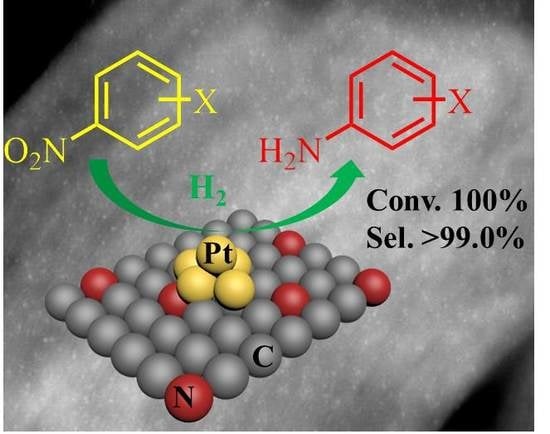
Highly Dispersed Pt Nanoparticles on N-Doped Ordered Mesoporous Carbon as Effective Catalysts for Selective Hydrogenation of Nitroarenes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Reaction
3. Experimental Section
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Reaction and Product Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Lu, H.; Geng, Z.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Metal-Free Reduction of Aromatic Nitro Compounds to Aromatic Amines with B2pin2 in Isopropanol. Org. Lett. 2016, 18, 2774–2776. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.C.; Chen, Y.; Li, X.Y. MoS2/reduced graphene oxide hybrid with CdS nanoparticles as a visible light-Driven photocatalyst for the reduction of 4-Nitrophenol. J. Hazard. Mater. 2016, 309, 173–179. [Google Scholar] [CrossRef]
- Deka, P.; Choudhury, R.; Deka, R.C.; Bharali, P. Influence of Ni on enhanced catalytic activity of Cu/Co3O4 towards reduction of nitroaromatic compounds: Studies on the reduction kinetics. RSC Adv. 2016, 6, 71517–71528. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, L.; Wang, F.; Deng, K.; Lv, K.; Zhang, Z. High performance of a cobalt–Nitrogen complex for the reduction and reductive coupling of nitro compounds into amines and their derivatives. Sci. Adv. 2017, 3, e1601945. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei Rezaei, S.J.; Khorramabadi, H.; Hesami, A.; Ramazani, A.; Amani, V.; Ahmadi, R. Chemoselective reduction of nitro and nitrile compounds with magnetic carbon nanotubes-supported Pt (II) catalyst under mild conditions. Ind. Eng. Chem. Res. 2017, 56, 12256–12266. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lin, K.C.; Liu, S.B. Well-Dispersed rhenium nanoparticles on three-Dimensional carbon nanostructures: Efficient catalysts for the reduction of aromatic nitro compounds. J. Colloid Interface Sci. 2017, 506, 271–282. [Google Scholar] [CrossRef]
- Nandi, D.; Siwal, S.; Choudhary, M.; Mallick, K. Carbon nitride supported palladium nanoparticles: An active system for the reduction of aromatic nitro-Compounds. Appl. Catal. A 2016, 523, 31–38. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef]
- Sudhakar, M.; Naresh, G.; Rambabu, G.; Anjaneyulu, C.; Padmasri, A.H.; Kantam, M.L.; Venugopal, A. Crude bio-Glycerol as a hydrogen source for the selective hydrogenation of aromatic nitro compounds over Ru/MgLaO catalyst. Catal. Commun. 2016, 74, 91–94. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Zhang, C.; Ren, K.; Xin, Y.; Zhao, M.; Xing, E. Selectivity Control on Hydrogenation of Substituted Nitroarenes through End-On Adsorption of Reactants in Zeolite-Encapsulated Platinum Nanoparticles. Chem.-Asian J. 2018, 13, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-Functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Mao, H.; Peng, S.; Yu, H.; Chen, J.; Zhao, S.; Huo, F. Facile synthesis of highly stable heterogeneous catalysts by entrapping metal nanoparticles within mesoporous carbon. J. Mater. Chem. A 2014, 2, 5847–5851. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Y.; Du, J.; Jing, C. Reductive transformation of nitroaromatic compounds by Pd nanoparticles on nitrogen-Doped carbon (Pd@NC) biosynthesized using Pantoea sp. IMH. J. Hazard. Mater. 2019, 366, 338–345. [Google Scholar] [CrossRef]
- Gao, D.; Li, S.; Wang, X.; Xi, L.; Lange, K.M.; Ma, X.; Lv, Y.; Yang, S.; Zhao, K.; Loussala, H.M.; et al. Ultrafine PtRu nanoparticles confined in hierarchically porous carbon derived from micro-Mesoporous zeolite for enhanced nitroarenes reduction performance. J. Catal. 2019, 370, 385–403. [Google Scholar] [CrossRef]
- Xu, G.; Wei, H.; Ren, Y.; Yin, J.; Wang, A.; Zhang, T. Chemoselective hydrogenation of 3-Nitrostyrene over a Pt/FeOx pseudo-Single-Atom-Catalyst in CO2-Expanded liquids. Green Chem. 2016, 18, 1332–1338. [Google Scholar] [CrossRef]
- Miao, H.; Hu, S.; Ma, K.; Sun, L.; Wu, F.; Wang, H.; Li, H. Synthesis of PtCo nanoflowers and its catalytic activity towards nitrobenzene hydrogenation. Catal. Commun. 2018, 109, 33–37. [Google Scholar] [CrossRef]
- Sorribes, I.; Liu, L.; Corma, A. Nanolayered Co–Mo–S catalysts for the chemoselective hydrogenation of nitroarenes. ACS Catal. 2017, 7, 2698–2708. [Google Scholar] [CrossRef]
- Yan, X.; Duan, P.; Zhang, F.; Li, H.; Zhang, H.; Zhao, M.; Zhang, X.; Xu, B.; Pennycook, S.J.; Guo, J. Stable single-Atom platinum catalyst trapped in carbon onion graphitic shells for improved chemoselective hydrogenation of nitroarenes. Carbon 2019, 143, 378–384. [Google Scholar] [CrossRef]
- Guo, X.; Yu, C.; Yin, Z.; Sun, S.; Seto, C. Hydrodehalogenation of Polyhalogenated Aromatics Catalyzed by NiPd Nanoparticles Supported on Nitrogen-Doped Graphene. ChemSusChem 2018, 11, 1617–1620. [Google Scholar] [CrossRef]
- Mane, G.P.; Talapaneni, S.N.; Anand, C.; Varghese, S.; Iwai, H.; Ji, Q.; Ariga, K.; Mori, T.; Vinu, A. Preparation of Highly Ordered Nitrogen-Containing Mesoporous Carbon from a Gelatin Biomolecule and its Excellent Sensing of Acetic Acid. Adv. Funct. Mater. 2012, 22, 3596–3604. [Google Scholar] [CrossRef]
- Karimi, B.; Behzadnia, H.; Bostina, M.; Vali, H. A Nano-Fibrillated Mesoporous Carbon as an Effective Support for Palladium Nanoparticles in the Aerobic Oxidation of Alcohols “on Pure Water”. Chem. Eur. J. 2012, 18, 8634–8640. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tan, M.; Wang, X.; Zhang, M.; Guo, S.; Zou, X.; Lu, X. Synthesis of Mesoporous γ-Alumina-Supported Co-Based Catalysts and Their Catalytic Performance for Chemoselective Reduction of Nitroarenes. ACS Appl. Mater. Interfaces 2018, 10, 5413–5428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Dong, Y.; Chi, B.; Cui, Z.; Deng, Y.; Shi, X.; Du, L.; Liao, S. UIO-66-NH2-Derived Mesoporous Carbon Catalyst Co-Doped with Fe/N/S as Highly Efficient Cathode Catalyst for PEMFCs. Small 2019, 15, 1803520. [Google Scholar] [CrossRef]
- Zhan, T.; Liu, W.; Teng, J.; Yue, C.; Li, D.; Wang, S.; Tan, H. Selective oxidation of glycerol to tartronic acid over Pt/N-Doped mesoporous carbon with extra framework magnesium catalysts under base-Free conditions. Chem. Commun. 2019, 55, 2620–2623. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, W.; Cheng, S.; Guo, S.; Shang, N.; Gao, S.; Feng, C.; Wang, C.; Wang, Z. Pd9Ag1-N-doped-MOF-C: An efficient catalyst for catalytic transfer hydrogenation of nitro-Compounds. Catal. Commun. 2017, 95, 50–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, P.; Zhang, H.; Yin, G.; Zhao, J. Cobalt nanoparticles anchoring on nitrogen doped carbon with excellent performances for transfer hydrogenation of nitrocompounds to primary amines and N-Substituted formamides with formic acid. Catal. Commun. 2019, 129, 105747. [Google Scholar] [CrossRef]
- Vakili, R.; Gibson, E.K.; Chansai, S.; Xu, S.; Al-Janabi, N.; Wells, P.P.; Hardacre, C.; Walton, A.; Fan, X. Understanding the CO oxidation on Pt nanoparticles supported on MOFs by operando XPS. ChemCatChem 2018, 10, 4238–4242. [Google Scholar] [CrossRef] [Green Version]
- Banu, K.; Shimura, T. A novel electroless method for the deposition of single-Crystalline platinum nanoparticle films on an organic solid matrix in the presence of gold single crystals. New J. Chem. 2011, 35, 1503–1514. [Google Scholar] [CrossRef]
- Wan, X.K.; Wu, H.B.; Guan, B.Y.; Luan, D.; Lou, X.W. Confining Sub-Nanometer Pt Clusters in Hollow Mesoporous Carbon Spheres for Boosting Hydrogen Evolution Activity. Adv. Mater. 2020, 32, 1901349. [Google Scholar] [CrossRef]
- Duan, Y.; Song, T.; Dong, X.; Yang, Y. Enhanced catalytic performance of cobalt nanoparticles coated with a N, P-codoped carbon shell derived from biomass for transfer hydrogenation of functionalized nitroarenes. Green Chem. 2018, 20, 2821–2828. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, C.; Chen, S.; Li, H.; Yang, H.; Zhang, X. In situ mosaic strategy generated Co-based N-doped mesoporous carbon for highly selective hydrogenation of nitroaromatics. J. Catal. 2017, 348, 212–222. [Google Scholar] [CrossRef]
- Li, X.; Zeng, C.; Jiang, J.; Ai, L. Magnetic cobalt nanoparticles embedded in hierarchically porous nitrogen-Doped carbon frameworks for highly efficient and well-Recyclable catalysis. J. Mater. Chem. A 2016, 4, 7476–7482. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C bifunctional catalysts for highly efficient Zn–Air batteries and water splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Du, A.; Amal, R.; Ng, Y.H. Decorating platinum on nitrogen-Doped graphene sheets: Control of the platinum particle size distribution for improved photocatalytic H2 generation. Chem. Eng. Sci. 2019, 194, 85–93. [Google Scholar] [CrossRef]
- Li, D.; Fang, H.; Yu, J.; Xu, M.; Li, T.; Wang, J. Porous carbon supported PtPd alloy nanoparticles derived from N-Heterocyclic carbene bimetal complex as efficient bifunctional electrocatalysts. Electrochim. Acta 2020, 337, 135855. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Chen, C.; Yang, X.; Huang, Y.; Cao, R. Defective Pt nanoparticles encapsulated in mesoporous metal–Organic frameworks for enhanced catalysis. Chem. Commun. 2018, 54, 8822–8825. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
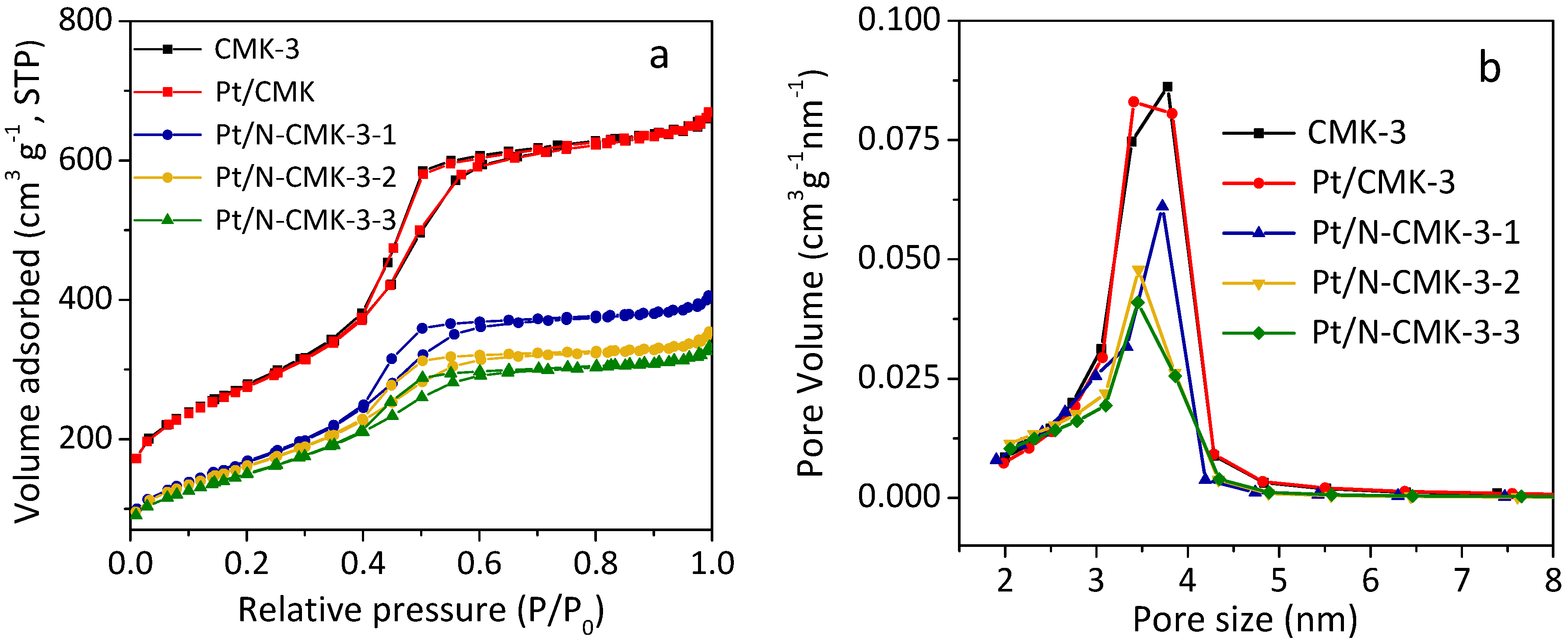




| Samples | Pt (wt %) | N (wt %) | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Pt crystallite Size by XRD (nm) |
|---|---|---|---|---|---|---|
| CMK-3 | - | - | 979 | 1.02 | 3.9 | - |
| Pt/CMK-3 | 1.95 | - | 971 | 1.02 | 3.9 | 5.5 |
| Pt/N-CMK-3-1 | 1.92 | 2.1 | 620 | 0.60 | 3.7 | 3.0 |
| Pt/N-CMK-3-2 | 1.89 | 3.5 | 590 | 0.52 | 3.5 | - |
| Pt/N-CMK-3-3 | 1.87 | 3.7 | 548 | 0.49 | 3.4 | - |
| Spent Pt/N-CMK-3-2 | 1.86 | 3.5 | 579 | 0.51 | 3.5 | - |
| Samples | Nitrogen Content (Atom %) a | Pyridinic-Type (%) b | Pyrrolic-Type (%) b | Graphitic-Type (%) b |
|---|---|---|---|---|
| Pt/N-CMK-3-1 | 2.0 | 9.0 | 68.2 | 22.8 |
| Pt/N-CMK-3-2 | 3.3 | 6.9 | 57.6 | 35.5 |
| Pt/N-CMK-3-3 | 3.6 | 7.0 | 41.9 | 51.1 |
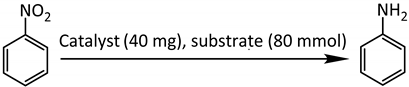 | |||||
|---|---|---|---|---|---|
| Entry | Solvent (mL) | H2 Pressure (MPa) | Temperature (°C) | Conv. (%) b | Sel. (%) |
| 1 | Ethylacetate (20 mL) | 2 | 40 | 49.2 | 100 |
| 2 | Toluene (20 mL) | 2 | 40 | 25.2 | 100 |
| 3 | Ethyl ether (20 mL) | 2 | 40 | 44.3 | 100 |
| 4 | Methanol (20 mL) | 2 | 40 | 53.7 | 100 |
| 5 | Ethanol (20 mL) | 2 | 40 | 79.5 | 100 |
| 6 | Ethanol (10 mL) | 2 | 40 | 65.1 | 100 |
| 7 | Ethanol (30 mL) | 2 | 40 | 63.6 | 100 |
| 8 | Ethanol (20 mL) | 0.5 | 40 | 24.4 | 100 |
| 9 | Ethanol (20 mL) | 1 | 40 | 56.8 | 100 |
| 10 | Ethanol (20 mL) | 4 | 40 | 80.3 | 100 |
| 11 | Ethanol (20 mL) | 6 | 40 | 81.4 | 100 |
| 12 | Ethanol (20 mL) | 2 | 60 | 98.2 | 100 |
| 13 | Ethanol (20 mL) | 2 | 80 | 100 (0.5 h) | 100 |
| 14 | Ethanol (20 mL) | 2 | 100 | 100 (0.2 h) | 100 |
| Entry | Catalyst | Conv. (%) | TOF (h−1) b | Sel. (%) |
|---|---|---|---|---|
| 1 | CMK-3 | 0 | 0 | - |
| 2 | N-CMK-3-2 | 0 | 0 | - |
| 3 | Pt/CMK-3 | 3.9 | 4680 | 100 |
| 4 | Pt/N-CMK-3-1 | 8.0 | 9750 | 100 |
| 5 | Pt/N-CMK-3-2 | 15.2 | 18819 | 100 |
| 6 | Pt/N-CMK-3-3 | 9.4 | 11763 | 100 |
| Entry | Substrate | Time (h) | Conv. (%) | Sel. (%) |
|---|---|---|---|---|
| 1 |  | 1.5 | >99.9 | 99.9 |
| 2 | 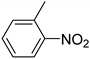 | 2.0 | >99.9 | 99.7 |
| 3 | 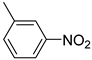 | 2.0 | >99.9 | 99.5 |
| 4 | 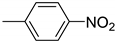 | 2.0 | >99.9 | 99.2 |
| 5 | 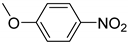 | 4.0 | >99.9 | 99.7 |
| 6 | 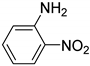 | 3.0 | >99.9 | 99.9 |
| 7 | 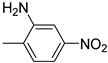 | 3.0 | >99.9 | 99.9 |
| 8 |  | 3.0 | >99.9 | 99.0 |
| 9 | 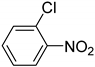 | 2.5 | >99.9 | 99.7 |
| 10 | 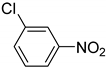 | 3.0 | >99.9 | 99.3 |
| 11 | 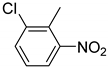 | 3.0 | >99.9 | 99.4 |
| 12 | 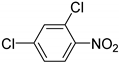 | 2.5 | >99.9 | 98.5 |
| 13 | 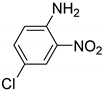 | 2.0 | >99.9 | 99.1 |
| 14 |  | 4.0 | >99.9 | 99.6 |
| 15 |  | 2.5 | >99.9 | 99.1 |
| 16 |  | 3.5 | >99.9 | 99.5 |
| 17 |  | 4.0 | >99.9 | 98.2 |
| 18 |  | 2.0 | >99.9 | 99.0 |
| 19 | 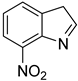 | 8.0 | >99.9 | 99.6 |
| 20 | 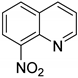 | 5.0 | >99.9 | 99.7 |
| 21 |  | 4.0 | >99.9 | 99.7 |
| 22 | 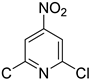 | 3.0 | >99.9 | 99.5 |
| 23 b | 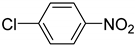 | 1.5 | 35.2 | 83.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Lin, X.; Wang, X.; Zou, X.; Zhang, C. Highly Dispersed Pt Nanoparticles on N-Doped Ordered Mesoporous Carbon as Effective Catalysts for Selective Hydrogenation of Nitroarenes. Catalysts 2020, 10, 374. https://doi.org/10.3390/catal10040374
Sheng Y, Lin X, Wang X, Zou X, Zhang C. Highly Dispersed Pt Nanoparticles on N-Doped Ordered Mesoporous Carbon as Effective Catalysts for Selective Hydrogenation of Nitroarenes. Catalysts. 2020; 10(4):374. https://doi.org/10.3390/catal10040374
Chicago/Turabian StyleSheng, Yao, Xinrui Lin, Xueguang Wang, Xiujing Zou, and Chunlei Zhang. 2020. "Highly Dispersed Pt Nanoparticles on N-Doped Ordered Mesoporous Carbon as Effective Catalysts for Selective Hydrogenation of Nitroarenes" Catalysts 10, no. 4: 374. https://doi.org/10.3390/catal10040374