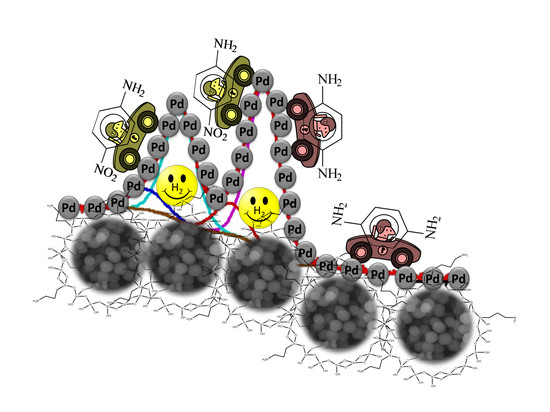
Amino-Modified Silica as Effective Support of the Palladium Catalyst for 4-Nitroaniline Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
2.1. FTIR Spectroscopy of Silica Supports
2.2. The Pulse Chemisorption
2.3. XPS of Catalysts before the Reaction
2.4. XPS of Used Catalysts
2.5. SEM
2.6. TEM
2.7. TPD of Matrices
2.8. The Activity of Synthesized Catalysts
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sol-Gel Method
3.2.1. Synthesis of Silica
3.2.2. Synthesis of Amino-Functionalized Silica
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.2. Hydrogen Pulse Chemisorption
3.4.3. XPS
3.4.4. SEM
3.4.5. TEM
3.4.6. Temperature Programmed Desorption (TPD) of NH3
3.4.7. Catalysts Activity Experiments Description
3.4.8. Deactivation Experiments Using Recovered Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gómez, J.E.; Kleij, A.W. Chapter Three-Catalytic nonreductive valorization of carbon dioxide into fine chemicals. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 71, pp. 175–226. [Google Scholar] [CrossRef]
- Gabriele, B. Chapter 3-Synthesis of Heterocycles by Palladium-Catalyzed Carbonylative Reactions. In Advances in Transition-Metal Mediated Heterocyclic Synthesis; Solé, D., Fernández, I., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 55–127. [Google Scholar] [CrossRef]
- Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S. Flow fine synthesis with heterogeneous catalysts. Tetrahedron 2018, 74, 1705–1730. [Google Scholar] [CrossRef]
- Yokoi, T.; Kubota, Y.; Tatsumi, T. Amino-functionalized mesoporous silica as base catalyst and adsorbent. Appl. Catal. A Gen. 2012, 421, 14–37. [Google Scholar] [CrossRef]
- Hocking, M.B. 19-PETROCHEMICALS. In Handbook of Chemical Technology and Pollution Control; Hocking, M.B., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 633–664. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 3-Industrial Organic Chemistry. In Environmental Organic Chemistry for Engineers; Speight, J.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 87–151. [Google Scholar] [CrossRef]
- Vedrine, J.C. 8-Main industrial processes using metal oxides as catalysts or support and future trends in heterogeneous catalysis. In Metal Oxides in Heterogeneous Catalysis; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–549. [Google Scholar] [CrossRef]
- Kelkar, A.A. Chapter 14-Carbonylations and Hydroformylations for Fine Chemicals. In Industrial Catalytic Processes for Fine and Specialty Chemicals; Joshi, S.S., Ranade, V.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 663–692. [Google Scholar] [CrossRef]
- Borah, B.J.; Mondal, M.; Bharali, P. Chapter 27-Palladium-Based Hybrid Nanocatalysts: Application toward Reduction Reactions. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 565–583. [Google Scholar] [CrossRef]
- Albéniz, A.C.; Casares, J.A. Chapter One-Palladium-Mediated Organofluorine Chemistry. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 62, pp. 1–110. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, M.; Wang, B.; Ling, L. Acetylene selective hydrogenation over different size of Pd-modified Cu cluster catalysts: Effects of Pd ensemble and cluster size on the selectivity and activity. Appl. Surf. Sci. 2019, 481, 421–432. [Google Scholar] [CrossRef]
- Yang, Q.; Hou, R.; Sun, K. Tuning butene selectivities by Cu modification on Pd-based catalyst for the selective hydrogenation of 1, 3-butadiene. J. Catal. 2019, 374, 12–23. [Google Scholar] [CrossRef]
- Eslava, J.L.; Gallegos-Suárez, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of Mo promotion on the activity and selectivity of Ru/Graphite catalysts for Fischer-Tropsch synthesis. Catal. Today 2019. [Google Scholar] [CrossRef]
- He, Z.-H.; Li, N.; Wang, K.; Wang, W.-T.; Liu, Z.-T. Selective hydrogenation of quinolines over a CoCu bimetallic catalyst at low temperature. Mol. Catal. 2019, 470, 120–126. [Google Scholar] [CrossRef]
- Chakoli, A.N.; Sadeghzadeh, M. Chapter 27-Recent Trends in Biomedical and Pharmaceutical Industry Due to Engineered Nanomaterials. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 499–519. [Google Scholar] [CrossRef]
- Zhu, J.; Wood, J.; Deplanche, K.; Mikheenko, I.; Macaskie, L.E. Selective hydrogenation using palladium bioinorganic catalyst. Appl. Catal. B Environ. 2016, 199, 108–122. [Google Scholar] [CrossRef]
- Akti, F. The effect of potassium modification on structural properties and catalytic activity of copper and iron containing SBA-16 catalysts for selective oxidation of ethanol. Mater. Chem. Phys. 2019, 227, 21–28. [Google Scholar] [CrossRef]
- Sánchez, G.; Friggieri, J.; Keast, C.; Drewery, M.; Dlugogorski, B.Z.; Kennedy, E.; Stockenhuber, M. The effect of catalyst modification on the conversion of glycerol to allyl alcohol. Appl. Catal. B Environ. 2014, 152, 117–128. [Google Scholar] [CrossRef]
- Zhang, H.; Ke, D.; Cheng, L.; Feng, X.; Hou, X.; Wang, J.; Li, Y.; Han, S. CoPt-Co hybrid supported on amino modified SiO2 nanospheres as a high performance catalyst for hydrogen generation from ammonia borane. Prog. Nat. Sci. Mater. Int. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Fernandes, A.E.; Jonas, A.M. Design and engineering of multifunctional silica-supported cooperative catalysts. Catal. Today 2018, 334, 173–186. [Google Scholar] [CrossRef]
- Feng, X.; Song, Z.; Guo, T.; Yang, R.; Liu, Y.; Chen, X.; Yang, C. Insights into the effect of surface functional groups on catalytic performance for hydrogen generation from sodium borohydride. RSC Adv. 2016, 6, 113260–113266. [Google Scholar] [CrossRef]
- Khalili, D.; Banazadeh, A.R.; Etemadi-Davan, E. Palladium Stabilized by Amino-Vinyl Silica Functionalized Magnetic Carbon Nanotube: Application in Suzuki–Miyaura and Heck–Mizoroki Coupling Reactions. Catal. Lett. 2017, 147, 2674–2687. [Google Scholar] [CrossRef]
- Miller, J.T.; Mojet, B.L.; Ramaker, D.E.; Koningsberger, D.C. A new model for the metal–support interaction: Evidence for a shift in the energy of the valence orbitals. Catal. Today 2000, 62, 101–114. [Google Scholar] [CrossRef]
- Jin, M.-H.; Park, J.-H.; Oh, D.; Park, J.-S.; Lee, K.-Y.; Lee, D.-W. Effect of the amine group content on catalytic activity and stability of mesoporous silica supported Pd catalysts for additive-free formic acid dehydrogenation at room temperature. Int. J. Hydrogen Energy 2019, 44, 4737–4744. [Google Scholar] [CrossRef]
- Vona, D.; Cicco, S.; Ragni, R.; Leone, G.; Lo Presti, M.; Farinola, G. Biosilica/polydopamine/silver nanoparticles composites: New hybrid multifunctional heterostructures obtained by chemical modification of Thalassiosira weissflogii silica shells. MRS Commun. 2018, 8, 911–917. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kusuma, D.Y.; Lee, P.S.; Srinivasan, M.P. Covalent Assembly of Gold Nanoparticles for Nonvolatile Memory Applications. ACS Appl. Mater. Interfaces 2011, 3, 4619–4625. [Google Scholar] [CrossRef]
- Alvarez-Toral, A.; Fernández, B.; Malherbe, J.; Claverie, F.; Pecheyran, C.; Pereiro, R. Synthesis of amino-functionalized silica nanoparticles for preparation of new laboratory standards. Spectrochim. Acta Part B At. Spectrosc. 2017, 138, 1–7. [Google Scholar] [CrossRef]
- Sugimura, H.; Hanji, T.; Takai, O.; Masuda, T.; Misawa, H. Photolithography based on organosilane self-assembled monolayer resist. Electrochim. Acta 2001, 47, 103–107. [Google Scholar] [CrossRef]
- Pasquardini, L.; Lunelli, L.; Potrich, C.; Marocchi, L.; Fiorilli, S.; Vozzi, D.; Vanzetti, L.; Gasparini, P.; Anderle, M.; Pederzolli, C. Organo-silane coated substrates for DNA purification. Appl. Surf. Sci. 2011, 257, 10821–10827. [Google Scholar] [CrossRef]
- Oliveira, R.L.; He, W.; Klein Gebbink, R.J.M.; de Jong, K.P. Palladium nanoparticles confined in thiol-functionalized ordered mesoporous silica for more stable Heck and Suzuki catalysts. Catal. Sci. Technol. 2015, 5, 1919–1928. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Mohammadsaleh, F. Triazole-Functionalized Silica Supported Palladium(II) Complex: A Novel and Highly Active Heterogeneous Nano-catalyst for C–C Coupling Reactions in Aqueous Media. Catal. Lett. 2018, 148, 1035–1046. [Google Scholar] [CrossRef]
- Yuan, M.; Yang, R.; Wei, S.; Hu, X.; Xu, D.; Yang, J.; Dong, Z. Ultra-fine Pd nanoparticles confined in a porous organic polymer: A leaching-and-aggregation-resistant catalyst for the efficient reduction of nitroarenes by NaBH4. J. Colloid Interface Sci. 2019, 538, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Bourane, A.; Elanany, M.; Pham, T.V.; Katikaneni, S.P. An overview of organic liquid phase hydrogen carriers. Int. J. Hydrogen Energy 2016, 41, 23075–23091. [Google Scholar] [CrossRef]
- Schrimpf, M.; Esteban, J.; Rösler, T.; Vorholt, A.J.; Leitner, W. Intensified reactors for gas-liquid-liquid multiphase catalysis: From chemistry to engineering. Chem. Eng. J. 2019, 372, 917–939. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Huang, T.; Fu, Y.; Peng, Q.; Yu, C.; Zhu, J.; Yu, A.; Wang, X. Catalytic hydrogenation of p-nitrophenol using a metal-free catalyst of porous crimped graphitic carbon nitride. Appl. Surf. Sci. 2019, 480, 888–895. [Google Scholar] [CrossRef]
- Sharma, S. Metal dependent catalytic hydrogenation of nitroarenes over water-soluble glutathione capped metal nanoparticles. J. Colloid Interface Sci. 2015, 441, 25–29. [Google Scholar] [CrossRef]
- Fu, Y.; Qin, L.; Huang, D.; Zeng, G.; Lai, C.; Li, B.; He, J.; Yi, H.; Zhang, M.; Cheng, M.; et al. Chitosan functionalized activated coke for Au nanoparticles anchoring: Green synthesis and catalytic activities in hydrogenation of nitrophenols and azo dyes. Appl. Catal. B Environ. 2019, 255, 117740. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Saeb, M.R.; Sefat, F.; Rezaeian, I.; Ganjali, M.R.; Ramakrishna, S.; Mozafari, M. Oligoaniline-based conductive biomaterials for tissue engineering. Acta Biomater. 2018, 72, 16–34. [Google Scholar] [CrossRef]
- Amer, I.; Mokrani, T.; Jewell, L.; Young, D.A.; Vosloo, H.C.M. Oxidative copolymerization of p-phenylenediamine and 3-aminobenzenesulfonic acid. Tetrahedron Lett. 2016, 57, 426–430. [Google Scholar] [CrossRef]
- Jin, J.-S.; Ning, Y.-Y.; Hu, K.; Wu, H.; Zhang, Z.-T. Solubility of p-Nitroaniline in Supercritical Carbon Dioxide with and without Mixed Cosolvents. J. Chem. Eng. Data 2013, 58, 1464–1469. [Google Scholar] [CrossRef]
- Nishioka, R.; Hiasa, T.; Kimura, K.; Onishi, H. Specific Hydration on p-Nitroaniline Crystal Studied by Atomic Force Microscopy. J. Phys. Chem. C 2013, 117, 2939–2943. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Lo Re, G.; Bertani, R.; Milani, R.; Sassi, A. Surface modification of poly(ethylene-co-acrylic acid) with amino-functionalized silica nanoparticles. J. Mater. Chem. 2011, 21, 3849–3857. [Google Scholar] [CrossRef]
- Jakša, G.; Štefane, B.; Kovač, J. XPS and AFM characterization of aminosilanes with different numbers of bonding sites on a silicon wafer. Surf. Interface Anal. 2013, 45, 1709–1713. [Google Scholar] [CrossRef]
- Latypova, A.; Tarasyuk, I.; Filippov, D.; Lefedova, O.; Bykov, A.; Sidorov, A.; Doluda, V.; Sulman, E. Synthesis, stability and activity of palladium supported over various inorganic matrices in the selective hydrogenation of nitroaniline. React. Kinet. Mech. Catal. 2019, 127, 741–755. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Wang, J.; Wang, R. Facile Fabrication of Ultrafine Palladium Nanoparticles with Size- and Location-Control in Click-Based Porous Organic Polymers. ACS Nano 2014, 8, 5352–5364. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, C.; Wang, Y.; Wang, R.; Hong, M. Tailor-made porosities of fluorene-based porous organic frameworks for the pre-designable fabrication of palladium nanoparticles with size, location and distribution control. Chem. Sci. 2016, 7, 2188–2194. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, C.; Tian, H.; Xiong, Y.; Zhang, H.; Qiao, P.; Fan, J.; Zhang, Z.; Li, Z.Y.; Li, J. An In situ TEM study of the surface oxidation of palladium nanocrystals assisted by electron irradiation. Nanoscale 2017, 9, 6327–6333. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef]
- Blosi, M.; Ortelli, S.; Costa, A.L.; Dondi, M.; Lolli, A.; Andreoli, S.; Benito, P.; Albonetti, S. Bimetallic Nanoparticles as Efficient Catalysts: Facile and Green Microwave Synthesis. Materials 2016, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Kha, N.T.T.; Merkin, A.A.; Komarov, A.A.; Korpatenkov, D.O.; Lefedova, O.V. Kinetics of catalytic hydrogenation of 4-nitroaniline in aqueous solutions of propan-2-ol with acid or base additives. Russ. J. Phys. Chem. A 2014, 88, 588–590. [Google Scholar] [CrossRef]
- Doluda, V.Y.; Sidorov, A.I.; Sulman, E.M.; Latypova, A.R.; Filippov, D.V.; Lefedova, O.V. Synthesis, structure and catalytic properties of Pd nanostructured materials in p-nitroaniline catalytic hydrogenation. Izv. Vyssh. Uchebnykh Zaved. Khimiya Khimicheskaya Tekhnol. 2019, 62, 60–68. [Google Scholar] [CrossRef]








| Simple | Metal Dispersion,% | Metallic Surface Area, m2/g Metal | Concentration of Active Center mmol/g |
|---|---|---|---|
| 5 wt.% Pd/SiO2-C3H6-(30%)NH2 | 5.0 | 21 | 0.017 |
| 5 wt.% Pd/SiO2 | 4.3 | 19 | 0.016 |
| Catalyst | Binding Energy, eV | Chemical State | at,% |
|---|---|---|---|
| 5 wt.% Pd/SiO2 [45] | 334.87 | Pd0 | 1.47 |
| 337.09 | PdO | 0.17 | |
| 5 wt.% Pd/SiO2-C3H6-NH2 | 334.44 | Pd0 | 2.12 |
| 336.59 | PdO/Pd0 | 0.15 |
| Catalyst | Binding Energy, eV | Chemical State | At,% |
|---|---|---|---|
| 5 wt.% Pd/SiO2 [45] | 335.04 | Pd0 | 1.04 |
| 337.25 | PdO | 0.06 | |
| 5 wt.% Pd/SiO2-C3H6-(30%)NH2 | 334.51 | Pd0 | 2.10 |
| 336.50 | PdO/Pd0 | 0.23 |
| Catalyst | The Hydrogen Consumption Rate 10−5 Mole(H2)/sec. | ||||
|---|---|---|---|---|---|
| 1 run | 2 run | 3 run | 4 run | 5 run | |
| 5 wt.% Pd/SiO2-C3H6-(30%)NH2 | 10.1 | 9.5 | 8.8 | 8.3 | 8.0 |
| 5 wt.% Pd/SiO2 [45,52] | 0.8 | 0.8 | 0.8 | 0.7 | 0.6 |
| Catalyst | Conversion * of 4-nitroaniline | Yields * of 1,4-phenylenediamine |
|---|---|---|
| 5 wt.% Pd/SiO2-C3H6-(30%)NH2 | 100 | 100 |
| 5 wt.% Pd/C | 100 | 100 |
| 5 wt.% Pd/SiO2 | 100 | 100 |
| 5 wt.% Pd/γ-Al2O3 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latypova, A.R.; Lebedev, M.D.; Rumyantsev, E.V.; Filippov, D.V.; Lefedova, O.V.; Bykov, A.V.; Doluda, V.Y. Amino-Modified Silica as Effective Support of the Palladium Catalyst for 4-Nitroaniline Hydrogenation. Catalysts 2020, 10, 375. https://doi.org/10.3390/catal10040375
Latypova AR, Lebedev MD, Rumyantsev EV, Filippov DV, Lefedova OV, Bykov AV, Doluda VY. Amino-Modified Silica as Effective Support of the Palladium Catalyst for 4-Nitroaniline Hydrogenation. Catalysts. 2020; 10(4):375. https://doi.org/10.3390/catal10040375
Chicago/Turabian StyleLatypova, Adele R., Maxim D. Lebedev, Evgeniy V. Rumyantsev, Dmitry V. Filippov, Olga V. Lefedova, Alexey V. Bykov, and Valentin Yu. Doluda. 2020. "Amino-Modified Silica as Effective Support of the Palladium Catalyst for 4-Nitroaniline Hydrogenation" Catalysts 10, no. 4: 375. https://doi.org/10.3390/catal10040375
APA StyleLatypova, A. R., Lebedev, M. D., Rumyantsev, E. V., Filippov, D. V., Lefedova, O. V., Bykov, A. V., & Doluda, V. Y. (2020). Amino-Modified Silica as Effective Support of the Palladium Catalyst for 4-Nitroaniline Hydrogenation. Catalysts, 10(4), 375. https://doi.org/10.3390/catal10040375