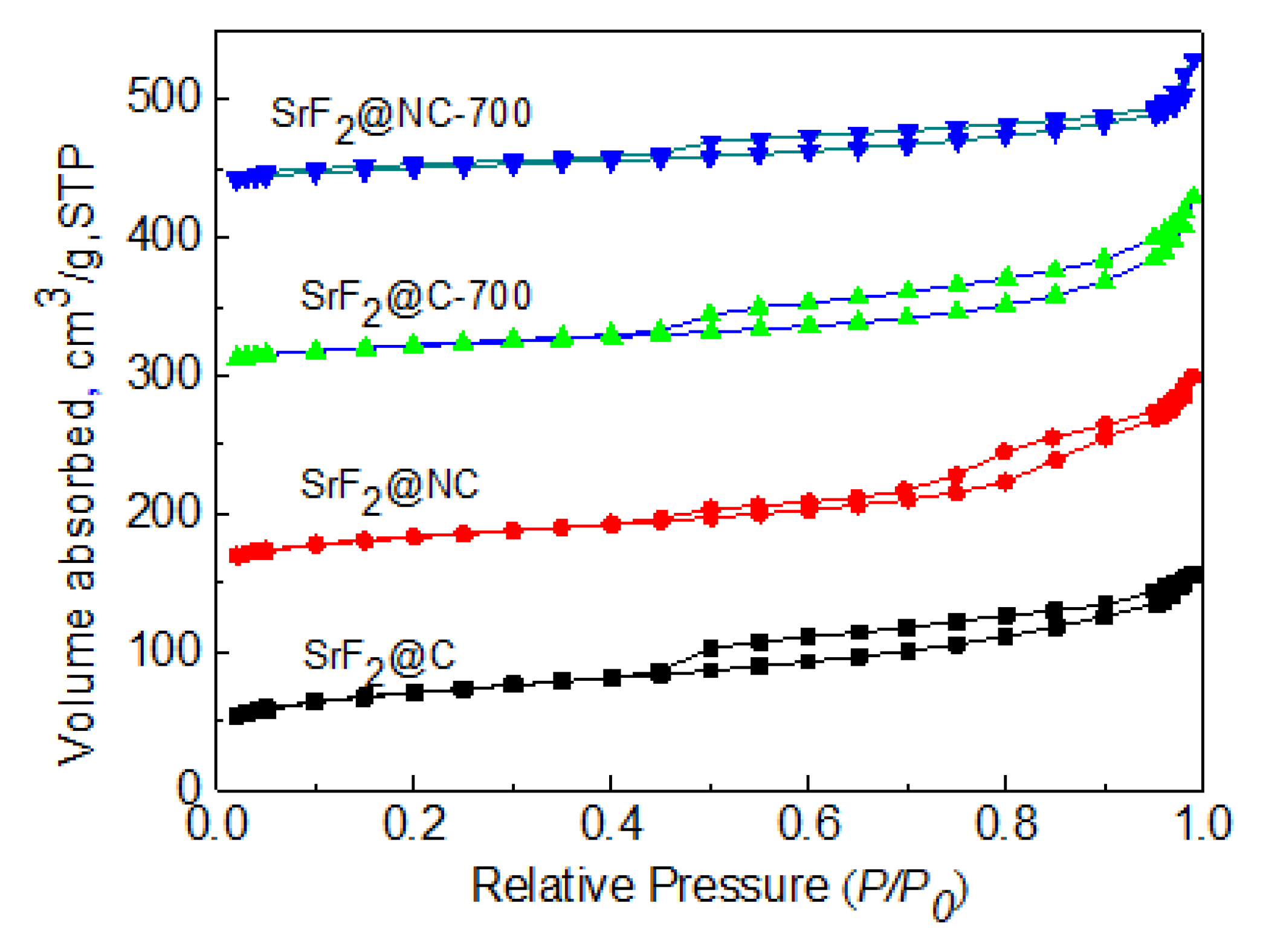
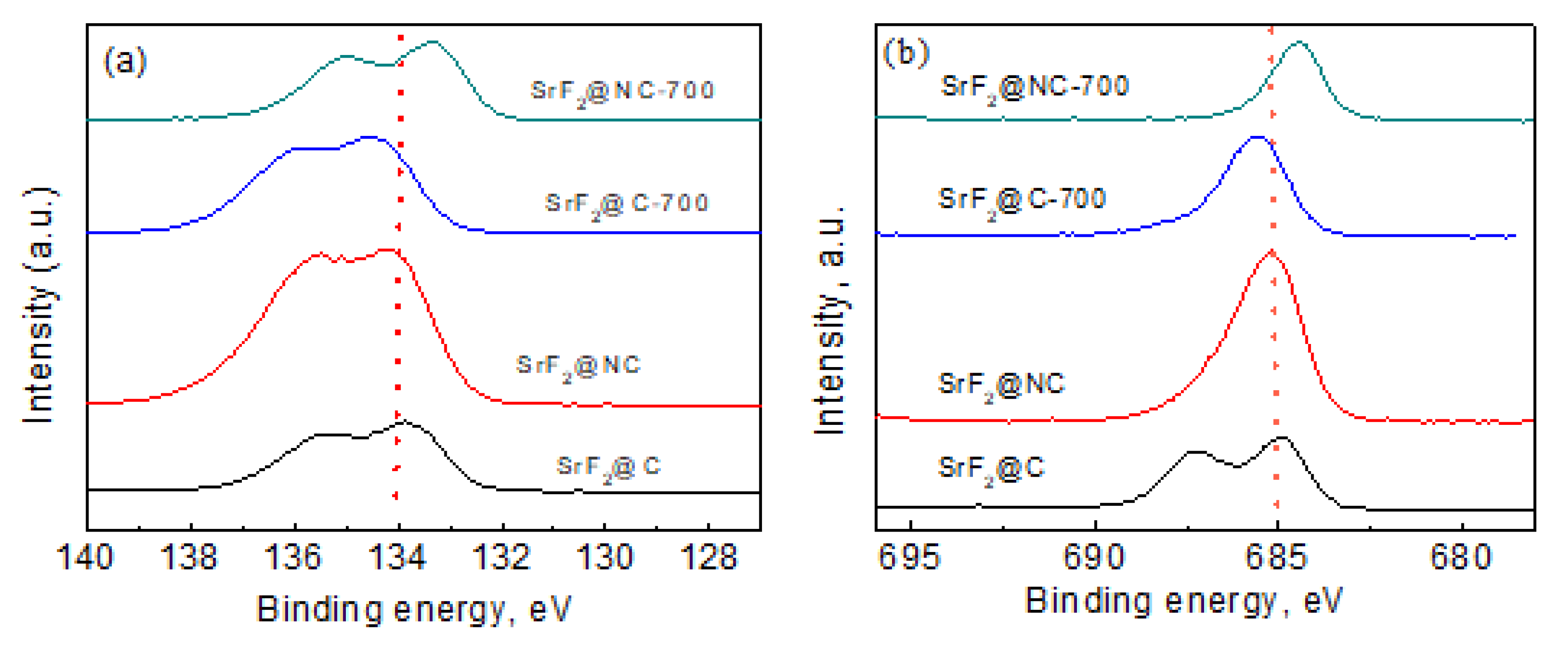
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Preparation of SrF2 Embedded in Carbon Catalyst
3.1.2. Preparation of SrF2 Embedded in N-Doped Carbon Catalyst
3.1.3. Preparation of SrF2 Catalyst
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [Green Version]
- Taguet, A.; Ameduri, B.; Boutevin, B. Crosslinking of vinylidene fluoride-containing fluoropolymers. In Crosslinking in Materials Science; Ameduri, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 184, pp. 127–211. [Google Scholar]
- Wang, L.; Liu, S.; He, H.Q.; Zhang, J.L. Dual-level direct dynamics study on the hydrogen abstraction reaction of fluorine atom with 1,1-difluoro-1-chloroethane. Can. J. Chem.-Revue Canadienne De Chimie 2011, 89, 1396–1402. [Google Scholar] [CrossRef]
- Huybrechts, G.; Van Assche, G.; Van der Auwera, S. Kinetics and mechanism of the pyrolysis of 1-chloro-1,1-difluoroethane in the presence of additives. Int. J. Chem. Kinet. 1998, 30, 359–366. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Zhang, C.; Zhou, S.; Wang, H.; Tang, H.; Liu, H. Preparation of N-Doped Activated Carbon for Catalytic Pyrolysis of 1-Chloro-1,1-difluoroethane to Vinylidene Fluoride. Chemistryselect 2018, 3, 1015–1018. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Tang, H.; Li, Y.; Liu, H. Preparation of N-doped ordered mesoporous carbon and catalytic performance for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. Microporous Mesoporous Mater. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Liu, H. EDTA-assisted hydrothermal synthesis of cubic SrF2 particles and their catalytic performance for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. Crystengcomm 2019, 21, 1691–1700. [Google Scholar] [CrossRef]
- Lan, G.J.; Wang, Y.; Qiu, Y.Y.; Wang, X.L.; Liang, J.; Han, W.F.; Tang, H.D.; Liu, H.Z.; Liu, J.; Li, Y. Wheat flour-derived N-doped mesoporous carbon extrudate as superior metal-free catalysts for acetylene hydrochlorination. Chem. Commun. 2018, 54, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, X.; Qin, Y.C.; Qiang, L.; He, Y.P.; Su, D.S.; Song, L.J.; Sun, Z.L. Direct Conversion of Acetylene and 1,2-Dichloroethane to Vinyl Chloride Monomer over a Supported Carbon Nitride Catalyst: Tunable Activity Controlled by the Synthesis Temperature. Ind. Eng. Chem. Res. 2019, 58, 5404–5413. [Google Scholar] [CrossRef]
- Liu, B.; Han, W.F.; Li, X.L.; Li, L.C.; Tang, H.D.; Lu, C.S.; Li, Y.; Li, X.N. Quasi metal organic framework with highly concentrated Cr2O3 molecular clusters as the efficient catalyst for dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Appl. Catal. B-Environ. 2019, 257, 117939. [Google Scholar] [CrossRef]
- Wang, H.L.; Han, W.F.; Li, X.L.; Liu, B.; Tang, H.D.; Li, Y. Solution Combustion Synthesis of Cr2O3 Nanoparticles and the Catalytic Performance for Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene. Molecules 2019, 24, 361. [Google Scholar] [CrossRef] [Green Version]
- Han, W.F.; Wang, Z.K.; Li, X.J.; Tang, H.D.; Xi, M.; Li, Y.; Liu, H.Z. Solution combustion synthesis of nano-chromia as catalyst for the dehydrofluorination of 1,1-difluoroethane. J. Mater. Sci. 2016, 51, 11002–11013. [Google Scholar] [CrossRef]
- Han, W.F.; Wang, J.C.; Chen, L.L.; Yang, L.T.; Wang, S.C.; Xi, M.; Tang, H.D.; Liu, W.C.; Song, W.Y.; Zhang, J.J.; et al. Reverting fluoroform back to chlorodifluoromethane and dichlorofluoromethane: Intermolecular Cl/F exchange with chloroform at moderate temperatures. Chem. Eng. J. 2019, 355, 594–601. [Google Scholar] [CrossRef]
- Han, W.F.; Liu, B.; Li, X.L.; Yang, L.T.; Wang, J.C.; Tang, H.D.; Liu, W.C. Combustion Synthesis of Amorphous Al and Cr Composite as the Catalyst for Dehydrofluorination of 1,1-Difluoroethane. Ind. Eng. Chem. Res. 2018, 57, 12774–12783. [Google Scholar] [CrossRef]
- Fang, X.X.; Liao, W.M.; Song, J.D.; Jia, W.Z.; Wang, Y.; Lu, J.Q.; Luo, M.F. Effect of Fe promotion on the performance of V2O5/MgF2 catalysts for gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Appl. Surf. Sci. 2019, 490, 365–371. [Google Scholar] [CrossRef]
- Mao, W.; Jia, Z.H.; Bai, Y.B.; Qin, Y.; Wang, B.; Han, S.; Zhang, W.; Kou, L.G.; Lu, J.; Kemnitz, E. Fe/hollow nano-MgF2: A green and highly-efficient alternative to classical Cr-based catalysts for the gas-phase fluorination reaction. Catal. Sci. Technol. 2019, 9, 3015–3019. [Google Scholar] [CrossRef]
- Han, W.F.; Liu, B.; Kang, Y.K.; Wang, Z.K.; Yu, W.; Yang, H.; Liu, Y.N.; Lu, J.Q.; Tang, H.D.; Li, Y.; et al. Experimental and DFT Mechanistic Study of Dehydrohalogenation of 1-Chloro-1,1-difluoroethane over Metal Fluorides. Ind. Eng. Chem. Res. 2019, 58, 18149–18159. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.K.; Jung, C.H.; Doh, W.H.; Park, J.Y. Effect of the metal-support interaction on the activity and selectivity of methanol oxidation over Au supported on mesoporous oxides. Chem. Commun. 2018, 54, 8174–8177. [Google Scholar] [CrossRef]
- Da Silva, A.B.; Jordao, E.; Mendes, M.J.; Fouilloux, P. Effect of metal-support interaction during selective hydrogenation of cinnamaldehyde to cinnamyl alcohol on platinum based bimetallic catalysts. Appl. Catal. A-Gen. 1997, 148, 253–264. [Google Scholar] [CrossRef]
- Jenness, G.R.; Schmidt, J.R. Unraveling the Role of Metal-Support Interactions in Heterogeneous Catalysis: Oxygenate Selectivity in Fischer-Tropsch Synthesis. ACS Catal. 2013, 3, 2881–2890. [Google Scholar] [CrossRef]
- Gross, E.; Somorjai, G.A. The Impact of Electronic Charge on Catalytic Reactivity and Selectivity of Metal-Oxide Supported Metallic Nanoparticles. Top. Catal. 2013, 56, 1049–1058. [Google Scholar] [CrossRef]
- Wang, Z.K.; Han, W.F.; Tang, H.D.; Liu, H.Z. CaBaFx composite as robust catalyst for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. Catal. Commun. 2019, 120, 42–45. [Google Scholar] [CrossRef]
- Han, W.F.; Zhang, C.P.; Wang, H.L.; Zhou, S.L.; Tang, H.D.; Yang, L.T.; Wang, Z.K. Sub-nano MgF2 embedded in carbon nanofibers and electrospun MgF2 nanofibers by one-step electrospinning as highly efficient catalysts for 1,1,1-trifluoroethane dehydrofluorination. Catal. Sci. Technol. 2017, 7, 6000–6012. [Google Scholar] [CrossRef]
- Al-Newaiser, F.A.; Al-Thabaiti, S.A.; Al-Youbi, A.O.; Obaid, A.Y.; Gabal, M.A. Thermal decomposition kinetics of strontium oxalate. Chem. Pap. 2007, 61, 370–375. [Google Scholar] [CrossRef]
- Dollimore, D.; Heal, G.R.; Passalis, N.P. The thermal analysis of strontium oxalate. Thermochim. Acta 1985, 92, 543–546. [Google Scholar] [CrossRef]
- Nagase, K.; Sato, K.; Tanaka, N. Thermal Dehydration and Decomposition Reactions of Bivalent Metal Oxalates in the Solid State. Bull. Chem. Soc. Jpn. 1975, 48, 439–442. [Google Scholar] [CrossRef]
- Han, W.F.; Wang, H.L.; Liu, B.; Li, X.L.; Tang, H.D.; Li, Y.; Liu, H.Z. PVDF mediated fabrication of freestanding AlF3 sub-microspheres: Facile and controllable synthesis of alpha, beta and theta-AlF3. Mater. Chem. Phys. 2020, 240, 122287. [Google Scholar] [CrossRef]
- Song, S.H.; Son, J.H.; Budiman, A.W.; Choi, M.J.; Chang, T.S.; Shin, C.H. The influence of calcination temperature on catalytic activities in a Co based catalyst for CO2 dry reforming. Korean J. Chem. Eng. 2014, 31, 224–229. [Google Scholar] [CrossRef]
- Harris, L.A.; Goff, J.D.; Carmichael, A.Y.; Riffle, J.S.; Harburn, J.J.; St Pierre, T.G.; Saunders, M. Magnetite nanoparticle dispersions stabilized with triblock copolymers. Chem. Mater. 2003, 15, 1367–1377. [Google Scholar] [CrossRef]
- Yang, X.N.; van Duren, J.K.J.; Janssen, R.A.J.; Michels, M.A.J.; Loos, J. Morphology and thermal stability of the active layer in poly(p-phenylenevinylene)/methanofullerene plastic photovoltaic devices. Macromolecules 2004, 37, 2151–2158. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.H.; Lu, G.Q. Nitrogen-Enriched Nonporous Carbon Electrodes with Extraordinary Supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]
- Ortega, K.F.; Arrigo, R.; Frank, B.; Schlogl, R.; Trunschke, A. Acid-Base Properties of N-Doped Carbon Nanotubes: A Combined Temperature-Programmed Desorption, X-ray Photoelectron Spectroscopy, and 2-Propanol Reaction Investigation. Chem. Mater. 2016, 28, 6826–6839. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Havecker, M.; Schlogl, R.; Su, D.S. Dynamic surface rearrangement and thermal stability of nitrogen functional groups on carbon nanotubes. Chem. Commun. 2008, 4891–4893. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.S.; Zhang, P.P.; Cui, Y.; Liu, G.B.; Wu, J.H.; Yang, G.H.; Yoneyama, Y.; Tsubaki, N. One-Pot Hydrothermal Synthesis of Nitrogen Functionalized Carbonaceous Material Catalysts with Embedded Iron Nanoparticles for CO2 Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 8331–8339. [Google Scholar] [CrossRef]
- Olive, G. (Ca, Sr)F2 surface-roughness effect on angle-resolved xps measurements. Surf. Sci. 1993, 297, 83–90. [Google Scholar] [CrossRef]
- Walas, M.; Lewandowski, T.; Synak, A.; Lapinski, M.; Sadowski, W.; Koscielska, B. Eu3+ doped tellurite glass ceramics containing SrF2 nanocrystals: Preparation, structure and luminescence properties. J. Alloys Compd. 2017, 696, 619–626. [Google Scholar] [CrossRef]
- Han, Y.J.; Park, S.J. Hydrogen Storage Behaviors of Porous Carbons Derived from Poly(vinylidene fluoride). J. Nanosci. Nanotechnol. 2017, 17, 8075–8080. [Google Scholar] [CrossRef]
- Wang, J.C.; Han, W.F.; Wang, S.C.; Tang, H.D.; Liu, W.C.; Li, Y.; Lu, C.S.; Zhang, J.J.; Kennedy, E.M.; Li, X.N. Synergistic catalysis of carbon-partitioned LaF3-BaF2 composites for the coupling of CH4 with CHF3 to VDF. Catal. Sci. Technol. 2019, 9, 1338–1348. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, Y.; Mardochee, K.M.; Wang, Z.; Wang, S.; Yu, W.; Zhang, J.; Han, W. Selectivity Dependence of 1,1-Difluoro-1-Chloroethane Dehydrohalogenation on the Metal–Support Interaction over SrF2 Catalyst. Catalysts 2020, 10, 355. https://doi.org/10.3390/catal10030355
Liu W, Liu Y, Mardochee KM, Wang Z, Wang S, Yu W, Zhang J, Han W. Selectivity Dependence of 1,1-Difluoro-1-Chloroethane Dehydrohalogenation on the Metal–Support Interaction over SrF2 Catalyst. Catalysts. 2020; 10(3):355. https://doi.org/10.3390/catal10030355
Chicago/Turabian StyleLiu, Wucan, Yongnan Liu, Kabozya M. Mardochee, Zhikun Wang, Shucheng Wang, Wei Yu, Jianjun Zhang, and Wenfeng Han. 2020. "Selectivity Dependence of 1,1-Difluoro-1-Chloroethane Dehydrohalogenation on the Metal–Support Interaction over SrF2 Catalyst" Catalysts 10, no. 3: 355. https://doi.org/10.3390/catal10030355




